Screening Lactobacillus plantarum Strains with Potential to Protect Human Intestinal Epithelium HT-29 Cells against Injury Caused by Escherichia coli or H2O2
2022-04-01MAChangluLIUQingLIShurongZHANGLijuanTUOYanfeng
MA Changlu, LIU Qing, LI Shurong, ZHANG Lijuan, TUO Yanfeng,*
(1. Department of Food and Biological Engineering, Beijing Vocational College of Agriculture, Beijing 102442, China;2. School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China)
Abstract: Probiotic Lactobacillus strains can exert anti-inflammatory or antioxidant activities after colonization in the gut,which is beneficial for the host’s intestinal health. In this study, eight L. plantarum strains isolated from traditional fermented dairy foods in Xinjiang, China, were evaluated for their protective effects on HT-29 human intestinal epithelial cells against injury caused by Escherichia coli or hydrogen peroxide (H2O2). Among these strains, L. plantarum 35 showed the highest adhesion capacity to HT-29 cell monolayers, and it reduced E. coli adhesion to HT-29 cells by displacement, competition,and exclusion, with inhibition rates of 42.60%, 59.17%, and 60.19%, respectively. L. plantarum 35 and its crude exopolysaccharide (EPS) inhibited E. coli from stimulating HT-29 cells to produce interleukin-8 (IL-8), protected HT-29 cells against injury induced by H2O2, increased the levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px),and decreased malondialdehyde (MDA) content in HT-29 cells. Therefore, L. plantarum 35 and its crude EPS have the potential to inhibit inflammatory bowel disease induced by E. coli O157.
Keywords: Lactobacillus; anti-adhesion; Escherichia coli O157; HT-29 cells
Human intestinal microbiota homeostasis and redox homeostasis play an important role in maintaining proper function of the gastrointestinal tract (GIT)[1]. Dysbiosis of the microbial population in the GIT may lead to inflammation and development of inflammatory bowel disease (IBD)[2]. The continuous formation of reactive oxygen species (ROS) in the GIT contributes to the pathogenesis of several gastrointestinal disorders, including IBD[1]. Significant increase in detrimental bacteria and ROS in GIT play an important role in the pathogenesis of IBDs[3]. The human intestinal cell lines Caco-2 and HT-29 isolated from colon adenocarcinoma are the most widely usedin vitroattachment and mechanism studies[4].As a goblet cell, HT-29 can secrete several mucins mainly expressed by theMUC5ACmucin gene. The HT-29 cell line grows in a single layer, with a brush-like edge on the top,and mucus production is similar to that of human intestinal mucosa. Compared with the Caco-2 or HT-29 single cell culture model, the mucus production and trans-epithelium electrical resistant (TEER) value of the co-culture model are closer to the human small intestine[5-6].
SomeLactobacillusstrains have been reported to restore GIT microbiota[2-3,7]or quench intestinal ROS[8-9],which benefit for GIT health. Pathogens growth and proinflammatory cytokines production in GIT could be inhibited by someLactobacillus strainsand their metabolites[10-12].Adequate intake of suchLactobacillusstrains had the therapeutic effects to alleviate the symptom of IBD in animal trial and clinical research[13-14].Lactobacillusstrains with anti-oxidative properties have been shown to be protective in some IBD models.Lactobacillus rhamnosusGG (LGG)-produced proteins protect the intestinal epithelial tight junctions and the barrier function from H2O2-induced insult[15].Lactobacillus fermentumLF1 could ameliorate the colitis of mouse by increasing the expression of anti-oxidative enzymes such as superoxide dismutase (SOD) and decreasing the malondialdehyde (MDA) level of the mouse[16].
In this study, eightLactobacillus plantarumstrains and their exopolysaccharides were studied for their protection for human intestinal epithelium cell model HT-29 cells againstE. coliadhesion and pro-inflammatory cytokine inducing interleukin-8 (IL-8) production, and against H2O2induced injury to find probiotic potential strains.
1 Materials and Methods
1.1 Materials and reagent
Escherichia coliO157 was obtained from State Key Laboratory of Dairy Biotechnology. TheLactobacillusstrains were isolated from traditional fermented dairy food in Yining,Xinjiang, China. LGG was provided by L. Zhang from Harbin Institute of Technology. Human colonic cancer cell line HT-29 was the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China).
Muir-Torre syndrome (MTS) assay kit England Abcam Co., Ltd.; de Man, Rogosa and Sharpe (MRS)medium Beijing Aobox Biotechnology Co., Ltd.; IL-8 enzyme-linked immunosorbent assay (ELISA) kit Shanghai Senxiong Co., Ltd.; Fetal bovine serum and McCoy’s medium USA Thermo Fisher Scientific Co., Ltd.; SOD,glutathione peroxidase (GSH-Px) and MDA assays kit Nanjing Jiancheng Bioengineering Institute; Luria-Bertani(LB) medium Beijing Land Bridge Technology Co. Ltd..
1.2 Instrument and equipment
SX-500 High temperature autoclave Japan Tomy Co.,Ltd.; Sartorius AG PB-10 pH meter Germany Sartorius Co., Ltd.; 5804R High speed freezing centrifuge Germany Eppendorf Co., Ltd.; MCO-18AIC(UV) HERAEUS Instruments Japan Sanyo Co., Ltd.; Multiskan GO Microplate Reader USA Thermo Fisher Scientific Co., Ltd.;Alpha 1-2 vacuum drier Germany Marin Christ Co., Ltd..
1.3 Methods
1.3.1 HT-29 cell culture
The HT-29 cells were cultured in McCoy’s medium supplemented with 10% (V/V) inactivated (56 ℃, 30 min)fetal calf serum in a humidified atmosphere of 5% CO2and 95% air at 37 ℃. The HT-29 cells were incubated to become fully differentiated. Then, cell viability was assessed by 0.2%(m/V) trypan-blue dye in phosphate buffered saline (PBS)(0.01 mol/L, pH 7.2) and the cell number was determined by hemocytometer. Cultures with cell viability greater than 80%were analyzed for subsequent assays. HT-29 cell monolayers were used as intestinal epithelium model to study theE. coliadhesion, IL-8 cytokine production, and injury by H2O2.
1.3.2 Identification of antibacterial activity ofLactobacillusstrains
Lactobacilluswere cultured anaerobically in MRS broth at 37 ℃ for 18 h.E. coliwas cultured aerobically in LB medium at 37 ℃ for 18 h. Serial dilutions of the suspension were plated onto MRS or LB plates and incubated at 37 ℃for 18 h to determine theLactobacillusorE. colibacterial number, separately. According to the method of Guo Yuanji[17], to pour 0.1 mL of cell suspensions ofE. coliinto LB agar medium. After the medium had solidified, punch a 5 mm pore size in the medium, and add 50 μL ofLactobacillusfermentation broth at 37 ℃ for 18 h. At the same time, MRS medium was used as positive control. Record the diameter of the inhibition zone and compared with the control group.
1.3.3 Determination ofLactobacillusstrains and EPS on theE. coliadhesion to HT-29 cells monolayers
The effect ofLactobacillusstrains on theE. coliadhesion on HT-29 cells was assessed by using HT-29 cells as an intestinal epithelial cell model[18]. HT-29 cells were seeded on 12-well cell culture plates at a concentration of 1 × 105cells per well. The plates were cultured at 37 ℃ in a humidified atmosphere of 5% CO2and 95% air until a confluent monolayer were obtained. HT-29 cell monolayers on the 12-well plates were washed twice with PBS before the adhesion assay.Lactobacillusstrains andE. coliwere harvested by centrifugation at 10 000 ×gfor 10 min at 4 ℃and washed twice with PBS, and then resuspended in McCoy’s medium, adjusted to 1 × 109CFU/mL, separately.
To study the adhering abilities ofLactobacillusstrains to HT-29 cell monolayers according to the method described by Lee et al.[19]with some modifications, 1.0 mL ofLactobacillusstrains McCoy’s medium suspension was added into microtitre wells with HT-29 cell monolayers. After 2 h of incubation at 37 ℃, each well of the plates was washed 4 times with PBS to remove free, nonattached bacterial cells.
To study the capacity ability ofLactobacillusstrains for adhering to HT-29 cells withE. coliaccording to the method described by Guo Yuanji[17], both 0.5 mL ofLactobacillusstrains McCoy’s medium suspension and 0.5 mL ofE. coliMcCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers. After 2 h of incubation at 37 ℃, each well of the plates was washed 4 times with PBS to remove free, nonattached bacterial cells.
To study the excluding capacity ofLactobacillusstrains fromE. coliadhering to HT-29 cells, 0.5 mL ofLactobacillusstrains McCoy’s medium suspension was added into microtitre wells with HT-29 cell monolayers, supplemented with 0.5 mL McCoy’s medium. After 1 h of incubation at 37 ℃, 0.5 mL ofE. coliMcCoy’s medium suspension was added into the same wells. After another 1 h of incubation,each well of the plates was washed 4 times with PBS to remove free, nonattached bacterial cells. To study the displacing capacity ofLactobacillusstrains fromE. coliadhering to HT-29 cells, 0.5 mL ofE. coliMcCoy’s medium suspension was added into microtitre wells with HT-29 cell monolayers, supplemented with 0.5 mL McCoy’s medium.After 1 h of incubation at 37 ℃, 0.5 mL ofLactobacillusstrains McCoy’s medium suspension was added into the same wells. After another 1 h of incubation, each well of the plates was washed 4 times with PBS to remove free, nonattached bacterial cells.
To study the inhibiting activity ofLactobacillusstrains’crude exopolysaccharide (EPS) againstE. coliadhering to HT-29 cells[17], both 0.5 mL ofLactobacillusstrains’EPS McCoy’s medium suspension (500, 250, 125 μg/mL,separately) and 0.5 mL ofE. coliMcCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers. After 2 h of incubation at 37 ℃, each well of the plates was washed 4 times with PBS to remove free,nonattached bacterial cells. The EPS of theLactobacillusstrains was extracted according to the reported method[19].TheLactobacillusstrains were cultured in MRS media at 37 ℃ for 18 h. The supernatants from cultures ofLactobacilluswere obtained by centrifugation (10 000 ×g, 15 min, 4 ℃).Trichloroacetic acid (TCA) was added to the supernatant for 12 h. Then centrifugation to remove protein (10 000 ×g,15 min, 4 ℃). EPS in the supernatant were precipitated by slowly adding 2 volumes of prechilled anhydrous ethanol(4 ℃) and stored at 4 ℃ for 24 h. The precipitation of EPS was obtained by centrifugation to solubilize in deionized water. The crude EPS was dialyzed (8-14 kDa) for 24 h.
In order to explore the effect of different concentration ofLactobacilluson the inhibition ofE. coliadhesion to HT-29 cells. Both 0.5 mL ofLactobacillusstrains (106, 107,108CFU/mL , respectively) McCoy’s medium suspension and 0.5 mL ofE. coliMcCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers. After 2 h of incubation at 37 ℃, each well of the plates was washed 4 times with PBS to remove free, nonattached bacterial cells.
Then 1 mL of 1% (V/V) Triton X-100 was added to each well, and the suspension was stirred to detach the bacterial cells from HT-29 cell monolayers. Serial dilutions of the suspension were plated onto MRS or LB plates and incubated at 37 ℃ to determine theLactobacillusorE. colibacterial cell number adhering to HT-29 cells.
The adhesion capacity of theLactobacillusstrains to HT-29 cell monolayers was expressed as adhesive number or the adhering rate. Adhesive number was expressed in logarithmic form, and adhering rate was calculated as equation (1).
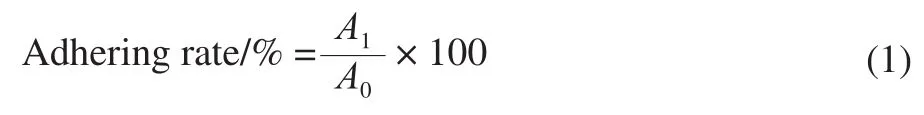
WhereA1is the number ofLactobacillusstrains adhering to HT-29 cell monolayers (lg(CFU/mL)) ;A0is the number ofLactobacillusstrains added into microtitre wells initially (lg(CFU/mL)) .
The inhibiting rate againstE. colifrom adhering to HT-29 cell monolayers was calculated as follows equation (2).

WhereN1is the number ofE. coliadhering to HT-29 cell monolayers/(CFU/mL);N0is the number ofE. coliadded into microtitre wells initially/(CFU/mL).
1.3.4 Determination ofLactobacillusstrains and their crude EPS on the IL-8 production of HT-29 cell monolayers induced byE. coli
The effect ofLactobacillusstrains and their EPS on the IL-8 production of HT-29 cells induced byE. coliwas assessed by using HT-29 cells as an intestinal epithelial cell model[20].
HT-29 cells were seeded in 12 well plates at 2 × 105cells/well. The plates were cultured at 37 ℃ in a humidified atmosphere of 5% CO2and 95% air for 48 h prior to infection.Lactobacillusstrains andE. coliwere harvested by centrifugation at 10 000 ×gfor 10 min at 4 ℃ and washed twice with PBS, and then resuspended in McCoy’s medium (antibioticfree, fetal bovine serum-free), adjusted to 1 × 109CFU/mL.
To study the inhibition ofLactobacillusstrains against IL-8 production of HT-29 cells induced byE. coli, a total of six experimental groups were determined according to the method of Roselli et al[21]. Control group: 1 mL of McCoy’s medium were added into microtitre wells with HT-29 cell monolayers for 3 h incubation;L. plantarumgroup: 1 mL ofLactobacillusstrains McCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers for 3 h incubation.E. coligroup: 1 mL ofE. coliMcCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers for 3 h incubation.L. plantarum+E. coligroup: both 0.5 mL ofLactobacillusstrains McCoy’s medium suspension and 0.5 mL ofE. coliMcCoy’s medium suspension were added into microtitre wells with HT-29 cell monolayers for 3 h incubation;L. plantarum/E. coligroup:0.5 mL ofLactobacillusstrains McCoy’s medium suspension was added into microtitre wells with HT-29 cell monolayers firstly, after 1 h incubation, 0.5 mL ofE. coliMcCoy’s medium suspension was added into the same wells for 2 h incubation;E. coli/L. plantarumgroup: 0.5 mL ofE. coliMcCoy’s medium suspension was added into microtitre wells with HT-29 cell monolayers firstly, after 1 h incubation, then 0.5 mL ofLactobacillusstrains McCoy’s medium suspension was added into the same wells for 2 h incubation.
To study the effect of different concentration ofLactobacillusEPS on the IL-8 production of HT-29 cells induced byE. coli, both 0.5 mL ofLactobacillusstrains EPS McCoy’s medium suspension (at the concentration of 500,250 and 125 μg/mL, respectively) and 0.5 mL ofE. coliMcCoy’s medium suspension (at the dose of bacteria:cell =100:1) were added into microtitre wells with HT-29 cell monolayers for 3 h incubation.
After 3 h of incubation at 37 ℃, the cell culture supernatants were harvested and the IL-8 levels were determined by using IL-8 ELISA kit.
1.3.5 Determination of protective effects ofLactobacillusstrains and their crude EPS on HT-29 cells against injury by H2O2
The protecting effects of theLactobacillusstrains and their crude EPS on HT-29 cells damaged by H2O2was assessed according to Zhang Li[22]and Li Jingyan[23]et al.The human colon cancer cell line HT-29 cell was routinely cultured at 37 ℃ in a 5% CO2atmosphere in McCoy’s medium supplemented with 10% (V/V) heat-inactivated foetal calf serum. After cell dissociation, the cells were added in 96 -well plates at the final density of 1 × 105cells per well and incubated to allow for cell attachment. After 24 h incubation, the growth medium was removed and the HT-29 cell monolayers were washed twice with PBS;1.0 mL ofLactobacillusstrains (LGG group: LGG strains only;L. plantarum35 group:L. plantarum35 strains only) McCoy’s medium suspension (1 × 109CFU/mL) orLactobacillusstrains EPS (EPS group:L. plantarum35 EPS: 500, 250,125 μg/mL, respectively; LGG EPS: 500, 250, 125 μg/mL,respectively) McCoy’s medium suspension was added into the same wells for 2 h incubation, and then H2O2was added to induce oxidative stress at the final concentration of 150 μmol/L. The control group cells were only treated with fresh medium; the H2O2group only treated with H2O2. After 24 h incubation, the damage was stopped by adding fresh medium.The survival rate of HT-29 cells was measured by MTS method. The MTS was added to the medium at 20 μL per well.After 4 h incubation, the absorbance at 490 nm was measured.The survival rate (%) of HT-29 cells was expressed as mean percentage of viable cells compared with control group.
To study the effects of theLactobacillusstrains and their crude EPS on the levels of SOD, GSH-Px and MDA of HT-29 cells damaged by H2O2, HT-29 cells were treated as described above. After 24 h incubation, each well of the plates was washed for 3 times with PBS, and then 0.5 mL of 0.5% (V/V)Triton X-100 was added to each well and incubated for 5 min at ice bath; the suspension was collected and centrifugated(10 000 ×g, 10 min, 4 ℃) to obtain the supernatant; the levels of SOD, GSH-Px and MDA in the supernatant were assessed by using assay kits.
1.4 Statistical analysis
All the statistical analyses were done in a triplicate manner and the data collected. Results are presented as mean ±standard deviation. Statistical treatment of the data was conducted using analysis of variances (ANOVA) of SPSS Statistics 22 software, and the Duncan’s test was used to compare the means when the overallPvalue of the experiment was below the value of significance (P< 0.05).
2 Results and Analysis
2.1 Antibacterial activity and adhesion capacity of Lactobacillus strains
In this study, 8 strains ofL. plantarumwere studied for their protective effects for HT-29 cell monolayers against the injury caused byE. coliand H2O2. TheLactobacillusstrains were isolated from traditional fermented dairy food in Yining,Xinjiang, China.
The supernatant of the 8Lactobacillusstrains’ MRS culture could inhibit the growth ofE. coliO157 in different degrees, among whichL. plantarum16 and 35 had higher antagonistic effect on the growth ofE. colias shown in Table 1. The lower pH value (approximately 3.6) of the strains’ culture supernatant accounted for the antagonistic activity. Organic acid and bacteriocin produced by LAB could inhibit the growth and colonization of pathogens in the gut[24]. The adhesion capacity ofLactobacillusstrains on intestinal epithelial cells is an important characteristic as probiotics against pathogens adhesion. As shown in Table 1,L. plantarum35, 2 and LGG had significantly higher(P< 0.05) adhering abilities on HT-29 cell monolayers than those of the otherLactobacillusstrains. TheL. plantarum35 was selected to do further study due to its higher antagonistic activity and adhesion capacity. TheL. plantarum22, which showed lower (P< 0.05) antagonistic activity and adhesion capacity than those ofL. plantarum35 and LGG, was selected for comparison withL. plantarum35 and LGG in the further study.

Table 1 Antibacterial effect against E. coli O157 and adhesion capacity of Lactobacillus strains
2.2 Protection of Lactobacillus strains and EPS against E. coli adhering to HT-29 cells
WhenL. plantarum22, 35 and LGG adhered to HT-29 cell monolayers prior to 1 h beforeE. coliwas added,L. plantarum35 and LGG inhibitedE. coliadhering to HT-29 cell monolayers with higher (P< 0.05) inhibiting rates (60.19% and 57.57%) thanL. plantarum22 did with inhibiting rate (26.31%) as shown in Table 2, indicating thatL. plantarum35 and LGG could preventE. coliadhering to HT-29 cell monolayers by previously occupying adhering site on HT-29 cell monolayers. WhenL. plantarum22,35 and LGG competed withE. colifor adhering to HT-29 cell monolayers,L. plantarum35 and LGG inhibitedE. coliadhering to HT-29 cell monolayers with higher(P< 0.05) inhibiting rate (59.17% and 51.26%) thanL. plantarum22 did with inhibiting rate (27.73%) as shown in Table 2. Maybe,L. plantarum35 and LGG had priority to adhere to HT-29 cell monolayers. WhenE. coliadhered to HT-29 cell monolayers previously,L. plantarum35 and LGG could displace the adheringE. coliwith higher (P< 0.05)inhibiting rate (42.60% and 31.32%) thanL. plantarum22 did with inhibiting rate (12.85%) as shown in Table 2, indicating thatL. plantarum35 and LGG could relieve some symptoms induced byE. coliadhering to intestinal epithelial cells.

Table 2 Three Lactobacillus strains inhibited the adhesion of E. coli to HT-29 cell monolayers by displacement, competition, and exclusion
In this study,L. plantarum35 exhibited the higher adhering rate on HT-29 cell monolayers and higher inhibiting activity againstE. coliadhesion. Furthermore,L. plantarum35, at different concentration (1 × 106CFU/well,1 × 107CFU/well and 1 × 108CFU/well, respectively),could decrease the amount ofE. coliadhering to HT-29 cell monolayers significantly (P< 0.05) in a dose-dependent manner, as shown in Fig. 1. TheL. plantarum35 was selected for further probiotic functional study. Probiotic strains could be selected according to some criteria, such as adhesion capacity, competition or inhibition against pathogen adhesion and colonization in intestinal epithelium[25].
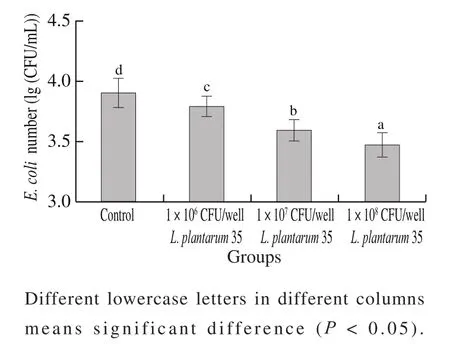
Fig. 1 Inhibitoion rates of different doses of L. plantarum 35 against E. coli adhesion to HT-29 cell monolayers
The EPS ofL. plantarum35 and LGG was extracted and found to exert inhibiting effect againstE. coliadhering to HT-29 cell monolayers, as shown in Table 3. Only at the highest concentration of 500 μg/mL, did the EPS ofL. plantarum35 and LGG inhibitE. coliadhering to HT-29 cell monolayers significantly (P< 0.05). At the concentration of 250 μg/mL and 125 μg/mL, the EPS of two strains had no inhibiting effect againstE. coliadhesion.

Table 3 Effects of different concentrations of L. plantarum 35 and LGG EPS against E. coli adhesion to HT-29 cell monolayers
2.3 Effects of Lactobacillus strains and EPS on the IL-8 production of HT-29 cells induced by E. coli
The further study found thatL. plantarum35 and its EPS could inhibitE. coliinducing HT-29 cells to express pro-inflammatory cytokine IL-8, as shown in Table 4 and Table 5. When the HT-29 cell monolayers were pretreated withL. plantarum35 and LGG respectively, or the HT-29 cell monolayers were treated with the twoLactobacillusand theE. coliat the same time, all the twoLactobacillusstrains inhibited IL-8 production of HT-29 cells induced byE. colisignificantly (P< 0.05); while when the HT-29 cell monolayers were treated with theE. colipreviously,L.plantarum35 could not decrease the IL-8 levels of HT-29 cells induced byE. coli.
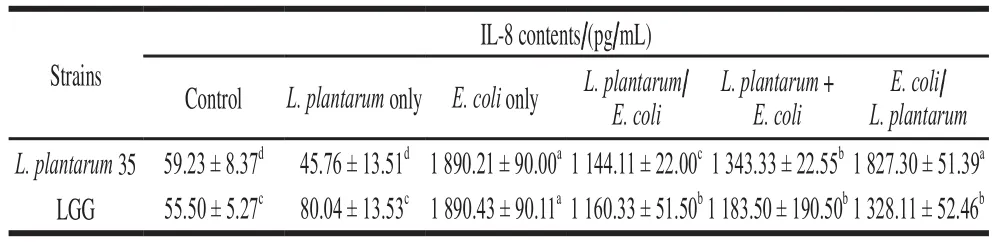
Table 4 Effects of Lactobacillus strains on IL-8 production by HT-29 cells induced by E. coli

Table 5 Effects of EPS of L. plantarum 35 and EPS of LGG on IL-8 production by HT-29 cells induced by E. coli
At the concentrations of 500, 250 and 125 μg/mL, the EPS ofL. plantarum35 and LGG could inhibit the IL-8 expression of HT-29 cells induced byE. colisignificantly(P< 0.05). The inhibiting effect ofL. plantarum35 and its EPS against IL-8 production of HT-29 cell monolayers induced byE. colicould be related with their capacity to inhibitE. coliadhering to HT-29 cell monolayers.Bifidobacterium lactisHN019[26]andLactobacillus crispatusK313[27]had been reported to inhibit the attachment of pathogenic bacteria to intestinal epithelial cells and to attenuate IL-8 expression level from intestinal epithelium induced by pathogens. Ligands such as flagellin on the surface of pathogenic bacteria promotes pathogens adhesion and invasion to host. And the metabolites of some beneficial bacteria, such as polysaccharide, could protect host from pathogen-induced-IBDs by promoting Tregcell function through special ligandreceptor interaction[28]. Maybe the special components and structure of theL. plantarum35 cell wall and its EPS accounts for its protection for HT-29 cells againstE. coliinvasion.
2.4 Protection of Lactobacillus strains and their EPS against H2O2 injury to HT-29 cells
Enhanced oxidative stress was shown to be tightly connected with the development and perpetuation of IBDs[29].ROS are important contributors to IBDs. In this study, the HT-29 cells were treated by H2O2at the concentration of 150 μmol/L, at which the survival rate of the HT-29 cells was up to 50%. When the HT-29 cells treated by H2O2at 150 μmol/L were intervened byL. plantarum35, LGG and their EPS, the survival rates of the HT-29 cells increased significantly (P< 0.05) as shown in Table 6, indicating thatL. plantarum35, LGG and their EPS protected HT-29 cells from H2O2damage.
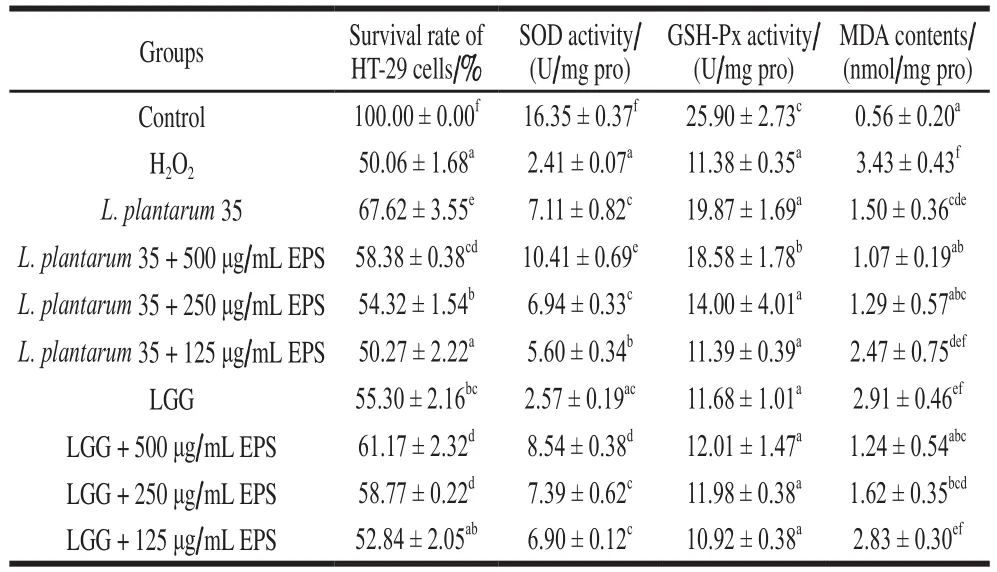
Table 6 Effects of L. plantarum 35 and LGG and their EPS on the survival rate, SOD, GSH-Px and MDA levels of H2O2-induced HT-29 cells
The intracellular catalase rather than GSH-Px in HT-29 cells was reported to protect against the cell injury induced by H2O2[29]. Our research found that the activities of SOD and GSH-Px of the HT-29 cells intervened by theLactobacillusstrains increased significantly (P< 0.05) than those of HT-29 cells treated only by H2O2, while the MDA levels decreased significantly (P< 0.05), as shown in Table 6. It was assumed that SOD and GSH-Px of the HT-29 cells could breakdown H2O2to rescue HT-29 cell from oxidative injury. One possible mechanism of probiotic-mediated protection against H2O2induced injury to intestinal epithelium was the degradation of H2O2[15]. L. fermentumLF1 was reported to ameliorate the colitis of mouse by increasing the expression of antioxidative enzymes such as SOD and decreasing the MDA level of the mouse[16]. Probiotics, which could help to promote the potency of the whole body antioxidative defense system, have recently emerged as the powerful microbial tools for novel therapeutic applications against IBDs[3,30].
Generation of excessive level of ROS in intestinal tract happens to be the hall mark of IBDs[31]. The increase of adherent invasive pathogens in GIT, such asE. coli,were found to be more prevalent in IBDs[32]. In this study,L. plantarum35 and its crude EPS inhibitedE. coliadhering to HT-29 cell monolayers, and enhanced the survival rate and enzyme activities of SOD and GSH-Px of HT-29 cells damaged by H2O2.
3 Conclusion
In this study, theL. plantarum35 had higher antagonistic effect on the growth ofE. coliand higher adhering abilities on HT-29 cell monolayers.L. plantarum35 and its crude EPS inhibited the pro-inflammatory cytokine IL-8 expression of HT-29 cells by inhibitingE. coliadhesion, and enhanced the survival rate of the H2O2-damaged HT-29 cells by enhancing SOD and GSH-Px expression. The results indicated thatL. plantarum35 and its crude EPS have the potential to ameliorate IBDs induced by pathogens and reactive oxygen.
