Isolation of Chloroplasts from Marine Microalga Isochrysis galbana Parke for Their Lipid Composition Analysis
2022-02-28ZHONGYeLIYanrongXUJilinCAOJiayiZHOUChengxuandYANXiaojun
ZHONG Ye ,LI Yanrong ,XU Jilin ,CAO Jiayi,ZHOU Chengxu,and YAN Xiaojun,
1) Key Laboratory of Applied Marine Biotechnology, Ningbo University, Ningbo 315211,China
2) Collaborative Innovation Center for Zhejiang Marine High-Efficiency and Healthy Aquaculture, Ningbo University,Ningbo 315211, China
3) Fujian Baozhi Aquatic Science and Technology Co.,Ltd., Zhangzhou 363503,China
4) Ningbo Institute of Oceanography,Ningbo 315211,China
Abstract Marine microalga Isochrysis galbana is an important feed species with a high nutritional value.Different from other unicellular algae,its cell contains two chloroplasts which are the major sites for lipid synthesis.Here,we optimized a chloroplast isolation approach suitable for the isolation of I.galbana chloroplasts and determined the purity and integrity of the isolated chloroplasts through microscopic observations and enzyme activity assay.The chloroplast lipids were analyzed with a ultrahigh-performance liquid chromatography-Q Exactive Orbitrap-mass spectrometry.This newly developed isolation approach is simple and reliable to isolate chloroplasts with high integrity and purity.The average yield of intact chloroplasts was 15.3% ± 0.1%.Glycolipids and acylglycerols were the main chloroplast lipids.Glycolipids accounted for 56.6% of chloroplast lipid.Digalactosyldiacylglycerol (DGDG),monogalactosyldiacylglycerol (MGDG) and sulfoquinovosyldiacylglycerol (SQDG) were the main glyceroglycolipids.The fatty acyl R1/R2 were mostly 18:4/16:1,18:3/16:1 and 18:4/18:5 in DGDGs,14:0/18:4,18:4/18:5,18:4/18:4 and 18:3/18:4 in MGDGs and 16:0/14:0,16:0/18:3,and 18:4/18:3 in SQDGs.In addition,diacylglycerol (DAG) was the most abundant acylglycerols;the content of 22:6/18:4-DAG was the highest.There was a little amount of glycosphingolipid (GSL) in chloroplast.Digalactosylmonoglyceride(DGMG),monogalactosylmonoglyceride (MGMG),sulfoquinovosylmonoacylglycerol (SQMG),monoglyceride (MAG),phospholipids (PLs),ceramide (Cer) and betaine lipids were nearly undetectable in chloroplast.The fatty acid proportions of DGDGs,MGDGs,SQDGs,DAGs,triglycerides (TAGs) and GSLs were either higher or lower than or similar to those of whole-cell.Collectively,our isolation approach is applicable to many aspects of chloroplast biology,and may offer a reference for the isolation of chloroplasts from other marine microalgae.
Key words Isochrysis galbana;chloroplast isolation method;lipid analysis;chloroplast lipid
1 Introduction
Chloroplasts are the feature components of microalgae,macroalgae and plants.As the sites for photosynthesis,they are important for organism survival and responsible for carbohydrate metabolism,amino acid biosynthesis,chlorophyll biosynthesis and lipid biosynthesis (Zakim and Herman,1969).The structure of chloroplasts can be divided into three sub-compartments,each performs their own functions.The envelope,a double-membrane system surrounding the chloroplasts of primary symbiotic origin contains enzymes involved in carbon metabolism and oxidative stress response,which are associated with lipid metabolism and communication between plastids (Ferroet al.,2003).Unlike most of microalgae,Isochrysis galbanaspeciated through the secondary endosymbiosis and its chloroplasts are surrounded by four membranes rather than two (Huerlimann and Heimann,2013).The stroma between the inner envelope and the thylakoid membrane contains soluble proteins involved in Calvin cycle and protein synthesis (Rollandet al.,2012).The thylakoid membrane consists of a number of thylakoids that stack on the top of each other to form grana and lamellae,and is the site for oxygenic photosynthesis.Therefore,chloroplasts are the main sites of biosynthesis and metabolic pathways and have a substantial control on cell survival.
Chloroplast lipids are indispensable for oxygenic photosynthesis.They are the integral components of photosynthetic protein complexes and constituents to protect the photosynthetic machinery (Kellyet al.,2003;Kobayashiet al.,2016).Additionally,lipids constitute the membranes for compartmentalization of chloroplasts,contribute to the flexibility of protein complexes and stabilize its bilayers(LaBrantet al.,2018).In general,chloroplast lipids play an important role in photosynthesis,which can modify their energetic metabolism and affect organism life activities.A comprehensive understanding of chloroplast lipid composition facilitates the clarification of lipid metabolic machinery,which should aid to rationally engineer oils and fatty acids for fuel,industrial feedstocks and nutritional improvement.
Until recently,a number of studies have focused on chloroplast lipid composition and metabolism in land plants.Chloroplasts are particularly rich in monogalactosyldiacylglycerol (MGDG),digalactosyldiacylglycerol (DGDG) and sulfoquinovosyldiacylglycerol (SQDG).In higher plantArabidopsis,galactolipids are essential to support the growth and photosynthesis (Jarviset al.,2000;Hölzl and Dörmann,2019).MGDG,DGDG,SQDG and phosphatidylglycerol (PG) constitute the thylakoid membrane,which are the integral ingredients of photosynthetic complexes(Kobayashiet al.,2007,2016).Lyso-phosphatidylcholine(lyso-PC),phosphatidylcholine (PC),or phosphatidic acid(PA) of chloroplast lipids can be produced in endoplasmic reticulum (ER) and chloroplast (Liu and Benning,2013).In unicellular green algaChlamydomonas reinhardtii,a model species,the building blocks for triglyceride (TAG) and membrane lipids are biosynthesized in chloroplast.MGDG and DGDG present in the chloroplast ofC.reinhardtii(Yanget al.,2017).Dephosphorylated product diacylglycerol(DAG) is produced in chloroplast and primarily functions as the precursor for structural lipids of photosynthetic membrane system (Moelleringet al.,2009).In starved cells,galactolipids are maintained to preserve sufficient chloroplast integrity (Simionatoet al.,2013).In general,neutral lipid metabolism of chloroplasts has been extensively studied inC.reinhardtii;however there is no comprehensive study on the lipid composition of microalgae chloroplasts.
Marine microalgae biomass plays a critical role in animal food chain.As a golden brown flagellate marine microalga,I.galbanais widely adopted as an aquaculture feed for young fish and bivalves due to its high content of polyunsaturated fatty acids (Wikforset al.,1992).Previous investigations have shown that the nutritional value ofI.galbanato mollusca larvae is greatly attributed to its unique lipid composition (Pernet and Tremblay,2004;Liuet al.,2009) and different cultivation temperatures or times can alter the lipid composition ofI.galbana.Therefore,many studies have focused on identifying growth conditions that can promote lipid production (Huanget al.,2017).Though the whole-cell lipidome ofI.galbanais widely studied,the lipid synthesis and biosynthesis of chloroplasts remain largely unknown.
A major deterrent in studying the lipid composition of chloroplast is the technical challenge associated with the isolation and purification of intact chloroplasts.The current techniques employ physical or chemical means to break cell wall and plasmalemma (Alhattabet al.,2018).The isolation techniques of chloroplasts have only employed in several higher plants and a model alga.ForArabidopsisspecies,a method for chloroplast isolation relies on protoplastation (Fitzpatrick and Keegstra,2001).However,this method is time-consuming and expensive.To resolve this problem,a simple and cost-effective approach has been developed to isolate a large number of intact chloroplasts fromArabidopsisseedlings (Aronsson and Jarvis,2002;Seigneurin-Bernyet al.,2008).For marine plantPosidonia oceanica,an optimized protocol has been adapted from terrestrial plants and can be used to isolate chloroplasts from minimal tissues (Piroet al.,2015).ForC.reinhardtiimutant,cells are broken by passing them through a 27-gauge syringe needle (Masonet al.,2006;Yanget al.,2017).However,thus far,no universal method for isolation and purification of chloroplasts is available due to the differences in the number,size,gravity,thylakoid membrane structure and lipid composition of the chloroplasts in different plant cells.Thus,developing a suitable chloroplast isolation protocol that can be applied to different plant species is essential.Marine microalgaI.galbanais different from others;it contains two chloroplasts each cell (Hori and Green,1985).
To our knowledge,there is no established technique for the isolation of intact chloroplasts fromI.galbana,and there is no study on its chloroplast lipid composition.In present study,we developed a rapid approach for the cellular fractionation ofI.galbana,by which intact chloroplasts could be isolated.The chloroplasts purified using this approach were mostly intact and contaminated minimally.The integrity and purity of the isolated chloroplasts were analyzed by phase-contrast fluorescence microscopy,transmission electron microscopy (TEM) and enzyme activity assay.The lipid composition of the chloroplasts isolated fromI.galbanawas identified by ultrahigh-performance liquid chromatography-Q Exactive Orbitrap-mass spectrometry (UHPLC-QE-MS).
2 Materials and Methods
2.1 Microalgal Culture and Sample Collection
I.galbanawas provided by the Marine Biotechnology Laboratory of Ningbo University,China.Seawater (pH 8.2,salinity 25) was filtered using 0.45-μm cellulose acetate membranes,followed by heat sterilization.The culture medium was supplemented with the following nutrients:100 mg L-1KNO3,10 mg L-1KH2PO4,2.5 mg L-1MnSO4·H2O,2.5 mg L-1FeSO4·7H2O,10 mg L-1EDTA-Na2,6 μg L-1vitamin B1and 0.05 μg L-1vitamin B12.Microalgae were maintained in 5000 mL conical flasks at 20℃ ± 2℃,with a light intensity of 4000 Lux (LED) under a 12 h:12 h light:dark (L:D) photoperiod.Cells were incubated at 4℃ overnight to metabolize the pyrenoid and starch granules in chloroplasts,followed by sample collection at the late stationary phase.The cells were centrifuged at 6000 ×gfor 10 min.A blood counting chamber was used to determine the cell density at each time point.
2.2 Isolation and Purification of Intact Chloroplasts
Chloroplasts fromI.galbanawere isolated and purified at 4℃ or on ice to protect the integrity of chloroplasts,and the pellet was washed and resuspended gently.At first,100 mg of fresh microalgae were washed twice with 2 mL of 50 mmol L-1HEPES-KOH (pH 8.2) and centrifuged at 800 ×gfor 10 min to remove the small metabolites surrounding the cells.The chlorophyll concentration of wholecell was measured with the method of Arnon (1949).The key step was to gently separate the cell membrane from plastids,and to this end,the cells were provided with a hypotonic environment by two steps.First,the cells were resuspended in 2 mL of solution A (300 mmol L-1sucrose,50 mmol L-1HEPES-Tris,10 mmol L-1EDTA-Tris,5 mmol L-1MgCl2,1% PMSF as a protease inhibitor,pH 7.8) and centrifuged.Second,the cells were subjected to a lower osmotic shock by resuspending the cell pellet in 2 mL of solution B (100 mmol L-1sucrose,50 mmol L-1HEPESTris,10 mmol L-1EDTA-Tris,5 mmol L-1MgCl2,1% PMSF,pH 7.8) and allowing to stand on ice for 20 min.Subsequently,the cells were successively homogenized with a homogenizer (Omni International,USA) at 699 ×gfor 10 s and 5169 ×gfor 15 s,followed by centrifugation at 800 ×gfor 10 min.For effective separation,the supernatant was set aside,and solution B was added to the cell pellet and subjected to homogenization for 3– 4 times.
The supernatant was centrifuged at 1000 ×gfor 10 min to remove nuclei and intact cells,and the crude chloroplasts were collected by centrifugation at 4500 ×gfor 10 min.The crude chloroplast pellet was gently resuspended in 1 mL of solution C (300 mmol L-1D-sorbitol,50 mmol L-1HEPES-Tris,10 mmol L-1EDTA-Tris,5 mmol L-1MgCl2,1% PMSF,pH 7.8) using a pipette to avoid disrupting the chloroplasts.The resuspended sample was gently loaded onto several Percoll density gradients (top to bottom:2 mL 10% Percoll,3 mL 30% Percoll,3 mL 50% Percoll).All gradient media were prepared in solution C.The Percoll gradients were centrifuged at 8000 ×gfor 30 min.The intact chloroplasts constituted a band at the 30%–50% interface.They were collected and diluted with three volumes of solution D (300 mmol L-1D-sorbitol,50 mmol L-1HEPES-Tris,10 mmol L-1EDTA-Tris,5 mmol L-1MgCl2,pH 7.8).Finally,they were centrifuged at 3500 ×gfor 10 min twice,and the pellets were stored at -80℃ for further analyses.The chlorophyll concentration of the chloroplast was determined with the same method as that for whole-cell.
2.3 Electron Microscopic Observation
The modified Spurr method (1969) was used to prepare the samples for TEM.Briefly,samples were incubated in 2.5% glutaraldehyde in phosphate buffer (0.1 mol L-1,pH 7.8) at 4℃ for 48 h,washed with phosphate buffer,and then post-fixed with 1% osmium tetroxide (OsO4) for an additional 3 h.Subsequently,samples were dehydrated in a graded series of ethanol from 30%,50%,70%,80% to 90% ethanol,followed by acetone.The specimens were incubated in acetone and finally Spurr resin mixture overnight,followed by incubation at 70℃ for more than 9 h.The samples were sectioned using a Leica EM UC7 ultramicrotome (Leica Microsystems,Germany),and the sections were stained by uranyl acetate and alkaline lead citrate,followed by observation using a Hitachi H-7650 TEM(Hitachi Ltd.,Japan) at an acceleration voltage of 80 kV.
2.4 Enzyme Activity Assaying
Proteins were extracted with a modified Rausch method (1981).Briefly,the samples were ground with liquid nitrogen in a pre-cooled mortar and subsequently sonicated in lysis buffer (8 mol L-1urea,1% PMSF) on ice three times.The remaining debris was removed by centrifugation at 12000 ×gat 4℃ for 10 min.The supernatant was collected,diluted with four volumes of cold acetone,and incubated at -20℃ for 24 h,followed by centrifugation at 12000 ×gat 4℃ for 10 min.Finally,the obtained pellet was dissolved in phosphate buffer (0.1 mol L-1,pH 7.2).
The purity of isolated chloroplasts was assessed by measuring the activity of cytochrome c oxidase (COX) which is a unique enzyme of mitochondria in haptophytes.The chloroplast-specific enzyme,Ribulose bisphosphate carboxylase oxygenase (rubisco),was used as a positive control.The enzyme activities were estimated using enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Meimian Biotechnology,China) following the manufacturer’s instructions,and the color change was measured spectrophotometrically at 450 nm.Each experiment was conducted three times.
2.5 Lipid Extraction
The total lipid was obtained from samples with a modified method developed by Bligh and Dyer (1959).Briefly,freeze-dried samples were extracted with chloroform/methanol/water (2:2:0.8,v/v/v) and dried under a gentle stream of N2,and the residue was preserved at -20℃.The lipid yield of the chloroplast ofI.galbanawas calculated according to the study by Silitongaet al.(2017).The final lipid residue was resuspended in CH3OH before further analysis.Samples were filtered using a 0.22-μm ultrafiltration membrane before injection.The above-mentioned procedures were carried out with eight replicates.
2.6 UHPLC Conditions
A Thermo Scientific™ UltiMate™ 3000 High-Performance LC system (UHPLC) with an ACQUITY UPLC BEH C8 analytical column (i.d.2.1 mm × 100 mm,particle size 1.7 μm,pore size 130 Å) was used to perform reversedphase analysis.Mobile phase A consisted of acetonitrile/water (6:4,v/v),mobile phase B consisted of isopropanol/acetonitrile (9:1,v/v),and both phases contained 0.1% formic acid and 10 mmol L-1ammonium acetate.The flow rate was set at 0.2 mL min-1.The gradient elution was programmed as follows:0– 15 min,60%– 45% A;15.0– 18.0 min,45%– 35% A;18.0– 26.0 min,35% A;26.0– 28.0 min,35%– 0 A;28.0– 30.0 min,0 A;30.0– 30.5 min,returning to an initial 60% A;30.5– 40 min,60% A.The temperature of the sample chamber was maintained at 8℃,and the column temperature was maintained at 45℃.
2.7 Mass Spectrometric Conditions
Mass spectrometry was carried out on a Thermo ScientificTMQ ExactiveTMhybrid quadrupole-Orbitrap mass spectrometer equipped with a HESI-II probe.The instrument was operated according to a data-dependent LC-MS/MS method in positive mode and negative mode,respectively.Data were obtained in a centroid mode from 200 to 2000 m/z at a resolution of 70 K,and in a high energy collisional dissociation (HCD) MS/MS mode at a resolution of 17.5 K.The automatic gain control (AGC) target was set at 1e6for MS and 2e5for MS2.The capillary voltage was maintained at 3.5 kV,and the capillary temperature was set at 350℃.The sheath gas was 45 arb while the aux gas was 10 arb.MS2analysis was carried out on the mass spectrometer with different collision energy and ramp of 25,30 V in a positive ion mode and 20,24,28 V in a negative ion mode based on the type of lipids.The instrument was previously calibrated in positive mode and negative mode,respectively.
2.8 Data Processing
The raw data acquired from LC-MS/MS runs were analyzed on the XcaliburTMversion 2.2 (Thermo Fisher Scientific,USA).LipidSearchTMsoftware version 4.1 (Thermo Fisher Scientific,USA) was used to perform peak identification,lipid identification,peak extraction,peak alignment,peak area and intensity.According to characteristic fragment ions and fragmentation pathways,glycolipids,phospholipids (PLs),betaine lipids,and non-polar lipids could be determined (Naumannet al.,2007;Roche and Leblond,2010;Xuet al.,2010;Yanet al.,2010;Liet al.,2014;Reyeset al.,2016;Huanget al.,2019).Identification of each lipid class and the acyl chains were further confirmed by MS/MS in positive or negative ion mode as previously described.The semi-quantitative determination of each lipid was based on the lipid standards (Avanti Polar Lipids Inc.,USA) area (Xuet al.,2010;Liet al.,2014).In lipid composition analysis,each lipid content is expressed as nmol lipid/mg dry weight,while the contents of all lipids were summed to get the amount of total lipid.Each lipid content was divided by the amount of total lipid and then converted to the percentage,which means the percentage of each lipid.The percentages of each fatty acid were calculated with the same method.
2.9 Statistical Analysis
In lipid composition analysis,data were analyzed using SPSS software version 22.0 (IBM Corp.,USA) and Student’st-test was performed to determine the significant difference between the two groups.All analyses were carried out with eight replicates and the differences were considered statistically significant ifP<0.05.Data were expressed as mean ± SE.
3 Results
3.1 Chloroplast Yield
The chlorophyll concentrations of whole-cell and chloroplast were determined.The average yield of intact chloroplasts was 15.3% ± 0.1% based on chlorophyll recovery(Table 1).

Table 1 The chlorophyll contents of whole-cell and chloroplasts

Table 2 Comparison of enzyme activities in cell homogenate,crude chloroplasts,and purified chloroplasts from I.galbana
3.2 Chloroplast Characterization and Integrity
Chloroplast debris was accumulated at the 10%– 30% Percoll gradient interface,the intact chloroplasts constituted a band at the 30%– 50% interface,and intact cells were found at the bottom of the tube (Fig.1).The microscopic pictures for each fraction are shown in Fig.2 and Fig.3.

Fig.1 Typical Percoll gradient after centrifugation,and the percentages of Percoll in buffer for the various layers.a,broken chloroplasts;b,intact chloroplasts;c,intact cells.

Fig.2 Microscopic images of the broken chloroplasts at 50000 × magnification (a) and the intact cell at 40000 × magnification (b).
Figs.3a and 3b show that there was no intact cell in the purified chloroplasts.Under a phase-contrast microscope,the intact chloroplasts were green and surrounded by a bright halo due to the presence of the chloroplast envelope,and had an integrity of >85% (Fig.3d).When observing the intact cells and isolated chloroplasts using fluorescence microscopy,the isolated chloroplasts emitted red light and were smaller than intact cells (Figs.3e and 3f).Examination of cell ultrastructure showed that purified chloroplasts retained their shape and were intact (Figs.3g and 3h).
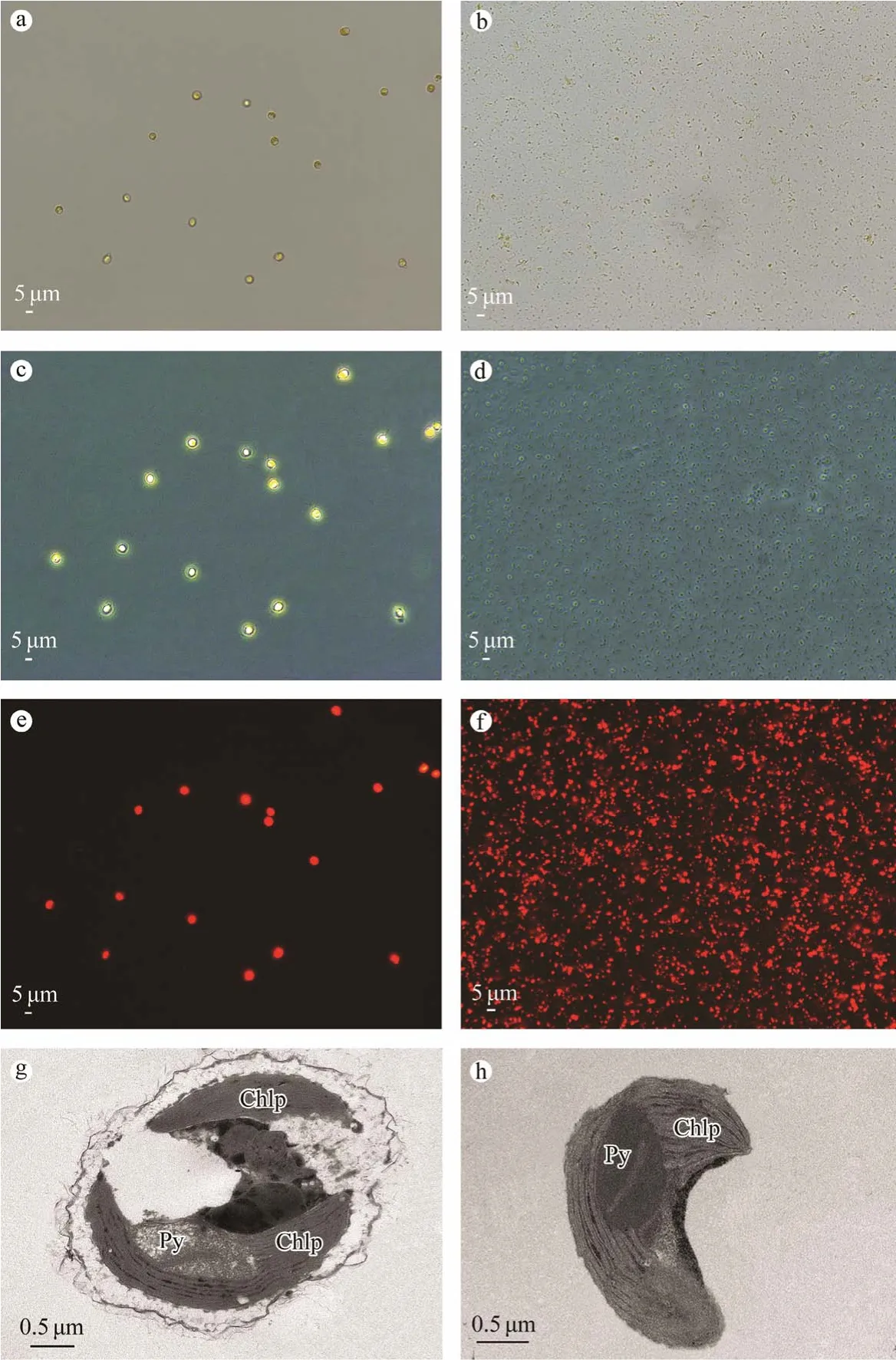
Fig.3 Micrographs of whole-cell and isolated chloroplasts from I.galbana.Light and electron micrographs of I.galbana:light micrograph of I.galbana at 400 × magnification (a);phase-contrast micrograph of I.galbana at 400 × magnification(c);fluorescence micrograph of I.galbana at 400 × magnification (e);and TEM image of I.galbana at 30000 × magnification (g).Light and electron micrographs of purified chloroplasts:chloroplasts purified from I.galbana were analyzed by phase-contrast fluorescence microscopy at 400 × magnification,light (b);phase contrast (d);and fluorescence (f) microscopy.The integrity of the chloroplasts was validated using TEM at 40000 × magnification (h).Chlp,chloroplast;Py,pyrenoid.
3.3 Chloroplast Purity
The activities of rubisco and COX in cell homogenates,crude chloroplasts and purified chloroplasts were determined,and the data are shown in Table 2.The differential centrifugation could reduce 64% mitochondrial contamination,and a discontinuous density gradient centrifugation could further reduce 83% mitochondrial.This method resulted in only low activities of COX,confirming that there was no significant mitochondria contamination in the isolated chlo-Notes:Activity is expressed as U per μg of chlorophyll with three independent replicates.Cell homogenate was obtained by homogenization.Crude chloroplasts were obtained by differential centrifugation.Purified chloroplasts were obtained by differential centrifugation followed by purification using discontinuous density gradient centrifugation.roplasts.Microscopic images and the rubisco activity data confirmed the integrity and purity of the isolated chloroplasts.
3.4 Lipid Composition of Whole-Cell and Chloroplast
The chloroplast contains up to 65.8% ± 4.3% of lipid inI.galbana,which is based on the lipid yield (the weight of dry chloroplast was 10.0 mg ± 0.1 mg and the weight of dry chloroplast lipid was 6.7 mg ± 0.3 mg).In the present study,we analyzed the contents of glycolipids,PLs,sphingolipids,betaine lipids,and acylglycerols in the whole-cell and chloroplast ofI.galbana.Fig.4 lists the relative amounts of lipid class in total lipid (a) and the relative amounts of lipid subclass in total lipid (b).
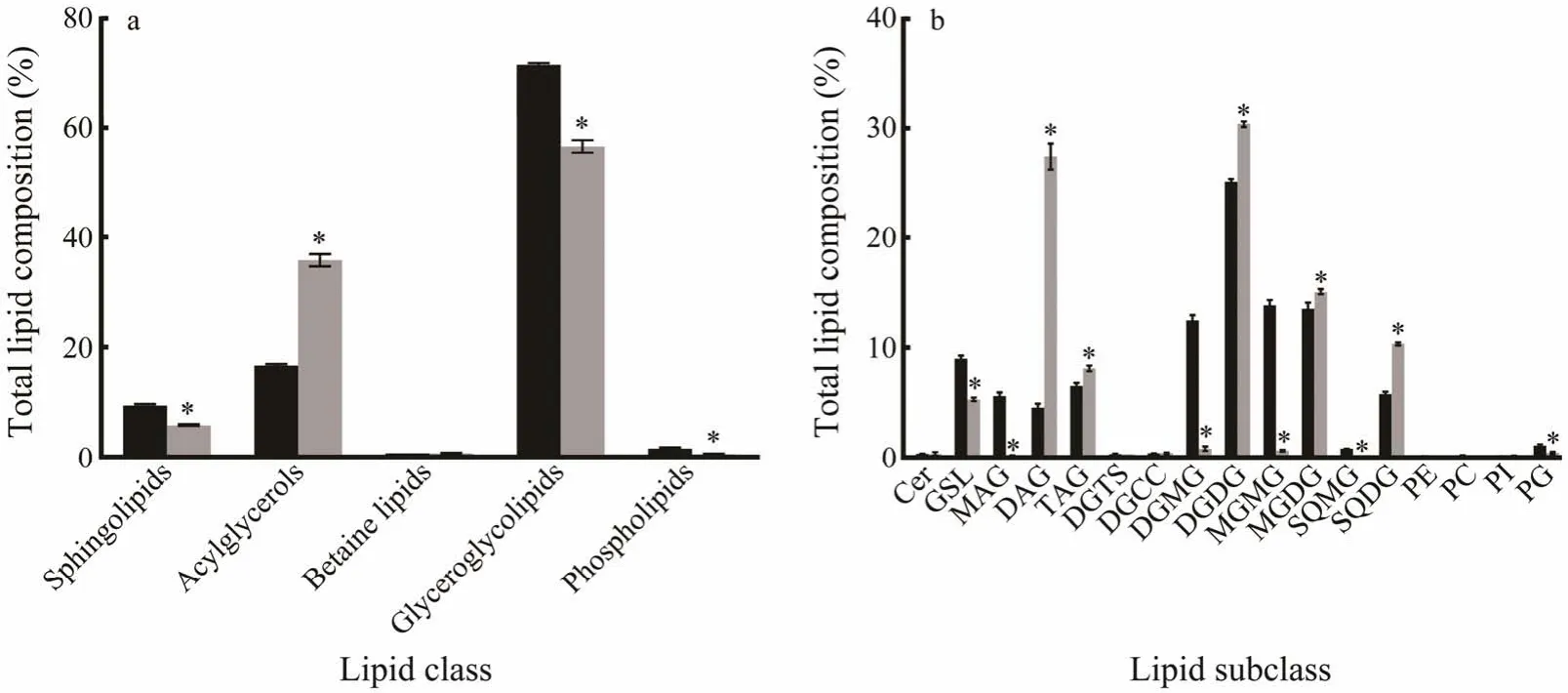
Fig.4 Total lipid composition of whole-cell (black bars) and chloroplast (gray bars) in I.galbana.The percentage of each lipid was shown as mean ± standard error (%,n=8).The significance of the differences between mean values was determined with Student’s t-test,while asterisk (*) indicates a significant difference at P <0.05.
Glycolipids were the main lipid components in the chloroplast and accounted for 56.6% of the total (Fig.4a).As the most abundant glyceroglycolipids in chloroplast,DGDG,MGDG and SQDG made up approximately 30.4%,14.2%and 10.4% of the total,respectively,which were much higher than those in whole-cell (Fig.4b).Compared to those in whole-cell,digalactosylmonoglyceride (DGMG),monogalactosylmonoglyceride (MGMG) and sulfoquinovosylmonoacylglycerol (SQMG) were nearly undetectable in chloroplasts (Fig.4b).The fatty acyl R1/R2 were mostly 18:4/16:1,18:3/16:1 and 18:4/18:5 in DGDGs (Fig.5a),14:0/18:4,18:4/18:5,18:4/18:4 and 18:3/18:4 in MGDGs (Fig.5b) and 16:0/14:0,16:0/18:3 and 18:4/18:3 in SQDGs (Fig.5c).
Based on our experimental results,the proportion of acylglycerols was the second after glycolipids,which were abundant in both whole-cell (16.7%) and chloroplasts(35.8%) ofI.galbana(Fig.4a).The content of fatty acyl R1/R2 with 22:6/18:4 was the highest among DAGs (Fig.5d).Microscopic observations confirmed that chloroplasts contained lipid droplets (LD),which were conserved neutral lipid storage organelles (Fig.6).The proportion of DAG in total chloroplast lipid was 27.4%,which was more than that in whole-cell (Fig.4b).Meanwhile,the proportion of TAG in chloroplasts (8.1%) was only slightly more than that in whole-cell (6.5%) and monoglyceride (MAG) was nearly undetectable in chloroplasts (Fig.4b).

Fig.5 Lipid content of fatty acyl R1/R2 with DGDGs,MGDGs,SQDGs and DAGs in the chloroplasts of I.galbana (nmol lipid per mg dry weight).Data are presented as mean ± SE (n=8).

Fig.6 TEM micrographs of lipid droplets in the chloroplast of I.galbana.Chlp,chloroplast;LD,lipid droplet;M,mitochondrion.
The proportion of PLs in the total lipid of chloroplasts was only slightly less than that in whole-cell (Fig.4a).The proportion of PG in PL was greater compared with the other PLs in whole-cell and chloroplasts,and other PLs in chloroplasts were detected at lower amounts (Fig.4b).PEs containing fatty acyl R1/R2 with 15:1/15:0,15:1/16:0,18:4/16:0,18:1/16:0,18:1/18:1 and 19:1/18:1 were not found in chloroplast compared with whole-cell,and PCs with 18:4/16:0,16:0/18:2,18:4/18:1 were also not found.
We also found sphingolipids which made up 9.4% in whole-cell and 5.8% in chloroplasts (Fig.4a).There was a little amount of glycosphingolipid (GSL,5.3%) and a small amount of ceramide (about 0.3%) of sphingolipids in chloroplasts (Fig.4b).The proportion of betaine lipids was low(Fig.4a),which was nearly the same in whole-cell (0.7%)and chloroplast (0.7%).Moreover,14:0 lyso-diacylglycerylcarboxyhydroxymethylcholine (lyso-DGCC) and 16:0 lyso-DGCC were not found in chloroplasts.
ForI.galbana,the fatty acid compositions of major lipid subclass in whole-cell and chloroplasts are given in Fig.7.Compared with those in whole-cell,in DGDGs of chloroplasts,the proportion of 14:0,16:0,16:1,18:1,18:2,18:3,and 22:6 fatty acids were substantially richer,16:2,18:4 and 18:5 fatty acids were substantially deficient and the proportions of other fatty acids showed no significant difference (Fig.7a).In MGDGs of chloroplasts,the proportion of 16:0,16:1,18:2,18:3,18:4,and 22:6 fatty acids were substantially richer,the proportions of 14:0,18:1,and 18:5 fatty acids were deficient and the proportions of other fatty acids showed no significant difference (Fig.7b).In SQDGs of chloroplasts,the proportion of 14:0,16:0,18:2,18:3,and 22:6 fatty acids were substantially richer,the proportions of 16:1 and 18:1 fatty acids were deficient,and no significant difference was observed in the proportions of other fatty acids (Fig.7c).From these images,it could be seen that chloroplast lipids ofI.galbanahad high amounts of unsaturated fatty acids,and were mostly 14:0,16:1,18:3,18:4 and 18:5 in DGDGs,14:0,16:0,18:2,18:3,18:4 and 18:5 in MGDGs and 14:0,16:0,18:3 and 18:4 in SQDGs.For acylglycerols,in DAGs of chloroplasts,the proportion of 16:1,18:4,and 22:6 fatty acids were richer,the proportions of 14:0,18:0,and 18:1 fatty acids were slightly deficient,and the proportions of 16:0,16:2,18:2,18:3 and 20:5 fatty acids in DAGs were similar (Fig.7d).Besides,in TAGs of chloroplasts,the proportion of 16:0,18:0 and 18:1 fatty acids were slightly richer,the proportions of 18:4,18:5 and 22:6 fatty acids were slightly deficient and the proportions of other fatty acids were similar (Fig.7e).The fatty acids were mostly 18:1,18:4,and 22:6 in DAGs,14:0,16:0,18:1,18:4,and 22:6 in TAGs.Moreover,in GSLs of chloroplasts,the proportions of d18:1,d18:3,d18:4,d19:3 and h22:1 fatty acids were substantially richer,the proportion of d19:4 and h22:0 fatty acids were sharply deficient,and the proportions of other fatty acids were similar (Fig.7f).

Fig.7 Fatty acid profiles of major lipid subclasses in whole-cell (black bars) and chloroplasts (gray bars) of I.galbana.The percentage of fatty acid was shown as mean ± standard error (%,n=8).The significance of the differences between mean values was determined with Student’s t-test.The asterisk (*) indicates a significant difference (P <0.05).
4 Discussion
I.galbanadiffers from other marine unicellular algae;it lacks cell wall.Due to this difference in structure,chloroplast isolation techniques need to be specifically adapted forI.galbana,and here,we report a new isolation approach.SDS is frequently used in isolation buffers to break up the cell wall;however,it was not included in the isolation buffer we used.Referred to the disruption method for animal cells (Andreyevet al.,2010),our improved method used two-step hypotonic buffers,which was more efficient compared with simplex lysis buffers.Buffer A contained 300 mmol L-1sucrose,and buffer B contained only 100 mmol L-1sucrose.The two-step hypotonic buffers allow the microalgae cells to gradually detach from the plasma membrane,and the proportion of the buffers needs to be strictly controlled.However,as the plasma membrane ofI.galbanawas extremely hard,separation could further be achieved by homogenization to save time.Meanwhile,the two-step hypotonic buffers provided conditions for the next homogenization.A rapid isolation method of chloroplast consists of two-step hypotonic extraction and homogenization,which can save time and increase the yield.
In particular,the multiple homogenization is a key feature of the isolation procedure and ensures a high yield of intact chloroplasts.During homogenization,a quartz sand mixture (0.1 and 0.4 mm) was added into the tube to break the cells ofI.galbana.The homogenization needs to be thorough,and several short-time homogenizations were used until very little sediment was observed after centrifugation at 800 ×gfor 10 min.The supernatant was examined using phase-contrast fluorescence microscopy to ensure the absence of intact cells.The speed of homogenization is also vital for intact chloroplast isolation,and we adopted the intermittent low-speed and high-speed methods.With this modification,we could not only isolate the intact chloroplasts,but also avoid thylakoid membrane fragmentation contamination,which is usually caused by mechanical heat during continuous homogenization.Additionally,continuous low-speed homogenization increases the isolation time substantially,which is not conducive to the separation and protection of intact chloroplasts.Therefore,our method of successive low-and high-speed homogenizations in this experiment mitigates these issues.The homogenization condition presented here is optimal forI.galbana,and we demonstrated that the osmotic shock and homogenization play fundamental roles for effective cell lysis,organelle separation and final isolation of intact chloroplasts.
After homogenization,intact chloroplasts were purified from damaged chloroplasts and intact cells by a Percoll density gradient centrifugation,and two-step gradients were employed for most experiments.Before gradients were added,the tube was rinsed with 1% BSA.Then the gradients were added slowly along the tube wall to form distinct interfaces.The gradient centrifugation step allowed the purification of intact,photosynthetically active chloroplasts fromI.galbana.
The methods of microscopic observation and enzyme activity assaying were used to estimate the integrity and purity of the isolated chloroplasts.According to the previous researches,mitochondria are common contaminants in the chloroplast isolates (Kleinet al.,1983;Masonet al.,2006;Piroet al.,2015).BecauseI.galbanais not a model species,we refer to the mitochondria and chloroplast markers ofEmiliania huxleyiwhich is the related prymnesiophyte alga ofI.galbana(Bendifet al.,2014).COX is the unique enzyme in mitochondria and chloroplasts do not contain this enzyme.ELISA is a powerful method for measuring the enzymatic activity and often applied in biochemical assays in algae (Yuet al.,2002;Sunet al.,2019).It is rapid,sensitive,specific and precise (Mosmann,1983).Therefore,we used it to quantify enzymatic activity.To avoid the inactivation of the enzymes,the samples should go through the entire experimental process,and the sampling process should be synchronous.Cell homogenate,rather than cell fractionation,was selected to determine 100% of individual enzymatic activity.The results of the COX activities in the cell homogenates,crude chloroplasts,and purified chloroplasts can detect the purity of the isolated chloroplast.
To fully understand the chloroplast lipid composition inI.galbana,we chose the late stationary phase of the microalga for analysis because it is the optimal period with the most lipid accumulation (Gnoumaet al.,2017).The chloroplast lipid composition ofI.galbanawas qualitatively similar to that of other photosynthetic plants.The results of the cellular and chloroplast lipid composition inI.galbanaindicated that chloroplasts were the major sites for fatty acid synthesis and lipid biosynthesis.The results of glycolipids were consistent with those reported on chloroplast from land plants likeArabidopsis(Gaudeet al.,2007;Granafeiet al.,2017).One important exception was that the content of DGDG was higher compared with the MGDG in this alga.This was probably a consequence of different plant species or cultivation conditions.It is well known that depending on the plant species,growth conditions can affect the components of DGDG and MGDG(Guschina and Harwood,2006;Simionatoet al.,2013).According to previous reports,there is a little quantity of acylglycerols inVicia faba(Yoshidaet al.,2009) and a high quantity inMimosa pudica(Lim,2014).Moreover,Choudhury and Chakrabarti (1980) had detected cerebroside in chloroplasts ofMimosa pudica.Sphingolipids are largely synthesized and localized in the plasma membrane.A small amount of sphingolipids were observed in chloroplast because of the lipid transportation between organelles (Lynch and Dunn,2004;Chenet al.,2008).Our results indicated that acylglycerol and cerebroside levels inI.galbanaandMimosa pudicawere similar.In eukaryotes,lipid droplets serve as the primary depot for energy and neutral lipid including TAG,DAG,and free fatty acids (Annaet al.,2015;Čopičet al.,2018;Xuet al.,2020).In this research,we also observed lipid droplets in the chloroplasts ofI.galbana,which isconsistent with previous studies (Eltgrothet al.,2005;Wilflinget al.,2013).Besides,the results indicated that DAG was the most abundant acylglycerols in the chloroplast ofI.galbana.According to previous reports,this is because DAG is not only the precursor for the synthesis of glycolipids,TAG and PG,but also the lipid signaling molecule involved in the growth and development of an organism (Kellyet al.,2003;Kalischet al.,2016).
5 Conclusions
To our knowledge,this is the first time to develop a method to isolate intact chloroplasts.The delivered lipid yield is sufficient for sub-cellular lipid composition studies inI.galbana.We compared the difference between cellular and chloroplast lipid composition inI.galbana,and analyzed the fatty acids of chloroplast lipids.Further work will focus on the changes in the chloroplast lipids during the entire growth stage.
Acknowledgements
This research was supported by the Ningbo Science and Technology Research Projects (No.2019B10006),the National Key Research and Development Program of China(No.2019YFD0900400),the Zhejiang Major Science Project (No.2019C02057),the China Agriculture Research System of MOF and MARA,the Natural Science Foundation of Ningbo (No.2019A610416),the Ningbo Science and Technology Research Projects (No.2019C10023),and the National Natural Science Foundation of China (No.31801724).
杂志排行
Journal of Ocean University of China的其它文章
- Numerical Simulation and Analysis of Storm Surges Under Different Extreme Weather Event and Typhoon Experiments in the South Yellow Sea
- Analysis of Wave Distributions Using the WAVEWATCH-III Model in the Arctic Ocean
- Multi-Objective Weather Routing Algorithm for Ships Based on Hybrid Particle Swarm Optimization
- Experimental Study on the Influencing Factors of Motion Responses on an Air-Floating Caisson with Multiple Compartments
- Placement Optimization Method of FPSO Gas Detectors Based on Leakage Risk
- Effect of Temperature on the Acoustic Reflection Characteristics of Seafloor Surface Sediments
