Phloretin-induced suppression of oxidative and nitrosative stress attenuates doxorubicin-induced cardiotoxicity in rats
2022-03-17ShivaniWaghKalpeshPatilUmeshMahajanPradnyaBagalAvinashWadkarBasavrajBommanhalliPrabhakarPatilSameerGoyalShreeshOjhaChandragoudaPatil
Shivani S. Wagh, Kalpesh R. Patil, Umesh B. Mahajan, Pradnya D. Bagal, Avinash R. Wadkar, Basavraj Bommanhalli, Prabhakar R. Patil, Sameer N. Goyal, Shreesh Ojha, Chandragouda R. Patil,6✉
1Department of Pharmacology, R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur- 425405, Dist. Dhule, Maharashtra, India
2Department of Pathology, Gadag Institute of Medical Sciences, Gadag, Karnataka, India
3Department of Pharmacology, Navodaya Medical College, Post Box No: 26, Mantralayam Road, Navodaya Nagar, Raichur-584 103, Karnataka,India
4Shri Vile Parle Kelavani Mandal's Institute of Pharmacy, Dhule -424001, Maharashtra, India
5Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain,Abu Dhabi, UAE
6Department of Pharmacology, Delhi Pharmaceutical Sciences and Research University, Mehrauli-Badarpur Road, Pushp Vihar Sector-3, New Delhi-110017, India
ABSTRACT
Objective: To compare the cardioprotective efficacy of equimolar doses (50 mM/kg, p.o.) of phloretin and genistein against doxorubicin-induced cardiotoxicity in rats.
Methods: Cardiotoxicity was induced in rats by intraperitoneal injection of 6 mg/kg doxorubicin on alternative days till the cumulative dose reached 30 mg/kg. This study included four treatment groups of rats (n=6): the control group (0.5% carboxymethyl cellulose solution-treated), the doxorubicintreated group (0.5% carboxymethyl cellulose solution along with doxorubicin), the genistein-treated group (50 mM/kg/day;p.o. along with doxorubicin) and phloretin-treated group (50 mM/kg/day; p.o. along with doxorubicin). On the 10th day of dosing, rats were anesthetized for recording ECG, mean arterial pressure, and left ventricular function. Oxidative stress, nitric oxide levels, and inflammatory cytokines were estimated in the cardiac tissue. Cardiac function parameters (creatine kinase MB,lactate dehydrogenase, aspartate aminotransferase, and alanine transaminase) were estimated in the serum samples.
Results: Phloretin treatment inhibited doxorubicin-induced oxidative stress and also reduced nitric oxide levels in cardiac tissues of rats. Phloretin administration attenuated doxorubicininduced alterations in hemodynamic parameters (heart rate, mean arterial blood pressure, and left ventricular function) and suppressed the expression of pro-inflammatory cytokines. The cardiac injury markers like creatine kinase MB, lactate dehydrogenase, aspartate aminotransferase, and alanine transaminase were reduced by both genistein and phloretin. All these effects of phloretin were more prominent than genistein.
Conclusions: Phloretin offers cardioprotection that is comparable to genistein, a clinically validated cardioprotectant against doxorubicin-induced cardiotoxicity. Further studies are needed to confirm and establish the therapeutic utility of phloretin as a chemopreventive adjuvant to doxorubicin chemotherapy.
KEYWORDS: Cardiotoxicity; Chemoprevention; Doxorubicin;Genistein; Phloretin; Phytoestrogens; Cardiac injury; Hemodynamic changes
Significance
Considering the reported chemopreventive potential of phloretin,we compared the cardioprotective effects of equimolar doses of phloretin and genistein in the rat model of doxorubicin-induced cardiotoxicity. We noted better cardioprotection exhibited by phloretin as compared with genistein. This interesting activity profile projects phloretin as an important phytoestrogen with chemoprotectant action needing further preclinical and clinical evaluation against cancer chemotherapy-induced cardiotoxicity.
1. Introduction
An anthracycline antibiotic, doxorubicin (DOX) is widely used for the therapeutic management of a variety of cancers including leukemia. Multi-organ toxicities like dose-dependent cardiotoxicity are the most serious side effect of DOX, which involve the generation of oxidative stress and induction of apoptosis[1,2].Earlier studies investigated the etiopathogenesis of DOX-induced cardiotoxicity and proposed several mechanisms. DOX-induced cardiotoxicity is mainly linked with the generation of reactive oxygen species (ROS), oxidative stress, stimulation of inducible nitric oxide synthase (iNOS) enzyme, altered calcium homeostasis,and subsequent lipid peroxidation[1,2]. DOX is predominantly stored in the liver, kidneys, and heart. However, the heart becomes more susceptible to oxidative stress due to increased mitochondria to cardiomyocyte ratio[3].
Phloretin (PHL) is a dietary flavonoid having weak estrogenic activity[4]. It interacts with the estrogen receptor beta (ERβ) and exhibits estrogenic effects[5,6]. Phytoestrogens like PHL ameliorate hormone-dependent prostate and breast cancer[7,8]. Phytoestrogens stimulate nuclear factor erythroid 2-related factor 2 (Nrf2)through ERβ receptors and induce the expression of antioxidant enzymes[9,10]. These effects of phytoestrogens resemble the action of 17β-estradiol-induced up-regulation of the Nrf2 signaling via phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway[11].Beyond estrogenic properties, PHL inhibits the Toll-like receptor 2 (TLR2) signaling pathway, suppresses the expression of proin flammatory cytokines, and thereby exerts anti-inflammatory activity in the HEK293-hTLR2 human embryonic kidney cells[12].Both ERβ and TLR2 are expressed in the heart and other parts of the cardiovascular system and mediate the DOX-induced cardiotoxicity [13,14].
Genistein (GEN), a phytoestrogen, has been clinically validated for its cardioprotective effect during DOX chemotherapy. GEN chemosensitizes the breast and prostate cancer cells towards DOX-induced cytotoxicity which is evident from in vitro and in vitvo preclinical studies[15,16]. GEN protected the heart against the toxicants like arsenic trioxide[17] and DOX[10].
PHL exhibits similar cancer cell chemosensitization through cyclin/cyclin-dependent kinases and thereby activates the mitochondria-mediated cell death and apoptosis[18]. However,the chemopreventive potential of PHL against DOX-induced cardiotoxicity needs to be evaluated. The present study aimed to compare the protective efficacy of equimolar (50 mM/kg, p.o.)doses of PHL and GEN against DOX-induced cardiotoxicity in male rats.
2. Materials and methods
2.1. Animals
Male Wistar rats (200-250 g) obtained from the central animal house facility of the host institute were used for this study. Rats were kept in a standard housing environment of (22±2) ℃ and relative humidity of 60%-70%. They had free access to standard pellet feed (Nutrimix-1020, supplied by Nutrivet Life Sciences,Pune, India) and water. The light-dark cycle was of 12:12 h.
2.2. Drug and chemicals
DOX was purchased from Shah Enterprises, Mumbai. PHL was purchased from the BLD Pharmatech Pvt. Ltd. India (Cat.No-BD147786-5g), GEN was purchased from A. K. Scientific,Mumbai, India (Batch No-SE20190411). The estimation kits for creatine kinase MB (CK-MB), lactate dehydrogenase (LDH),aspartate aminotransferase (AST), and alanine transaminase (ALT)were procured from the ERBA Diagnostics, Germany, ELISA kits(TNF-α, IL-6 & IL-1β) were obtained from e-Biosciences, USA.
2.3. Experimental protocol
Male Wistar rats were randomly divided into four groups containing six animals per group. The treatment schedule and protocol are shown in Supplementary Figure 1. For induction of cardiotoxicity, DOX was injected at 6 mg/kg intraperitoneal dose on alternative days till the cumulative dose of 30 mg/kg was achieved. The dose of PHL was selected considering the prior reports by Zuo et al[1]. and the effects of equimolar doses of GEN were compared.
2.3.1. ECG and hemodynamic parameters analysis
Following the last dosing on the 10th day, the hemodynamic parameters and electrocardiogram (ECG) were recorded in rats under urethane (1.25 g/kg, intraperitoneally) anesthesia. AD instrument’s PowerLab system (Australia) and LabChart Pro 7.0 software were used for these recordings. Blood pressure and left ventricular function were determined by cannulating the right carotid artery. The body temperature of animals was retained at 37 ℃ throughout the recordings.
Pressure changes in the carotid artery were analyzed to determine the heart rate (HR) and mean arterial pressure (MAP). Pressure changes in the left ventricle [+ dp/dt, - dp/dt, left ventricular enddiastolic pressure (LVEDP)] were determined by progressing the carotid cannula up to the left ventricle.
Following these recordings, the blood samples were collected.Rats were sacrificed with overt anesthesia and hearts were isolated,weighed, homogenized, and subjected to the assessment of proinflammatory cytokines and oxidative stress.
2.3.2. Estimation of the oxidative and nitrosative stress
Oxidative stress parameters including nitric oxide (NO), catalase(CAT), superoxide dismutase (SOD), and reduced glutathione(GSH) were estimated in the 10% cardiac tissue homogenates prepared in PBS (pH 7.4). The 10% cardiac tissue homogenates prepared in KCl (pH 7.4) were used to estimate the level of lipid peroxidation as malondialdehyde (MDA) content.
2.3.3. Estimation of cardiac injury markers
Serum samples were used for the estimation of biochemical markers of cardiotoxicity including CK-MB, LDH, AST, and ALT, These estimations were carried out according to the protocol prescribed by the manufacturer.
2.3.4. Estimation of proinflammatory cytokines
The proinflammatory cytokines (TNF-α, IL-6 & IL-1β) were determined in the cardiac tissues using commercially available ELISA kits according to mentioned protocol.
2.3.5. Histopathological examination
A portion of the heart, preferably from the tip of ventricles, was kept in formaldehyde solution (10%) and fixed in the paraffin.The sections were cut using a microtome and transferred to microscopic slides and subjected to hematoxylin and eosin staining protocol. The histological examination was carried out under a light microscope (Model- Motic, DMWB 2-223, Canada) at 400×magnifications. The microscopic images were captured using Motic Images 2.0 software.
2.4. Statistical analysis
Data were expressed as a mean ± standard deviation (SD). Data analysis was performed using GraphPad Prism 8.0.2 software(GraphPad, San Diego, CA, USA). Data were analyzed by oneway analysis of variance (ANOVA), and Bonferroni’s test was applied for post hoc analysis. A value of P<0.05 was considered to be statistically significant.
2.5. Ethical statement
All the experimental procedures were approved by the Institutional Animal Ethics Committee (IAEC) and conducted as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (IAEC/CPCSEA/RCPIPER/2019-20/11).
3. Results
3.1. Effects of PHL and GEN on ECG and hemodynamic parameters
DOX demonstrated a significant rise in the ST-height (P<0.05)as well as elevated heart rate, QT-interval and MAP (P<0.001)as compared with control rats. PHL and GEN reserved the DOX-induced increase in ST-height (P<0.05) and QT-interval (P<0.001)to a similar extent (Figures 1 & 2). GEN was not as effective as PHL in reducing the heart rate (Figure 2C) and MAP (Figure 2D).
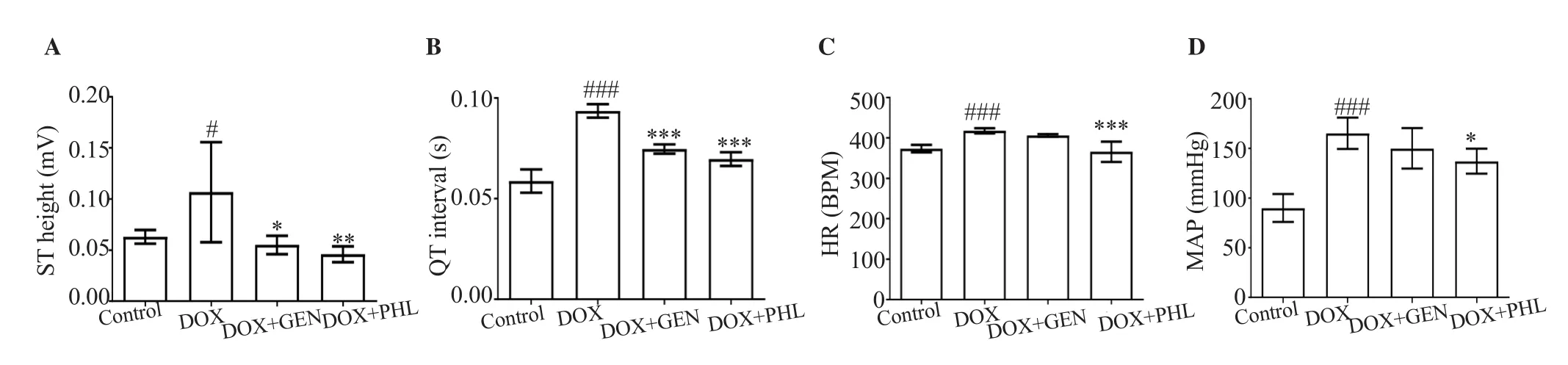
Figure 2. Effect of GEN and PHL on DOX-induced changes in ECG. A: ST height; B: QT interval; C: heart rate (HR); D: mean arterial pressure (MAP). Data expressed as mean ± SD. One-way ANOVA and Bonferroni’s test are used. For ST height: P<0.001, F(3, 20)=6.72, n=24; For QT interval: P<0.001, F(3,20)=82.06, n=24; For HR: P<0.001, F(3, 20)=19.48, n=24; For MAP: P<0.001, F(3, 20)=24.79, n=24; #P<0.05, ###P<0.001 compared with the control group;*P<0.05, **P<0.01, ***P<0.001 compared with the DOX-treated group.
The left ventricular function was determined as the rate of the left ventricular pressure changes i.e. -dp/dt and +dp/dt. DOX treatment almost halved the +dp/dt and doubled the -dp/dt (P<0.001). Both PHL and GEN reversed these effects of DOX. The effect of PHL on LVEDP was more prominent and significant (P<0.001) as compared with GEN (P<0.01) (Figure 3).

Figure 3. Effect of GEN and PHL on DOX-induced deterioration of the left ventricular function. A. +dp/dt; B. -dp/dt; C. left ventricular end-diastolic pressure(LVEDP). Data are expressed as mean ± SD. One-way ANOVA and Bonferroni’s test are used. For +dp/dt: P<0.001, F(3, 20)=16.23, n=24; For -dp/dt:P<0.001, F(3, 20)=19.55, n=24; For LVEDP: P<0.001, F(3, 20)=133.20, n=24; ###P<0.001 compared with the control group; **P<0.01, ***P<0.001 compared with the DOX-treated group.
3.2. Effects of PHL and GEN on oxidative stress
DOX administration induced a more than two-fold rise in the NO level in the cardiac tissue (P<0.001). PHL significantly (P<0.001)reduced the DOX-induced NO levels to almost normal as observed in the control group. The SOD activity was reduced significantly in the DOX-treated group (P<0.001). PHL preserved the SOD activity more prominently (P<0.05) than GEN (statistically not significant).The GSH levels in the cardiac tissue were suppressed significantly by DOX administration (P<0.001). These suppressed levels of GSH by DOX treatment were significantly preserved by PHL(P<0.001) and GEN (P<0.01). CAT activity in the cardiac tissue was suppressed in the group treated with DOX (P<0.001). GEN was effective (P<0.01) in retaining CAT activity, and PHL also increased the CAT activity significantly (P<0.001) as compared with the DOX treated group. Extensive lipid peroxidation was observed as a significant rise in the MDA levels in the cardiac tissue of DOX-treated animals (P<0.001). DOX-induced lipid peroxidation was significantly reduced by PHL and GEN treatment(P<0.001). The effect of PHL was more pronounced than that of GEN (Table 1).
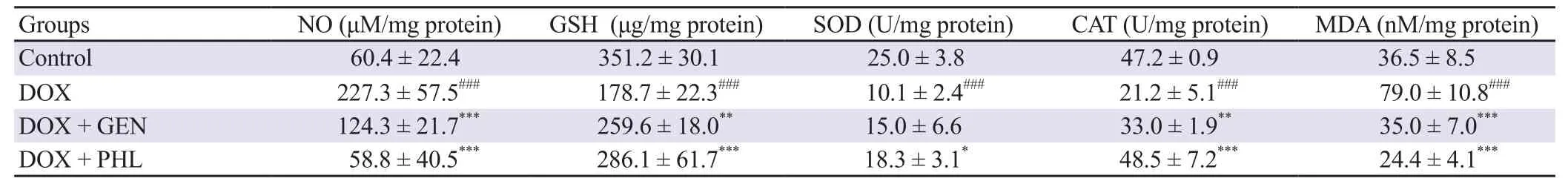
Table 1. Effect of PHL and GEN on the DOX-induced oxidative stress.
3.3. Effects of PHL and GEN on cardiac injury markers
The serum samples were analyzed for the cardiac injury markers.DOX had prominently increased levels of CK-MB (P<0.001),LDH (P<0.001), AST (P<0.05), and ALT (P<0.001). PHL reduced the DOX-induced elevation of CK-MB, LDH, ALT (P<0.001),and AST (P<0.05). The reducing effect of GEN on ALT level was comparable to that of PHL (Figure 4D). However, GEN reduced the level of LDH to a less significant extent as compared with the PHL (Figure 4B).
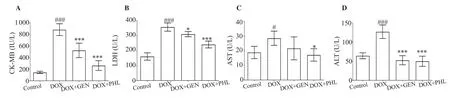
Figure 4. Effect of GEN and PHL on DOX-induced alterations in cardiac injury markers. Data are expressed as mean ± SD and analyzed by one-way ANOVA followed by Bonferroni’s test. For creatine kinase-MB (CK-MB): P<0.001, F(3, 20)=76.33, n=24; For lactate dehydrogenase (LDH): P<0.001, F(3, 20)=80.28,n=24; For aspartate aminotransferase (AST): P<0.01, F(3, 20)=5.03, n=24; For alanine transaminase (ALT): P<0.001, F(3, 20)=45.39, n=24;#P<0.05,###P<0.001 compared with the control group; *P<0.05, ***P<0.001 compared with the DOX-treated group.
3.4. Effects of PHL and GEN on proinflammatory cytokines
The DOX-induced significant rise of pro-inflammatory cytokines(TNF-α, IL-6 & IL-1β) in the cardiac tissue was observed(P<0.001). Pretreatment of rats with PHL and GEN reduced the DOX-induced rise in proinflammatory cytokine levels to a similar extent (P<0.001) (Table 2).
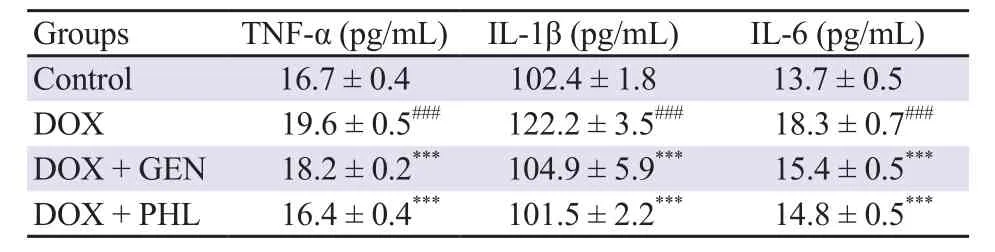
Table 2. Effects of PHL and GEN on the DOX-induced proinflammatory cytokine levels.
3.5. Effects of PHL and GEN on the DOX-induced histological perturbations
DOX-induced marked interstitial hemorrhage along with vacuolization of the cardiomyocytes was observed. There was interstitial edema with neutrophil infiltration. GEN treated group showed interstitial edema along with moderate infiltration of neutrophils and occasional vacuolated cardiomyocytes. The PHL treated group showed mild interstitial edema along with occasional vacuolated cardiomyocytes (Figure 5).
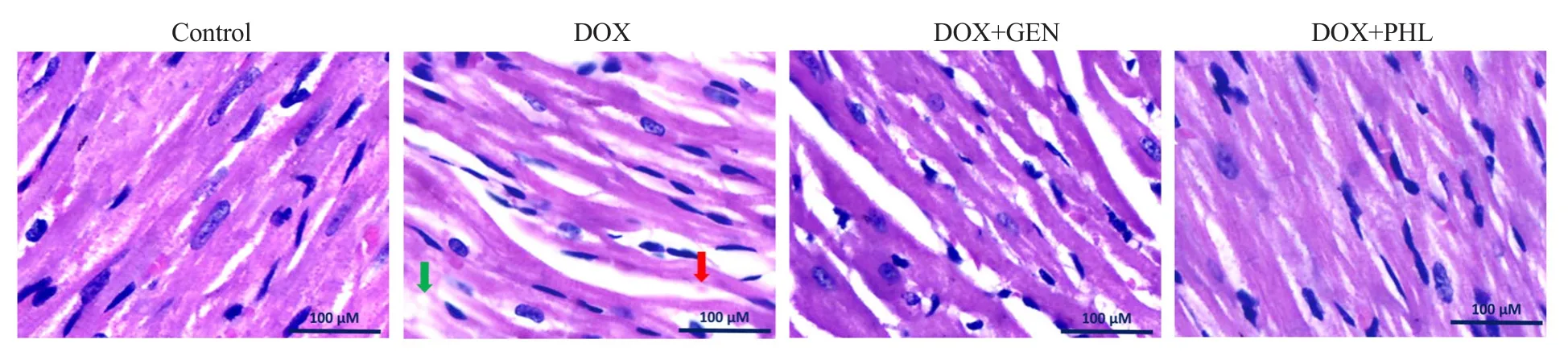
Figure 5. Histopathological changes in hematoxylin and eosin stained sections of the cardiac tissue. The green arrow shows vacuolation and red arrow shows extravasation of red blood cells. Microscopic examinations were performed under 400× light microscopy, scale bar: 100 μm.
4. Discussion
The pathogenesis of DOX-induced cardiotoxicity is multifactorial which involves several mechanisms[1-3]. Many detrimental effects have been related to DOX-induced cardiotoxicity, including oxidative stress, lipid peroxidation, DNA/RNA damage, autophagy suppression, endoplasmic reticulum-mediated apoptosis, and calcium homeostasis disruption. Superoxide anions and hydroxyl radicals generated during the metabolism of DOX lead to cellular membrane injury[19,20]. Elevated ROS levels decrease the production of the Nrf2, making cells more vulnerable to oxidative stress and apoptosis[21]. Furthermore, cardiac inflammatory changes due to DOX are also documented[2]. Generation of ROS, oxidative stress, and imbalanced antioxidant enzyme system are responsible for the DOX-induced cardiotoxicity. DOX-administration is reported to decline the level of GSH in cardiac tissues and activities of antioxidant enzymes like CAT, SOD, and glutathione peroxidase[2]. In this study, we compared the cardioprotective efficacy of equimolar doses of the PHL and GEN against DOX-induced cardiotoxicity in Wistar rats. The PHL showed better cardioprotection than GEN. Pre-treatment of rats with PHL and GEN restored the levels of antioxidant enzymes including CAT, SOD, and GSH and reduced the NO and MDA levels in cardiac tissues. PHL is reported to induce glutathione synthesis and to increase heme oxygenase-1 and glutamate-cysteine ligase expression as phase-Ⅱ enzymes via ERK2/Nrf2 pathway and protect hepatocytes from oxidative stress[22]. Preclinical studies suggested that PHL restores the activity of Mn-SOD through deacetylation[23]. These findings are in congruence with the earlier reports, where GEN is reported to increase the antioxidant gene expression, inhibit ERK1/2 and NF-κB activation and elevate the expression of Mn-SOD[24].
DOX increases the NO production via stimulation of iNOS which is linked with dilated cardiomyopathy[1]. NO is a vasodilator and a key mediator in myocardial contraction which is associated with the development of cardiac illnesses including heart failure, and cardiomyopathy. NO interacts with the superoxide to produce peroxynitrite anion which leads to lipid peroxidation and subsequent DNA damage[1,3]. PHL and GEN both suppressed the DOX-induced NO release. In the isoproterenolinduced cardiotoxicity, GEN prevented cardiac hypertrophy by inhibiting iNOS and stimulating e-NOS expression[25]. PHL was also reported to reduce the NO level in RAW-264.7 cells challenged by bacterial endotoxin[26]. The NO reducing effects of PHL and GEN in the DOX challenged rat hearts were in tune with these reports. PHL and GEN have been reported to possess chemosensitizing potentials against different types of cancers.PHL exhibits cytotoxicity against a variety of cancers[19,27,28].Similarly, GEN also exerts cytotoxicity against breast, prostate,ovarian and intestinal cancers[15,29,30]. Both these drugs are known to exert organ protective effects against chemical toxicants including anticancer agents. PHL prevents streptozotocin-induced cardiomyopathy in mice through its anti-inflammatory effects and restoration of sirtuin 1 (SERT1)[9]. PHL also offered protection against mitochondrial dysfunction induced by arsenic trioxide in the cardiomyoblasts through the modification of electron transport chain complexes and membrane permeability[31]. PHL protected against the D-galactosamine (D-GL) induced liver damage in mice through its antioxidant effects[1]. Similarly, GEN inhibits the D-GL-induced hepatotoxicity by inhibiting TGF-β/SMAD signaling pathway[32]. GEN confers cardioprotection against arsenic trioxide through inhibition of apoptosis against the DOX-induced cardiotoxicity via activation of Nrf-2/heme oxygenase-1 signaling[10,17]. GEN reduced the streptozotocin-induced cardiac inflammation and oxidative stress in rats[33]. Recently, GEN is reported to protect against burn-induced myocardial injury via suppression of oxidative and nitrosative stress[34].
In tune with the earlier reports[2,35], in our biochemical study, we observed induction of inflammatory reaction by DOX, manifested as elevated levels of cytokines like TNF-α, IL-1β, and IL-6. DOX has been found in preclinical studies to increase the levels of TNF-α and trigger the expression of neutrophil adhesion molecules on vascular endothelial cells, resulting in an inflammatory response in cardiac tissues[1,36,37]. An earlier study reported that DOX-overdose increased the production of transforming growth factor-β1, a protein released by cardiac myofibroblasts that promotes apoptosis and cardiac fibrosis[1,38]. Interestingly these elevated cytokine levels were significantly reduced by the pretreatment of experimental animals with PHL and GEN.
Furthermore, DOX treatment showed a significant rise in the level of CK-MB, LDH, AST, and ALT. However, the PHL and GEN treatment showed a decrease in the level of these cardiac injury markers. The effects of PHL were more prominent as compared with the effect of GEN on these inflammatory and cardiac injury markers. It was noted that both PHL and GEN exert significant cardioprotection against DOX-induced toxicity and underlying antioxidant and anti-inflammatory activities contribute to such protection. The efficacy of PHL was either better than or at least comparable to that of GEN. The preclinical safety studies have established that GEN exerts significant gonadal organ toxicity in rats of either sex at the doses of 50-500 mg/kg[39]. Clinical toxicities of GEN have been reported in the form of spermatotoxicity which becomes prominent in the presence of daidzein[40]. Long-term treatment of GEN disturbs the pituitary gonadotrophin axis in adult male rats[41]. Compared to GEN, the toxicities of PHL are less prominent and it has been reported to be a weak estrogen with a mild inhibitory effect on the pituitary gonadotropin axis[42]. Thus, PHL is a phytoestrogen that possesses anticancer and organ protective effects similar to GEN but exerts comparatively lesser adverse effects. The histological changes like interstitial edema with neutrophils infiltrations, interstitial hemorrhage, and occasional vacuolated cardiomyocytes which have been noted earlier by many investigators are suggestive of oxidative stress, lipid peroxidation, and apoptosis as the underlying mechanisms for these observations[2,43,44]. Apoptotic mechanism of DOX that was not executed in this study includes caspasemediated intrinsic apoptosis pathway and calcium homeostatic imbalance-mediated extrinsic apoptosis pathway in cardiac tissues[1]. Our findings indicate that, PHL offered cardioprotection against DOX-induced toxicity and its efficacy was more prominent than that of GEN. PHL more effectively reduced the DOX-induced oxidative stress as compared to GEN. It effectively restored the GSH level and improved the CAT and SOD activities. The lipid peroxidation was also inhibited by PHL more efficiently than GEN.All the cardiac injury parameters like CK-MB, LDH, ALT, and AST were more prominently reduced by PHL than GEN. These findings indicate that, PHL offered better protection against DOX-induced cardiotoxicity than GEN in rats. However, data generated by us does not compare the chemosensitizing potentials of PHL and GEN.
The present study established that equimolar doses of PHL and GEN exert comparable cardioprotection in the in vivo model of DOX-induced cardiotoxicity in rats. It may be speculated that this data does not directly conclude the utility of PHL in place of GEN as an adjuvant to DOX chemotherapy. To establish the utility of PHL as a therapeutic agent in place of GEN, further confirmatory and exploratory studies on chemosensitizing potential of PHL as well as investigation of detailed molecular mechanisms including apoptosis are desirable. Such studies may be extended to explore whether PHL provides organ-specific protection against other chemotherapeutic agents.
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
SSW performed literature search, conducted experiments, data acquisition, statistical analysis, and manuscript preparation. CRP designed the concept and study, literature search, conducted and supervised experimental studies, data, and statistical analysis,manuscript preparation, editing, and review. KRP performed literature search, study design, and concept, experimental studies,data and statistical analysis, preparation, editing, and review of the manuscript. UBM contributed in biochemical estimations, data acquisition, prepared figures, and manuscript editing. SNG assisted in study design, and performed data and statistical analysis, and manuscript review. SO, BB, and PRP contributed in chemicals,data analysis, and manuscript review. PDB and ARW executed literature search, performed experiments, and data acquisition,assisted in preparing the first manuscript draft. All authors have read, contributed, and approved the final version of manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Non-alcoholic fatty liver disease: Epidemiology, pathophysiology and an update on the therapeutic approaches
- Aqueous extract of freeze-dried Protaetia brevitarsis larvae promotes osteogenesis by activating β-catenin signaling
- Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress
