Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress
2022-03-17KantSangpairojRapeewanSettacomkulTanapanSiangchamKraiMeemonNakornNiamnontNilubonSornkaewMontakanTamtinPrasertSobhonPornpunVivithanaporn
Kant Sangpairoj, Rapeewan Settacomkul, Tanapan Siangcham, Krai Meemon, Nakorn Niamnont, Nilubon Sornkaew, Montakan Tamtin, Prasert Sobhon, Pornpun Vivithanaporn✉
1Division of Anatomy, Department of Preclinical Science, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
2Thammasat University Research Unit in Neutraceuticals and Food Safety, Pathum Thani, Thailand
3Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan, Thailand
4Faculty of Allied Health Sciences, Burapha University, Chonburi, Thailand
5Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand
6Department of Chemistry, Faculty of Science, King Mongkut's University of Technology Thonburi, Bang Mod, Bangkok, Thailand
7Department of Fisheries, Phetcha Buri Coastal Aquaculture Research and Development Center, Phetcha Buri, Thailand
ABSTRACT
Objective: To investigate the effect of the hexane solvent fraction of Halymenia durvillei (HDHE) on triple-negative breast cancer.
Methods: The phytochemical profile of HDHE was investigated by GC-MS. The cytotoxicity of HDHE against MDA-MB-231 cells was determined. The apoptotic and autophagic effects of HDHE were analyzed. The expression of molecular markers controlling apoptosis, autophagy, DNA damage, and endoplasmic reticulum (ER)stress was determined.
Results: HDHE contains a mixture of fatty acids, mainly hexadecanoic acid. HDHE at a cytotoxic concentration induced apoptotic death of MDA-MB-231 cells through mitochondrial membrane dysfunction, and induction of apoptosis markers, and increased the expression of proteins related to DNA damage response. HDHE also induced the expression of LC-3, a marker of autophagic cell death at a cytotoxic concentration. Moreover, HDHE modulated the expression of ER stress genes.
Conclusions: The hexadecanoic acid-enriched extract of Halymenia durvillei promotes apoptosis and autophagy of human triple-negative breast cancer cells. This extract may be further explored as an anticancer agent for triple-negative breast cancer.
KEYWORDS: Halymenia durvillei; Triple-negative breast cancer;Apoptosis; Autophagy; Endoplasmic reticulum stress; Red algae;Hexadecanoic acid
Significance
Marine red algae synthesize a variety of bioactive compounds with potential anticancer activity. The present study demonstrates that the hexadecanoic acid-enriched extract of red alga Halymenia durvillei could induce both caspase-dependent apoptotic and autophagic death of MDA-MB-231 cells. Halymenia durvillei at a cytotoxic concentration caused mitochondrial membrane dysfunction, DNA damage and ER stress.
1. Introduction
Breast cancer is the most common cancer in women worldwide.Triple-negative breast cancer (TNBC) is characterized by the lack of estrogen, progesterone, and epidermal growth factor receptors,leading to the resistance to endocrine therapy and targeted monoclonal therapy. TNBC, which accounts for about 15% of global breast cancer cases, displays poor prognosis and long-term outcomes; therefore, it is considered the most lethal subtype[1].TNBC demonstrates a high histological grade with increased proliferative and metastatic abilities[2]. Cytotoxic chemotherapy is the typical treatment for the malignant stage and TNBC. However,TNBC often shows resistance to cytotoxic drugs and develops metastasis[3]. Therefore, the search for novel effective therapeutics for breast cancer is urgently needed.
It is evidenced that natural compounds can decrease the aggressiveness of breast cancer, inhibit cancer cell proliferation, and modulate cancer-related pathways and may be applied as alternative medicine or adjuvant treatment. Marine seaweeds synthesize a variety of bioactive compounds with potential anticancer activity,including carotenoids, flavonoids, sulfated polysaccharides,terpenoids, and fatty acids[4]. The potential use of algae extracts for the prevention of various cancers including breast cancer has gained great interest. Dietary seaweed consumption reduced the incidence and mortality of postmenopausal breast cancer, which was associated with the decreased concentration of urinary human urokinasetype plasminogen activator receptor (uPAR), a tumor metastasis biomarker[5]. Therefore, the extracts of culinary seaweed might have the potential to be used for cancer prevention and treatment.
Halymenia durvillei (H. durvillei) classified as red marine algae is widely found in the tropical areas around the Indo-Pacific region[6]. It can be used as food and natural coloring substances in cosmetics and pharmaceuticals. Red pigments of the algae are rich in chlorophyll,carotenes, xanthophyll, zeaxanthins, luteins, and phycocyanins[7].Their cell walls contain carrageenan, porphyrans, and phycocolloids as sulfated polysaccharides[8]. The present study aimed at evaluating the anticancer effects of H. durvillei extracts against human TNBC.
2. Materials and methods
2.1. Preparation of seaweed extracts
H. durvillei was cultured and collected from Phetcha Buri coastal aquaculture research and development center, Phetchaburi,Thailand. The authentication of H. durvillei (voucher specimens No.SPFR16040) was performed by Dr. Montakan Tamtin, Department of Fisheries, Coastal Aquatic Feed Research Institute, Coastal Fisheries Research, and Development Bureau, Petchaburi, Thailand.The dried whole crude H. durvillei was soluted and macerated in 95% ethanol for 7 d. The ethanolic extracts were partitioned in hexane and evaporated to remove residual solvents. The hexane solvent extract of H. durvillei (HDHE) was freeze-dried and collected for further experiments.
2.2. Gas chromatography-mass spectrometry (GC-MS)analysis
The phytochemical investigation of HDHE extracts was performed by gas chromatography coupled to mass spectrometry (GC-MS)using Agilent 7890B GC system (Agilent Technologies, USA). The experimental conditions of the GC-MS system were as follows: HP-5 column 30 m × 0.25 mm i.d. with 0.25 μm film thickness. Flow rate of mobile phase (carrier gas: He) was set at 1 mL/min. In the gas chromatography part, the oven temperature was raised from 50 ℃to 250 ℃ at 5 ℃/min and the injection volume was 1 μL. Samples dissolved in chloroform were fully run at a range of 50-400 m/z and the results were compared by using Wiley Spectral library search program NIST MS search 2.0.
2.3. Cell culture
MDA-MB-231 cells were obtained from the American Type Culture Collection (ATCC, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) containing 10% fetal bovine serum and 1% penicillin/streptomycin cocktail antibiotics in an incubator with an atmosphere of 5% CO2at 37 ℃.
2.4. Determination of cytotoxicity
HDHE was dissolved in dimethyl sulfoxide (DMSO). Cultured cells in 96-well plates were incubated with HDHE diluted in serumfree media at a final concentration of 10-1 000 μg/mL for 24 h.The final concentration of DMSO was 0.25%. The cytotoxicity of treated cells was determined by the methyl thiazolyl tetrazolium(MTT) assays. MTT solution (Bio Basic, Canada) was dissolved in treated wells to the final concentration of 30 μg/mL, followed by 3 h incubation. DMSO was added to dissolve MTT formazan. The reaction was measured by a spectrophotometer (Varioskan Flash Microplate Reader, Thermo Fisher Scientific) at the absorbance of 562 nm and 630 nm.
2.5. Apoptotic cell detection
The effects of HDEH on percentage of apoptotic cells were analyzed by annexin V staining. After 24 h treatment with HDHE,cells were collected and incubated with annexin V-FITC (BD Biosciences) for 15 min in dark at room temperature. The number of annexin V-positive cells was measured using a Guava easyCyte flow cytometer (Luminex Corporation) at 5 000 events/sample.
2.6. Analysis of mitochondrial membrane potential
The change of mitochondrial membrane potential in MDA-MB-231 cells after HDHE treatment was analyzed using JC-1 mitochondrial membrane potential assay (Luminex Corporation).After 24 h treatment with HDHE, cells were washed with phosphatebuffered saline before the incubation of JC-1 solution for 10 min.Fluorescence signals were detected using a Guava easyCyte flow cytometer (Luminex Corporation) at 2 000 events/sample.
2.7. Caspase 3/7 activation assay
Cultured MDA-MB-231 cells in 6-well plates were treated with HDHE for 24 h. After that, treated cells were collected and stained with caspase 3/7 carboxyfluorescein (FAM) reagents in dark at 37 ℃ for 1 h according to the manufacturer’s protocol (Luminex Corporation). Cells were washed with apoptosis washing buffer and stained with 7-aminoactinomycin D. Population of activated caspases and dead cells were determined by a flow cytometer(Luminex Corporation) at 5 000 events/sample.
2.8. DNA damage analysis
Cultured MDA-MB-231 cells in 6-well plates were treated with HDHE for 24 h. After that, cells were collected, fixed, and stained with anti-phospho-histone H2AX-Alexa488 and anti-phosphoataxia-telangiectasia mutated kinase (ATM)-PE (Merck Millipore)in dark at room temperature for 30 min. The phosphorylated-ATM and phosphorylated-H2AX populations were determined by a flow cytometer (Luminex Corporation) at 5 000 events/sample.
2.9. Real-time PCR
HDHE-treated MDA-MB-231 cells at 24 h were washed and RNA was extracted using Total RNA purification Kit (Geneaid,Taiwan). After that, cDNA was amplified by mixing total RNA with Primer “random” (Sigma) and Superscript Ⅲ reverse transcriptase(Invitrogen). Gene expression was measured using SensiFAST SYBR LO-ROX (Bioline, Canada) on a real-time PCR machine(Applied Biosystems Real-time PCR 7500 system ABI 7500,Applied Biosystems). For the analysis of endoplasmic reticulum stress, the expressions of activating transcription factor 6 (ATF6),activating protein kinase RNA-like endoplasmic reticulum kinase(PERK), activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), growth arrest, and DNA damage-inducible protein(GADD34), inositol-requiring enzyme 1α (IRE1α), spliced X-Box binding protein 1 (XBP1s) and 78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP) were analyzed. Gene expression was normalized to the expression of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primer sequences of all genes are summarized in Supplementary Table 1.
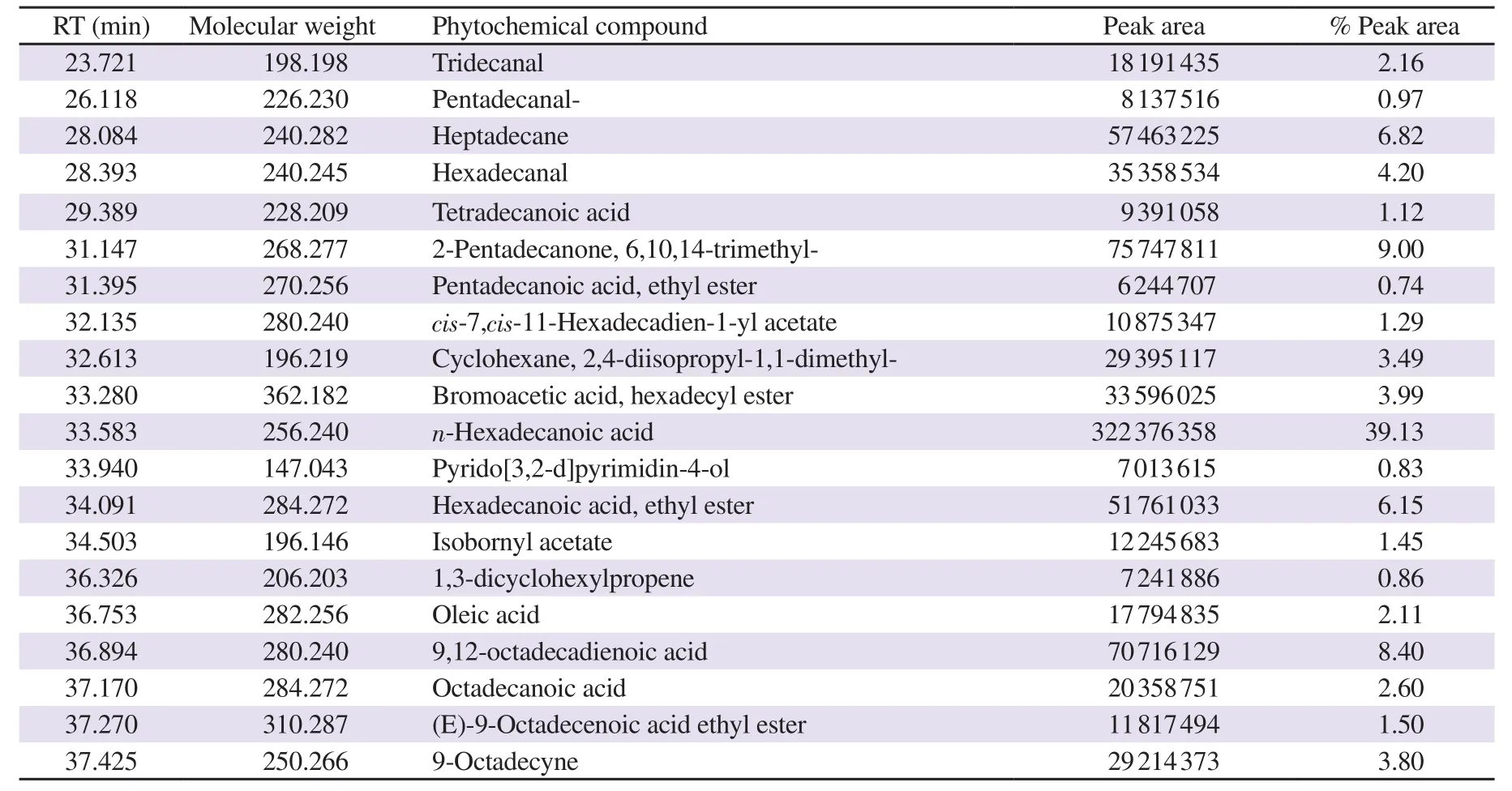
Table 1. Compounds identified in hexane solvent fraction of Halymenia durvillei (HDHE) by GC-MS.
2.10. Immunoblotting
Whole proteins of treated MDA-MB-231 cells were extracted using a lysis buffer [20 mM Tris, 1% NP-40, 50 mM NaCl and 0.1% protease inhibitor cocktail (Calbiochem)]. Protein concentrations were determined using Bradford protein assays (Bio-Rad, USA).Whole proteins at 30 μg were separated by 15% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked in tris-buffered saline and Tween-20 (TBS-T), containing 5% non-fat dry milk for 1 h, then incubated with the primary antibodies including rabbit anti-Bax (5023S, 1:400 dilution), rabbit anti-Bcl-2 (2872T, 1:600 dilution), rabbit anti-LC3 (12741T, 1:1 000 dilution), rabbit anti-beclin-1 (3495T, 1:1 000 dilution) and rabbit anti-β-actin (4970S, 1:8 000 dilution) (Cell Signaling Technology).The membranes were washed with TBS-T, then incubated with corresponding HRP-conjugated secondary antibodies (4010-05,1:5 000 dilution) (Southern Biotech) diluted in TBS-T at room temperature for 1 h. The expressions of targeted proteins were visualized by ECL chemiluminescence system (Bio-Rad). The relative expression level of targeted proteins was normalized to β-actin expression compared with the control group.
2.11. Statistical analysis
Data were expressed as mean ± SD. Statistical variations of experiments were analyzed by GraphPad Prism statistical analysis software (GraphPad Software Inc, USA) using the Student’s t-test. A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. Characterization of bioactive compounds in HDHE
GC-MS was performed to characterize the major bioactive compounds in the extract. Top twenty bioactive compounds were identified as shown in Figure 1. The main compounds were identified as the major peaks, including n-hexadecanoic acid [retention time(RT) 33.583 min, 39.13%], 6,10,14-trimethyl-2-pentadecanone(RT 31.147 min, 9.0%), 9,12-octadecadienoic acid (RT 36.894 min, 8.40%), heptadecane (RT 28.084 min, 6.82%), hexadecenoic acid ethyl ester (RT 34.091 min, 6.15%), octadecanoic acid (RT 37.170 min, 2.6%) and oleic acid (RT 36.753 min, 2.11%) (Table 1). The results revealed that HDHE contains a mixture of bioactive components.
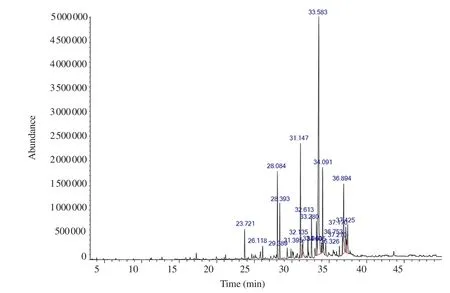
Figure 1. GC-MS chromatogram of HDHE.
3.2. HDHE induces cytotoxicity in MDA-MB-231 cells
The effects of HDHE on cell viability of MDA-MB-231 were determined using MTT assays. The increased concentrations of HDHE decreased the viability of MDA-MB-231 cells after treatment for 24 h when compared with the untreated control group that was considered as 100% viability (Figure 2). The IC50of the extract was (50.35±17.72) μg/mL. The results indicated that HDHE was cytotoxic to MDA-MB-231 cells. HDHE at 50 μg/mL was used in further experiments as the cytotoxic concentration.
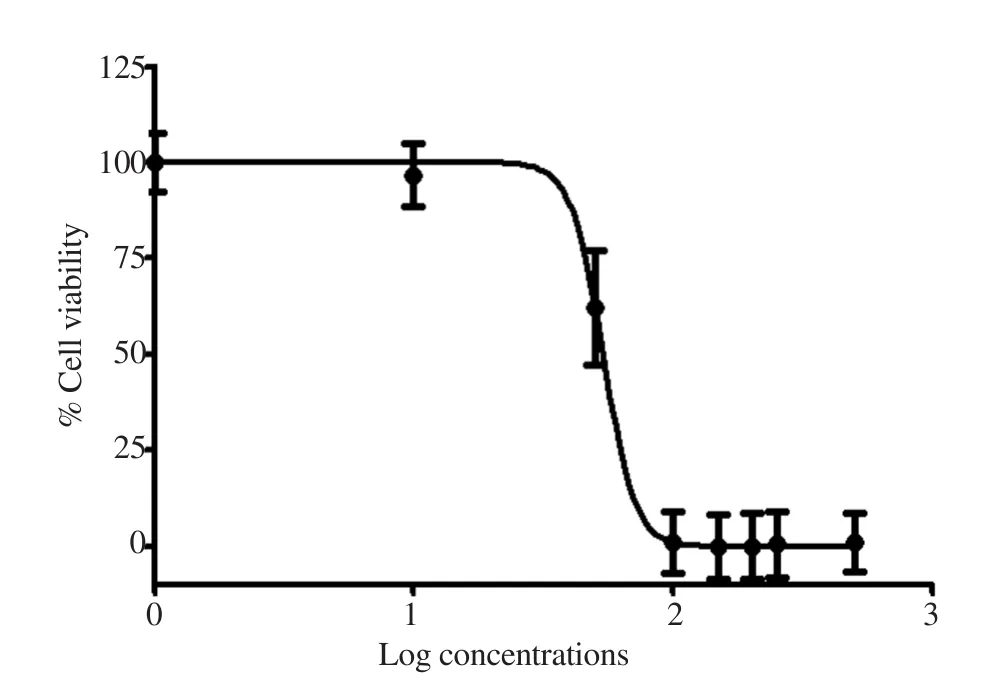
Figure 2. Cytotoxic effects of HDHE on MDA-MB-231 cells. Cell viability was measured by MTT assays after incubation with various concentrations of HDHE for 24 h (n=4).
3.3. HDHE promotes mitochondria-mediated apoptosis
MDA-MB-231 cells treated with HDHE at 50 μg/mL significantly increased the annexin V-positive cell numbers when compared with the untreated control (Figure 3A). This result indicated that HDHE could induce apoptotic cell death in MDA-MB-231 cells.
The effect of HDHE on mitochondrial membrane potential was detected by measuring JC-1 fluorescence intensity. HDHE at 50 μg/mL significantly increased the percentage of depolarized cells as shown by the intensity of JC-1 green fluorescence (Figure 3B). This result implied that HDHE could induce mitochondrial damage in MDAMB-231 cells as a target of apoptotic cell death.
3.4. HDHE modulates the expression of Bcl-2 and Bax
The expression of molecules mediated intrinsic pathway of apoptotic cell death after HDHE treatment on MDA-MB-231 cells was investigated. HDHE treatment increased Bax expression but decreased Bcl-2 expression in MDA-MB-231 cells (Figure 3C).
Furthermore, the activation of effector caspase-3 and caspase-7 cascades was detected by caspase 3/7 and 7-aminoactinomycin D fluorescent labeling using flow cytometry. At 24 h post-treatment,HDHE at 50 μg/mL increased the percentage of activated caspase 3/7, indicated by the shift of the population of HDHE-treated cells into the right upper quadrant (Figure 3D). The results affirmed that HDHE promoted the expression of key molecules contributing to apoptotic cell death including Bax, Bcl-2, and active caspase-3 and caspase-7 in MDA-MB-231 cells.
3.5. HDHE induces autophagy in MDA-MB-231 cells
The effects of HDHE on autophagic cell death were analyzed by detecting a change of autophagic flux in treated MDA-MB-231 cells using flow cytometry. HDHE treatment at 50 μg/mL for 24 h enhanced the shift of LC3 fluorescence with increased intensity(Figure 4A, red curve) compared with the untreated control group(Figure 4A, black curve), indicating the induction of autophagic cell death after HDHE treatment.
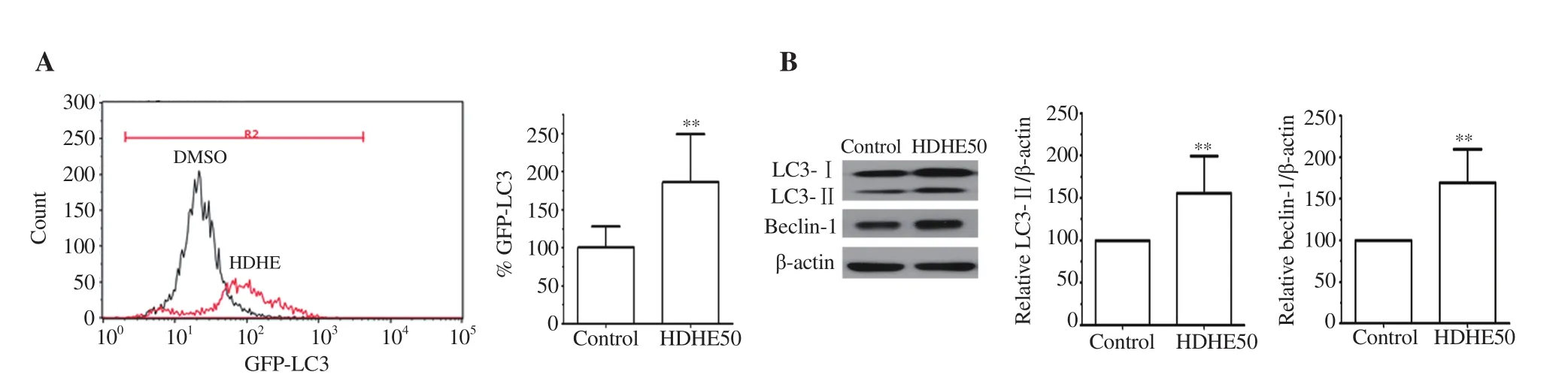
Figure 4. HDHE induces autophagy in MDA-MB-231 cells. (A) A representative histogram of autophagic flux detection in the HDHE-treated group for 24 h compared with the DMSO-treated control group. A bar graph represents mean fluorescence intensity of LC3. (B) Representative blots show immunoreactive expression of LC3-Ⅰ, LC3-Ⅱ, and beclin-1 in MDA-MB-231 cells after 50 μg/mL HDHE treatment for 24 h. Bar graphs represent relative expression of LC3-Ⅱ and beclin-1 that is normalized against β-actin. All data are expressed as mean ± SD and analyzed by Student’s t-test, n = 4. **P < 0.01 compared with the DMSO-treated control group.
The expressions of autophagy-related molecules LC3 and beclin-1 were analyzed by immunoblotting. The expression levels of LC3-Ⅱand beclin-1 were significantly increased in the HDHE-treated group compared with the untreated group (Figure 4B). The result indicated that HDHE could induce autophagic cell death by upregulating the expression of autophagic molecules in MDA-MB-231 cells.
3.6. HDHE promotes DNA damage
The effect of HDHE on DNA damage in MDA-MB-231 cells was analyzed by measuring the levels of phosphorylated forms of ATM and H2AX signaling molecules as DNA damage markers using flow cytometry. After 24 h treatment, 50 μg/mL HDHE increased the levels of phospho-ATM (pATM) and phospho-H2AX (pH2AX)compared with the control group (Figure 5). This implied that HDHE could promote DNA damage in MDA-MB-231 cells.
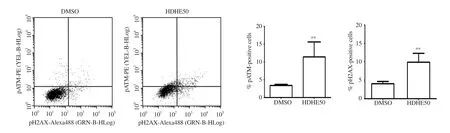
Figure 5. HDHE promotes DNA damage in MDA-MB-231 cells. A representative histogram shows the level of pATM and pH2AX in MDA-MB-231 cells after treatment with 50 μg/mL of HDHE for 24 h. A bar graph represents the percentage of pATM and pH2AX-positive cells. All data are expressed as mean ±SD and analyzed by Student’s t-test, n = 4. **P < 0.01 compared with the DMSO-treated control group.
3.7. HDHE modulates ER stress in MDA-MB-231 cells
The expression of ER stress-associated molecules in MDA-MB-231 cells after HDHE treatment for 24 h was detected by qPCR. HDHE increased ATF4, ATF6, CHOP, GADD34, IRE1α, XBP1s, and GRP78/BiP mRNA levels (Figure 6). The result suggested that HDHE mediates ER stress response through ATF6 and IRE1 activating pathways.
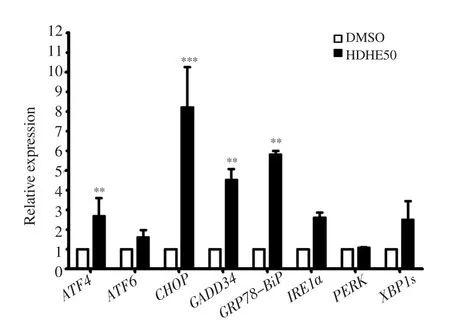
Figure 6. HDHE induces endoplasmic reticulum stress. A bar graph represents the relative expression of endoplasmic reticulum stress-related molecules in MDA-MB-231 cells after 50 μg/mL HDHE treatment for 24 h.The data are expressed as mean ± SD and analyzed by Student’s t-test, n = 3.**P < 0.01, ***P < 0.001, compared with the DMSO-treated control group.
4. Discussion
TNBC represents the most aggressive subtype of breast cancer with poor prognosis and low overall survival. The therapeutic potential of marine algae for breast cancers is being investigated, especially for TNBC treatment. The present study explored the in vitro anticancer effect of HDHE on apoptotic and autophagic death against MDAMB-231 cells. HDHE promoted cytotoxicity to MDA-MB-231 cells with an IC50value of (50.35±17.72) μg/mL. The cytotoxic activity of HDHE may be due to several bioactive components in the extracts, which may mediate cell death. Several fatty acids were found in marine algae as their main constituents. The GCMS analysis of HDHE revealed several bioactive ingredients,mainly n-hexadecanoic acid (palmitic acid), 6,10,14-trimethyl-2-pentadecanone, heptadecane, and hexadecenoic acid ethyl ester.
Fatty acids found in HDHE extracts may serve as the bioactive ingredients modulating anticancer activity. Hexadecanoic acid, also called palmitic acid, is a saturated fatty acid found in various species of green and red seaweeds[9,10]. Several studies claimed potent anticancer effects of hexadecanoic acid derived from seaweeds.Extracts of red algae Amphiroa zonata containing n-hexadecanoic acid promoted cytotoxicity and induced apoptosis in human leukemic cells by inhibiting DNA topoisomeraseⅠ as its molecular target[11]. Methanolic extracts of green seaweeds Ulva intestinalis,Halimeda macroloba, and brown seaweed Sargassum ilicifolium with n-hexadecanoic acid as the major fatty acid enhanced the in vitro cytotoxic activity against human breast, colorectal, and hepatocellular carcinoma cells[12]. In breast cancers, palmitic acid sensitized MCF-7 breast cancer cells and induced apoptosis through upregulating caspase-3, caspase-9, Bax, and p53[13]. In addition,n-hexadecanoic acid purified from the sea pens Virgularia gustaviana inhibited growth and promoted apoptosis in MDA-MB-231 breast cancer cells by increasing the expression of caspase-3, caspase-8 and Bax proteins[14]. The Antarctic macroalgae Adenocystis utricularis extracts containing the mixtures of fatty acids inhibited growth and induced apoptosis in MCF7 and MDA-MB-231 breast cancer cells[15]. Moreover, other fatty acids found in HDHE extract may also provide antitumor activity. Cis-9-octadecenoic acid (oleic acid),a monounsaturated fatty acid found in HDHE, promoted anticancer activity against breast, prostate, and colorectal cancers through the induction of apoptosis and increased ROS production[16]. Oleic acid also induced apoptosis and autophagic cell death in tongue squamous cell carcinoma[17].
Cytotoxic effects of HDHE in TNBC cells might be modulated by apoptotic and/or autophagic cell death. Mitochondrial membrane potential in MDA-MB-231 cells was disrupted following the HDHE treatment, which correlated with the increased apoptotic cell population in HDHE-treated cells. Mitochondrial dysfunction is the early event of apoptosis induction, which is mediated through the increased pro-apoptotic protein Bax as well as the decreased anti-apoptotic proteins Bcl-2. Mitochondrial disruption after HDHE exposure is associated with the upregulation of Bax and the downregulation of Bcl-2 level. In addition, HDHE activated the effector caspase-3/7 cascade that modulated phenotypic change of apoptosis. The present study indicated that HDHE induced mitochondrial-mediated apoptotic cell death in MDA-MB-231 cells through Bax and Bcl-2 cascade, resulting in the activation of caspase-3/7 cascade.
Autophagy is another signaling pathway that mediates the degradation of cellular components by lysosomal activity. Recently,autophagy is recognized as a tumor cell death inducer and the impairment of autophagy is associated with anticancer therapy resistance[18]. There are dual roles of autophagy in breast cancer.The expressions of autophagy markers, including LC3 and beclin-1,are higher in TNBC cells than other subtypes of breast cancer[19].A previous study showed that the suppression of key autophagy genes LC3 and beclin-1 could inhibit the proliferation and invasion of MDA-MB-231 cells[20]. However, several studies presented autophagy-promoting effects of conventional chemotherapeutic agents in both pre-clinical and clinical breast cancer models,including MDA-MB-231 cells[21]. Our data showed that HDHE treatment can promote the autophagy of MDA-MB-231 cells through the conversion of LC3-Ⅰ to LC3-Ⅱ and the upregulation of beclin-1. Fatty acids trigger autophagy that contributes to diverse physiological and pathological processes including apoptosis of cancer cells[22]. A previous study showed that high concentrations of palmitic acid induced MDA-MB-231 cell apoptosis and inhibited the invasion by promoting autophagy[23]. Therefore, the promotion of autophagy in TNBC cells treated with HDHE may be mediated by n-hexadecanoic acid (palmitic acid).
Many studies evidenced that some natural bioactive compounds promoted prolonged ER stress and may contribute to the activation of pro-death signaling in several cancers[24]. The ER is a dynamic organelle primarily responsible for post-translational protein synthesis and folding as well as calcium signaling. Cytotoxic insults cause ER stress by the accumulation of unfolded proteins through the trigger of the unfolded protein response (UPR) pathways.Major molecular sensors of the UPR include IRE1α, ATF6, and PERK[25]. All three UPR pathways concertedly trigger apoptotic and autophagic cell death by the activation of transcription factor CHOP as key pro-death signaling, especially in cancer cells[26]. HDHE upregulated the expression of active ER-resident chaperone GRP78/BiP. HDHE could activate downstream UPR signaling markers ATF6 and IRE1α, which activate the effector CHOP molecule. The present study reported the upregulation of ATF4 with no change in PERK mRNA level. ER stress induces the phosphorylation of PERK, leading to the phosphorylation of eIF2a and the subsequent overexpression of ATF4. No change in PERK total level in the present study did not rule out the activation of PERK, which should be detected by measuring the levels of phosphorylated PERK using immunoblotting. Some marine algal products could stimulate apoptosis and tumor-suppressing autophagy in cancer cells through the upregulation of ER stress molecules[27]. A previous study showed the ethanolic extract of the marine brown alga Dictyopteris undulata induced ER stress in human colon cancer cells and caused apoptosis through CHOP expression[28]. Therefore, the increase of ER stress by HDHE may play a role in the induction of apoptotic and autophagic cell death of MDA-MB-231.
Tumor cells exposed to cellular stressors result in apoptosis that may be triggered by their DNA damage[29]. DNA damage response signals ATM/ATR family activates H2AX phosphorylation at damaged DNA sites and is involved in DNA repair, which has been used as DNA damage markers[29]. The increase of apoptotic cells in MDA-MB-231 cells under HDHE treatment is associated with DNA damage by the increase of p-ATM and p-H2AX levels. Our result suggested that HDHE induced DNA damage in TNBC. DNA damage can be triggered by CHOP expression after ER stress[26].Therefore, the upregulation of CHOP by HDHE could contribute to the increased DNA damage in MDA-MB-231 cells.
These fatty acids found in HDHE extracts possessed potent antiinflammatory activity[30]. Chronic inflammation is closely associated with the establishment and progression of tumor microenvironment by promoting the expression of inflammasome components,including breast cancers[31]. Therefore, HDHE extract could also reduce inflammation and further investigation is warranted.
In conclusion, the present study demonstrated the anticancer effects of the n-hexadecanoic acid-enriched fraction of H. durvillei against MDA-MB-231 cells. The limitation of the study is the lack of information on the toxicity of HDHE on normal human breast cells.The use of a single TNBC cell line may not represent the variation of TNBC. Moreover, further investigation of this extract in in vivo models is needed.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
This research was supported by Thammasat University Research Unit in Neutraceuticals and Food Safety.
Funding
This research was supported by grants from the National Research Council of Thailand to Rapeewan Settacomkul (No. 13/2563); and Faculty of Medicine, Thammasat University to Kant Sangpairoj(No.2-20/2563).
Authors’ contributions
KS, PS, and PV contributed to the conceptualization and design of the research; TS, KM, and NN contributed to the design of the research; KM, NN, and MT contributed to resource provision; KS,RS, NS, and PV contributed to the acquisition and analysis of the data; KS, TS, NN and PV contributed to the interpretation of the data; and KS, RS, and PV drafted the manuscript. All authors read and approved the manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Non-alcoholic fatty liver disease: Epidemiology, pathophysiology and an update on the therapeutic approaches
- Aqueous extract of freeze-dried Protaetia brevitarsis larvae promotes osteogenesis by activating β-catenin signaling
- Phloretin-induced suppression of oxidative and nitrosative stress attenuates doxorubicin-induced cardiotoxicity in rats
