Enhanced Biodegradation of High-Salinity and Low-Temperature Crude-Oil Wastewater by Immobilized Crude-Oil Biodegrading Microbiota
2022-02-28HUANGXiaoZHOUTingCHENXiBAIJieandZHAOYangguo
HUANG Xiao ,ZHOU Ting ,CHEN Xi, ,BAI Jie ,and ZHAO Yangguo
1) Collaborative Innovation Center of Atmospheric Environment and Equipment Technology, Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, School of Environmental Science and Engineering,Nanjing University of Information Science and Technology,Nanjing 210044,China
2) The Key Laboratory of Marine Environmental Science and Ecology, Ministry of Education, Ocean University of China,Qingdao 266100,China
3) Harbin Institute of Technology (Shenzhen), Shenzhen Key Laboratory of Water Resource Utilization and Environmental Pollution Control, Shenzhen 518055,China
Abstract High salt and low temperature are the bottlenecks for the remove of oil contaminants by enriched crude-oil degrading microbiota in Liaohe Estuarine Wetland (LEW),China.To improve the performance of crude-oil removal,microbiota was further immobilized by two methods,i.e.,sodium alginate (SA),and polyvinyl alcohol and sodium alginate (PVA+SA).Results showed that the crude oil was effectively removed by the enrichment with an average degrading ratio of 19.42–31.45 mg (L d) -1.The optimal inoculum size for the n-alkanes removal was 10% and 99.89%.Some members of genera Acinetobacter,Actinophytocola,Aquabacterium,Dysgonomonas, Frigidibacter,Sphingobium,Serpens,and Pseudomonas dominated in crude-oil degrading microflora.Though the removal efficiency was lower than free bacteria when the temperature was 15℃,SA and PVA+SA immobilization improved the resistance to salinity.The composite crude-oil degrading microbiota in this study demonstrated a perspective potential for crude oil removal from surface water under high salinity and low temperature conditions.
Key words crude-oil degrading microbiota;microbial community;immobilization;high salinity;low temperature
1 Introduction
Wetlands,as ecologically sensitive and vulnerable ecotone,locate at the junction of aquatic and terrestrial ecosystems to provide many economic and ecological services for human being (Saha and Pal,2019).Flood prevention,wildlife habitat,sediment fixation,contaminant removal and landscape value are considered irreplaceable roles and functions of wetland,however,human disturbance and climatic change have destroyed this special ecological environment (Woodward and Wui,2001;Saha and Pal,2019).Liaohe Estuarine Wetland (LEW) is typical natural wetland owns many extensive industries depending on the abundant oil and natural gas resources and petroleum hydrocarbons pollutants frequently generated from petrochemical industries during the processes of crude-oil storage,spill,rinse water and clean-outs from vessel processing operation (Luo and Xing,2010;Linet al.,2013;Duanet al.,2019).
The common methods for treating oil pollution include physical adsorption (Pourmandet al.,2015),chemical dispersant (Kujawinskiet al.,2011) and biodegradation(Mahmoudiet al.,2013;Princeet al.,2013).In most cases,petroleum hydrocarbons could be adsorbed by physical methods,instead of being degraded completely.The adsorbent with crude-oil still need to be treated harmless depending on biodegradation.Chemical methods are not economically feasible due to the generation of secondary pollutants (Liuet al.,2016).In contrast,biodegradation is considered to be eco-friendly and costeffective technologies for crude-oil removal (Xueet al.,2015).With the development of microbial biotechnology,many microbial strains are applied to degrade crude oil(Hassanshahianet al.,2012;Bayatet al.,2015;Santisiet al.,2015).However,crude-oil biodegradation completely usually depends on the perfect cooperation of different types of microorganisms.During biodegradation process,some microorganisms such as,Arthrobactersp.,Acinetobactersp.,Streptomycessp.,Pseudomonassp.,andBacillussp.could degrade alkanes effectively (Shahiet al.,2016).Due to different microbes in degrading petroleum hydrocarbons play different roles,microbial consortium is required to cooperate and transform the total petroleum hydrocarbons compounds to CO2and H2O.
On the other hand,environment is the key factor to restrict biodegradation.LEW is located in estuarine area in Northeast China with high-salinity (range from 10 to 35)and low temperature (lower than 0℃) conditions limit biodegradation.By now,few previous studies focus on crude-oil biodegradation under high-salinity and low temperature conditions,and how to improve biodegradability and break the environment limit is necessary to be studied.Hence,choosing appropriate measure that can improve the activity of functional bacteria during oil pollutants removal is significant for overcoming above disadvantages.Immobilization technology of oil degradation bacteria has been studied for several years (Gentiliet al.,2006;Zhenet al.,2012).It could keep microflora from adverse environmental conditions of threatening microbial survival (Lauet al.,2003).Sodium alginate (SA) and sodium alginate-polyvinyl alcohol (PVA+SA) were the crosslinking materials and they could form a network structure to protect the bacteria against the impact of external adverse environment (Angelimet al.,2013).Moreover,this technology would prevent microbiota from being washed out and diluted in flowing water (Houet al.,2013),and help microbiota treat high pollution loadings (Sekaranet al.,2013).In this study,immobilization of crude-oil degrading microbiota is quite necessary strategy to avoid the impacts of temperature and salinity.
Therefore,the objective of this study was to enrich crude-oil degrading microbiota,and evaluate the degradation performances of total petroleum hydrocarbons and n-alkanes,and discuss the changes of microbial community composition during the enrichment process by highthroughput sequencing technology.Meanwhile,immobilized crude-oil degrading microbiota was investigated to observe its resistance to low temperature and high salinity.Thus,this study will deepen our understanding of compound oil degrading bacteria and enhance their application in wetland oil pollution control.
2 Materials and Methods
2.1 Sampling
To enrich crude-oil degrading microbiota,fresh soil or sediment samples were taken from four stations in LEW.Liaohe oilfield is located near four sampling stations.Table 1 showed the sampling sites and some environmental parameters of the soil samples,which were collected from surface soil (0 to 10 cm) with a sterile knife,and then transported to the laboratory under 4℃ condition.One part of samples was stored at -20℃ for DNA extraction,and the others were mixed and stored at 4℃for the enrichment of crude-oil degrading microbiota.

Table 1 The coordinates of the sampling stations and environmental parameters in Liaohe Estuarine Wetland
2.2 Enrichment of Crude-Oil Degradation Bacteria
Inorganic salt liquid medium with crude-oil was used to enrich the crude-oil degradation microbiota.The chemical ingredients contained (per liter):3 g NaNO3,0.5 g KCl,0.5 g K2HPO4,0.5 g KH2PO4,0.5 g MgSO4·7H2O,0.001 g FeSO4·7H2O,0.002 g CaCl2,and pH 7.2 250 mL flasks containing 100 mL inorganic salt medium was used for crude-oil degradation microbiota enrichment with adding soil samples (10 g) and supplying crude oil to the concentration of 0.2 g L-1,and then incubated for 10 d at 30℃ in a shaker.Then 5 mL supernatant was added into a fresh inorganic salt medium with higher concentration oil every 7 d.The above steps were repeated three times with the increasing concentration of the oil from 0.5,1.0,to 2.0 g L-1,until the crude-oil degradation microbiota was enriched.
2.3 Change of Microbial Community Composition
High-throughput sequencing technology was applied for analyzing the changes of microbial diversity between original soil sample and crude-oil degradation microbiota and the total DNA of soil and crude-oil degradation bacteria were extracted with Power Soil DNA kit (Mobio,CA USA) as the described by Simisteret al.(2015).The total DNA was dissolved in 2 mmol L-1Tris-HCl (pH was 8) eventually,and 5 µL DNA sample was collected for electrophoresis detection by 1% agarose gel electrophoresis and Nanodrop (2000c,Thermo,USA).
After that,PCR amplification was conducted with universal primers and DNA extracts as a template for amplifying V4 region of 16S rDNA.The DNA amplification was generated using the methods described in Lauberet al.(2008).Here,the forward primer 515F (5’-GTGCCAG CAGCAGCCGCGGTAA-3’) and a combination of reverse primers 806R (5’-GGACTACCAGGGTATCTAAT-3’).All PCR reactions were carried out in 30 μL reactions and the steps were following by Huanget al.(2017b) and then determined the sequencing on an Illumina MiSeq platform by Novogene (Beijing,China).The paired-end reads merged and species diversity and relative abundance were analyzed by Huanget al.(2017b).
2.4 Immobilization of the Crude-Oil Degradation Microbiota
SA and PVA+SA immobilized crude-oil degradation microbiota measures are according to Wanget al.(2016)and Zhanget al.(2014),respectively.SA immobilized measure is described as following:2% CaCl2and SA aqueous solutions (2 g and 3 g CaCl2and SA were souled in 100 mL deionized water,respectively) were prepared,and then sterilized at 121℃ for 20 min.Both of aqueous solutions were cooled to room temperature for later using.The crude-oil degradation microbiota was washed by deionized water for three times and centrifuged at 4000 r min-1for 10 min.Above steps were repeated for three times to remove the nutrients on the surface of organisms.Bacteria suspension and SA aqueous solutions were mixed by the proportion of 1:2 and transferred to 2%CaCl2aqueous solutions by peristaltic pump.The prepared immobilized particles were stored to complete the crosslinking.
PVA+SA immobilized measure uses sodium alginate and sodium alginate-polyvinyl alcohol as embedding material.2 g SA and 12 g PVA were dissolved in 100 mL distilled water to form the embedding medium,which was sterilized at 121℃ for 20 min.The bacteria suspension,which was washed,centrifuged,and added into PVA+SA aqueous solutions by the proportion of 1:2.The mixture was dropped into saturated boric acid and CaCl2aqueous solutions by peristaltic pump and stored at 4℃ for 24 h to form beads.The formed beads were washed three times by deionized water and stored at 4℃ for using.In order to deduct the pollutants removed by the adsorption of materials in the experimental group,three control groups without crude-oil degradation bacteria were set up,and deionized water was then used to replace bacteria solutions (control,SA-control,and PVA+SA-control,respectively).
2.5 Degradation of Crude Oil
For exploring the degradation characteristic of free and immobilized crude-oil degrading microbiota,a series of batch experiments were performed.0.5g L-1crude oil liquid medium was selected and 1%,3%,5%,and 10%(V/V) crude-oil degrading microbiota (OD600=0.8) were inoculated to investigate the degradation efficiency of free crude-oil degrading microbiota.The same inoculation amount of immobilized particles was added to investigate the crude oil degradation performance of immobilization methods.Meanwhile,different gradient temperatures (15,20,25,30,and 35℃) and salinities (2,5,15,25,and 35) were designed to observe the tolerance to temperature and salinity by different immobilization methods.The nutrient solution was then incubated in a shaker at 30℃ and 180 r min-1for 15 d.The amount of total petroleum hydrocarbon and the composition of n-alkanes in the different conditions were analyzed after 15 d experiment.
2.6 Detection Method of n-alkanes
The residual n-alkanes were analyzed by gas chromatographic mass spectrometry (GC-MS) with an Agilent 6890 Gas Chromatograph (DB-5 column 30 m × 0.25 mm ID × 0.25 μm film thickness) coupled to an Agilent 5975 mass spectrometer,as described by Zhaoet al.(2014).The column temperature kept 50℃ for 2 min and then increased to 300℃ at a rate of 6℃ min-1,held for 16 min.The carrier gas was high-purity Helium at a flow rate of 1.0 mL min-1.The injection volume was 1μL with a pressure of 66.9 kPa and an inlet temperature of 290℃.Mass spectrometry conditions:the interface,ion source,and quadrupole temperature was 280,230,and 150℃,respectively.The ionization energy was 70 eV,solvent delay was 5 min,box temperature equilibrium time was 0.5 min,and the total running time was 59 min.
2.7 Analytical Methods
Ammonia nitrogen (NH4+-N),total nitrogen (TN) and total phosphorus (TP) were measured according to the standard method standard protocol of State Environmental Protection Administration of China (SEPA,2002).The detection of total petroleum hydrocarbon was conducted by ultraviolet (UV) spectrophotometry (Qinet al.,2014) using a UV spectrophotometer (TU-1810,Beijing).The removal efficiency of total petroleum hydrocarbon was calculated as follows:

whereηis the removal efficiency,C0is the initial concentration of total petroleum hydrocarbon andCdis the concentration of total petroleum hydrocarbon after 15 days experiment.
The removal efficiency of n-alkanes was calculated as follows:
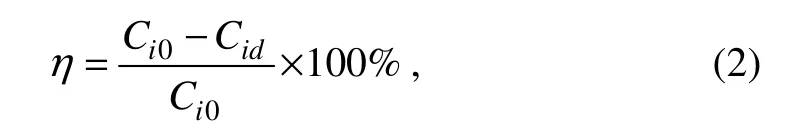
whereηis the removal efficiency,Ci0is the initial concentration of n-alkanes andCidis the concentration of n-alkanes after 15 d experiment.
3 Results and Discussion
3.1 Degradation of Crude-Oil by Microbiota
Crude-oil degrading microbiota capable was enriched and its total petroleum hydrocarbons removal performance was confirmed.Fig.1 showed that the suspended crude-oil degrading microbiota (inoculum size from 1%to 10%) was capable to remove 500 mg L-1total petroleum hydrocarbon in 15 d at an average ratio of 19.42-31.45 mg (L d)-1.Inoculum size affected the degradation of total petroleum hydrocarbon.The total petroleum hydrocarbons degradation efficiency was improved from 58.26% to 94.35% with the increasing inoculum size from 1% to 10% (V/V).This performance was similar to the results of Sujaet al.(2014) who found that the enriched microbiota could degrade petroleum hydrocarbons at a removal efficiency of 84.61%–92.04% in the microcosm tank experiment.In this study,it demonstrated that the double inoculum size did not result in double degradation efficiency.The reason leading to this result may be ascribed to the limit of nutrient elements.Shetaiaet al.(2016) isolated two halotolerant microbial strains,i.e.,RS-Y1 and RS-F3 from polluted Red Sea area,68.3% and 58.15% total petroleum hydrocarbons degradation efficiency was obtained with inoculum size 5% (V/V).Liuet al.(2014) isolated aBacillus licheniformisstrain Y-1,which could achieve 60.2% crude-oil degradation rate in only 5 d.

Fig.1 Degradation of crude-oil by the enrichment.
n-alkanes are important constituent of petroleum hydrocarbons.The gas chromatograms for residual N-alkanes (C10–C26) are presented in Fig.2 and the changes of concentrations are shown in Fig.3A.From Fig.2,almost all n-alkanes in the crude oil were degraded at the group of 10% inoculum size.The crude-oil degrading microbiota could achieve 50%–70% n-alkanes removal efficiencies even as small as 1% inoculum size.Varjani and Upasani (2016) isolatedPseudomonas aeruginosaNCIM 5514,61.03% C8–C36 hydrocarbons were degraded,respectively.As shown in Fig.3B,medium length n-alkanes (C12–C18) were more easily degraded than short chains (C10–C11) or long chains (C19–C25),which is consistent well with the research of Hassanshahianet al.(2014).They concluded that long-chain n-alkanes inhibited the degradation of crude-oil due to their low solubility and high toxic for bacteria was the characteristic of short-chain n-alkanes as they dissolve cellular membrane.

Fig.2 Gas chromatograms of n-alkanes extracted from the liquid medium.Gray line,blank;Red line,after 15 d.
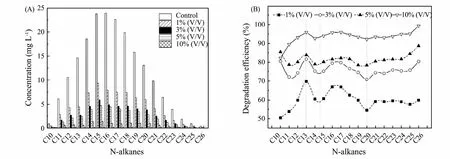
Fig.3 The effect of inoculum size on n-alkanes degradation efficiency.
However,the higher inoculum sizes presented different degrading characteristics.When inoculum sizes were 3%and 5%,C10 revealed the similar degradation efficiency with medium length (C12–C18) n-alkanes,but the C11 degradation was lower than others.For the 10% inoculum size group,short chains (C10–C11) n-alkanes presented less degradation efficiency than medium length (C12–C18) and long chains (C19–C25).
3.2 Composition and Dynamic Changes of Crude-Oil Degrading Microbiota
3.2.1 Composition of crude-oil degradingmicrobiota
Crude-oil degrading microbiota was a composite microbial community and it is of great significance to analyze its composition when revealing oil degradation mechanism.The main genus (relative abundance more than 0.5%) in crude-oil degrading bacteria was shown in Fig.5D.And some research on crude-oil degrading microbiota was presented in Table 2.There are seven genera dominating the community,i.e.,Acinetobacter(47.59%),Actinophytocola(9.68%),Aquabacterium(4.09%),Dysgonomonas(3.49%),Frigidibacter(1.28%),Sphingobium(0.86%),Serpens(0.80%),andPseudomonas(0.50%)and they could be found in others’ research.The relative abundance ofAcinetobacterapproached to about half of total microbial communities.Jeonget al.(2015) reported that some members ofAcinetobacterin oil-contaminated soil were able to grow by using diesel,kerosene and gasoline as energy and carbon sources.Lalet al.(1996)also showed that a major degradation of C17–C24 was conducted byAcinetobacter.Besides,Aquabacterium,who can degrade a wide range (C9–C18,C10–C34,C12–C30,C13–C44) n-alkanes,is another important crude-oil degrading population (Yusteet al.,2000;Komaet al.,2001).The high abundant existence ofAquabacterium(4.09%) might explain why long-chain n-alkanes were effective removed in this study.

Table 2 Related researches on dominant genus in containing crude-oil degrading bacteria communities environment samples
3.2.2 Dynamic changes of microbial community structure
Dynamic changes of microbial community structure of sediment/soil microbial sample and crude-oil degrading microbiota were revealed by high throughput sequencing technology.At two groups,65373 and 31971 high-quality sequences were obtained and grouped into 1836 and 2208 OTUs,respectively.Significant change in diversity was found during the process of enrichment according to the Shannon index (Fig.4).Sediment/soil microbial sample contained higher Shannon index (9.87).Comparatively,less diverse bacterial populations and low Shannon index(4.31) was found in crude-oil degrading microbiota.The change of Shannon index indicated the microbial diversity of soil obviously reduced by treatment of high concentration crude oil.Zhaoet al.(2016) got the same conclusion where the Shannon index of the sediment (Shannon index=9.4) was higher than sulfide removal microbiota (Shannon index=6.0).
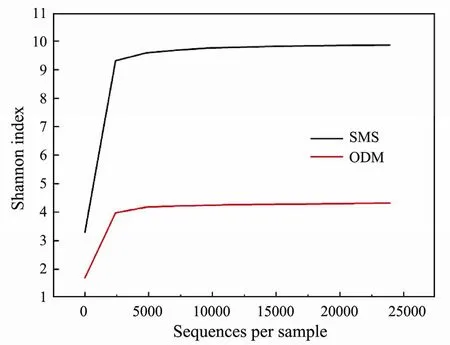
Fig.4 Shannon index based the bacterial community 16S rDNA sequences.SMS was from sediment/soil microbial sample bacteria and ODM was from oil degrading microbial sample.
Microbial community composition and abundance in different levels (class,order,family and genus) are shown in Fig.5.At the class level (Fig.5A),sediment/soil microbial sample was dominated by Gammaproteobacteria,Alphaproteobacteria,Deltaproteobacteria,andBetaproteobacteria.In crude-oil degrading microbiota,the largest abundant class wasGammaproteobacteria with approximately 69.62% of all sequences.The relative abundance of Alphaproteobacteria,Betaproteobacteria,Bacteroidia,and Deltaproteobacteriawere 7.87%,7.13%,3.83%,and 2.38%.Gammaproteobacteria is an important class in marine microbels and is widely found in the oil polluted seawater or sediments (Curtiset al.,2018).Grayet al.(2011) inoculated estuarine sediment into oil-polluted sea water and foundMarinobacterin class Gammaproteobacteria was largely enriched.
At order level (Fig.5B),the first five largest abundant populations were Xanthomonadales (7.31%),Rhizobiales(5.40%),Rhodospirillales (4.43%),Myxococcales (3.83%)and Rhodobacterales (3.55%) in sediment/soil sample.The dominant bacterial populations are similar to class level.However,a significant change occurred in crude-oil degradation microbiota at order level.The relative abundance of Pseudomonadales increased from 1.11% in soil/sediment sample to 65.88% in crude-oil degradation microbiota.There was also a slight increase of Burkholderiales from 2.25% to 5.68%.On the contrary,Rhodobacterales (2.88%),Xanthomonadales (2.00%),and Sphingomonadales(1.81%) reduced by different degrees,which might be ascribed to high crude oil in the enriching medium.Pseudomonadales are common enrichments in oil pollution environment and they are able to degrade both short-chain and long-chain n-alkanes (Kostkaet al.,2011;Neethuet al.,2019).

Fig.5 Composition and relative abundance of microbial communities at different levels.A,class level;B,order level;C,family order;D,genus level.SMS,sediment/soil microbial sample;ODM,oil degrading microbiota.
Similarly,the difference at family level was shown in Fig.5C.The top five most predominant bacterial groups belonged to Xanthomonadaceae (4.84%),Rhodobacteraceae (3.09%),Rhodospirillaceae (3.07%),Hyphomicrobiaceae (2.47%),and Sphingomonadaceae (2.28%) in sediment/soil sample.Moraxellaceae accounted for 53.68% of total sequences in crude-oil degrading microbiota with 25.78 times as large as sediment/soil sample.Pseudomonadaceae (12.20%),Comamonadaceae (5.12%),and Porphyromonadaceae (3.78%) accounted for a relative large percentages in crude-oil degrading microbial.
Microbial community composition at genus level was investigated (Fig.5D),and the dominant groups (relative abundance more than 0.5%) were shown in Table 3.Lysobacter(1.69%) andAcinetobacter(47.59%) were the largest dominant genera in sediment/soil sample and crude-oil degradation bacteria sample.Enrichment process changed microbial community composition and relative abundance.Especially,the proportion of OUT-1 to OUT-5 significantly increased in crude-oil degradation bacteria sample compared with sediment/soil sample.In order to further compare the microbial communities of sediment/soil microbial sample and oil degrading microbial sample,the Mega software was used to conduct all-against-all comparison.The dominant genus evolutionary tree is shown in Fig.6.In this figure,the five gray ball sizes (descending order) represent relative abundance of corresponding genus >2,1–2,0.5–1,0.1–0.5,and <0.1.It could clearly see that microbial abundance changed in the process of crude-oil degradation bacteria enrichme.
Table 3 Changes in abundance of the bacterial genera with the enrichment
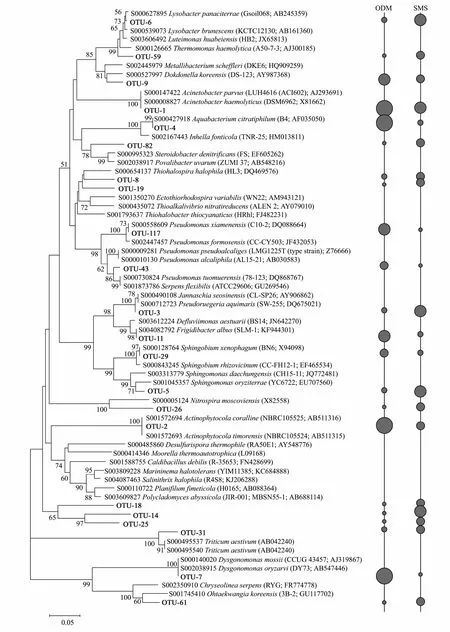
Fig.6 Sequences from sediment/soil microbial sample and crude-oil degrading microbial sample assigned into NCBI taxonomies with Blast and Mega software.Gray ball indicate the relative amount for each genus.The gray ball size in descending order represent relative abundance of corresponding genus >2,1–2,0.5–1,0.1–0.5,and <0.1.SMS was from sediment/soil microbial sample bacteria and ODM was from oil degrading microbial sample.
3.3 Immobilization of the Crude-Oil Degrading Microbiota
The crude-oil degradation microbiota was immobilized into SA and SA-PVA beads with a diameter of 3–5 mm.Crude oil degradation efficiency was affected by immobilized methods (Fig.7).The free degradation microbiota and immobilized method with PVA+SA could remove 500 mg L-1crude oil effectively and obtained good removal efficiency (93.69% and 96.53%) after 15 d degradation.However,88.93% removal efficiency was obtained by SA immobilized microbiota.
From Fig.7A,the mobilized crude-oil degrading microbiota presented better degradation efficiency than free degradation microbiota in the first 9 d,while the opposite effect is obtained from 9 to 15 d.Much crude oil was adsorbed onto the surface at the first period and an obvious advantage of immobilization was shown up compared with free degradation microbiota.At the later period of the degradation experiment,the free microbiota degradation efficiency exceeded adsorption and resulted in improving degradation efficiency.Due to the weathering of crude oil,the natural attenuation was observed in control groups.However,the removal of crude oil by immobilizing crude-oil degrading bacteria (SA-control and PVA+SA-control groups) was more than non-immobilized(control group) due to the adsorption occurred on beads(Taoet al.,2009;Zhaoet al.,2016).The difference of degradation efficiencies were not obvious (Fig.7B) and 28.78 mg (L d)-1,26.92 mg (L d)-1,and 30.35 mg (L d)-1crude oil were removed by the free degradation microbiota,SA,and PVA+SA immobilized microbiota,respectively.Nieet al.(2019) immobilized cells ofP.aeruginosaNY3,and the residual of oil in the effluent kept at 10–20 mg L-1with the removal efficiency reaching above 80%.
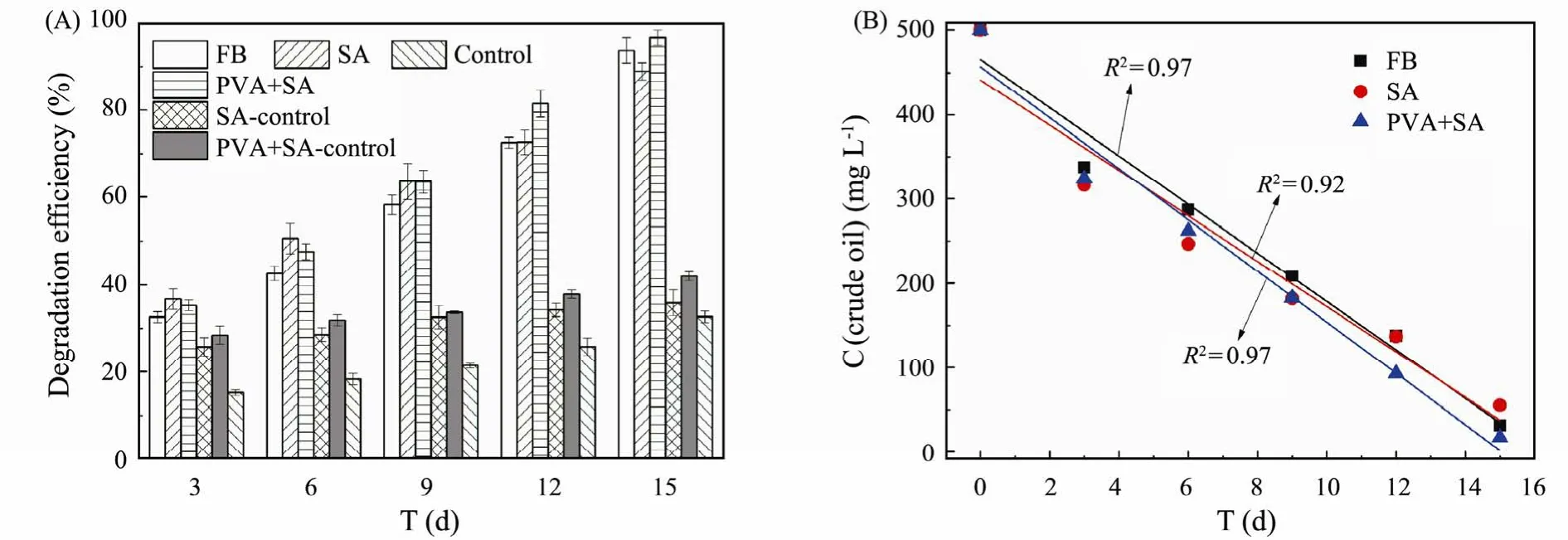
Fig.7 The effect of immobilized methods on degradation efficiency.FB,free bacteria experiment group;SA,immobilizing crude-oil degradation bacteria by SA experiment group;PVA+SA,immobilizing crude-oil degradation bacteria by SA+PVA experiment group;Control,SA-control,and PVA+SA-control,the control experiment groups without crude-oil degradation bacteria.
3.4 Effect of Salinity and Temperature on Crude-Oil Degradation Efficiency
3.4.1 Effect of salinity on crude-oildegradation efficiency
LEW is a typical estuarine wetland ecosystem where high salinity and low temperature are the major limiting factors for microbial activity.This study shows that salinity had a significant effect on free degradation microbiota and immobilized microbiota (Fig.8A).The crude oil removal efficiency reduced with the increase of salinity,which suggested that higher salinity obviously inhibited the microbial activity.The removal efficiency decreased from 90% to 31.85%,54.84%,and 58.13% when salinity approached to 35.Compared with the immobilized microbiota,the free degradation microbiota was impacted stronger and they almost lost the activity.Thus,it can be seen that under high salinity condition,the immobilized microbiota were effective for crude oil degradation.
The resistance of immobilized microorganisms to salt has been studied.Liuet al.(2020) found that the partial nitrification and anammox sludge was immobilized by polyethylene glycol (PEG)-modified PVA-SA immobilization technology achieved nitrite accumulation rate(NAR) reaching 81.03% at a concentration of 10 g L-1NaCl.Gaoet al.(2019) concluded that salinity tolerance could be improved by immobilized microbial granules and 92% NH4+-N removal efficiency was achieved at salinity up to 35.0 g L-1.For Free bacteria,high salt could destroy the stability of metabolic system and the osmotic pressure balance of cell membrane,even destroy the cell membrane structure and block the material transport (Heet al.,2017;Vyrides and Stuckey,2017).Meanwhile,Na+could also snatch the protein binding sites and reduced the enzyme activity of functional flora (Vyrides and Stuckey,2017).Immobilized microorganisms could keep enough quantities of microbiology and improved tolerance to salinity,and the close floc structure was formed among microorganisms to resist the impact of Na+.
3.4.2 Effect of temperature on crude-oil degradation efficiency
The effect of temperature on immobilized crude-oil degrading microbiota is shown in Fig.8B.Compared with salinity,the effect of temperature on crude-oil degrading microbiota was not significant.The crude oil removal efficiency of free degradation microbiota and immobilized microbiota increased from 15 to 25℃ and then decreased with increasing temperature.The optimum temperature was 25℃,under which,the removal efficiency was 94.23%,92.53%,and 86.75% by free degradation microbiota,SA and PVA+SA immobilized microbiota,respectively.To our surprised,temperature affected little on free degradation microbiota.It might be because that the crude-oil degrading microbiota was enriched from soil/sediment of LEW and the microbes were genetically cold-tolerant (Huanget al.,2017a).

Fig.8 Effect of salinity (A) and temperature (B) on crude-oil degradation efficiency by immobilizing crude-oil degrading microbiota.FB,free bacteria experiment group;SA,immobilizing crude-oil degradation bacteria by SA experiment group;PVA+SA,immobilizing crude-oil degradation bacteria by SA+PVA experiment group.
4 Conclusions
Crude-oil degrading microbiota was enriched from LEW and an ideal removal effective for the n-alkanes was achieved when the optimal inoculum size was 10%,and almost all n-alkanes could be removed effectively.For the complex microbial flora,the main genuses wereAcinetobacter,Actinophytocola,Aquabacterium,Dysgonomonas,Frigidibacter,Sphingobium,SerpensandPseudomonas.SA and PVA+SA immobilization were conducted,and 88.93% and 96.53% removal efficiency was obtained respectively.Meanwhile,the immobilized microbiota showed higher salinity-tolerance,but temperature has a strong influence on its degradation effect.This study deepened our understanding of crude-oil degrading microbiota and provided a new strategy for control of crude oil in high salinity and low temperature wetland.
Acknowledgements
This work was supported by the National Key R&D Program of China (No.2018YFD0900805) and the Startup Foundation for Introducing Talent of Nanjing University of Information Science and Technology.
杂志排行
Journal of Ocean University of China的其它文章
- Numerical Simulation and Analysis of Storm Surges Under Different Extreme Weather Event and Typhoon Experiments in the South Yellow Sea
- Analysis of Wave Distributions Using the WAVEWATCH-III Model in the Arctic Ocean
- Multi-Objective Weather Routing Algorithm for Ships Based on Hybrid Particle Swarm Optimization
- Experimental Study on the Influencing Factors of Motion Responses on an Air-Floating Caisson with Multiple Compartments
- Placement Optimization Method of FPSO Gas Detectors Based on Leakage Risk
- Effect of Temperature on the Acoustic Reflection Characteristics of Seafloor Surface Sediments
