An efficient route towards R-2-phenoxypropionic acid synthesis for biotransformative production of R-2-(4-hydroxyphenoxy)propionic acid
2021-06-26HaiyanZhouYizuoLiRuiJiangXianlinWangYuanshanWangYapingXueYuguoZheng
Haiyan Zhou,Yizuo Li,Rui Jiang,Xianlin Wang,Yuanshan Wang,Yaping Xue,Yuguo Zheng
Key Laboratory of Bioorganic Synthesis of Zhejiang Province,College of Biotechnology and Bioengineering,Zhejiang University of Technology,Hangzhou 310014,China
Engineering Research Center of Bioconversion and Biopurification of Ministry of Education,Zhejiang University of Technology,Hangzhou 310014,China
The National and Local Joint Engineering Research Center for Biomanufacturing of Chiral Chemicals,Zhejiang University of Technology,Hangzhou 310014,China
*Corresponding author at:College of Biotechnology and Bioengineering,Zhejiang University of Technology,18 Chaowang Road,Hangzhou 310014,China.
Keywords:R-2-phenoxypropionic acid R-2-(4-hydroxyphenoxy)propionic acid Biosynthesis S-2-chloropropionic acid
ABSTRACT R-2-(4-hydroxyphenoxy)propionic acid(R-HPPA) is a key intermediate for the synthesis of classic herbicides with high selectivity against grassy weed.The main route for R-HPPA biosynthesis is to hydroxylate the substrate R-2-phenoxypropionic acid (R-PPA) at C-4 position with microbes.In order to provide sufficient R-PPA for the industrial production of R-HPPA,an effective R-PPA synthesis method was established and optimized in this work.The synthesis process mainly consisted of two steps:(1) synthesis of S-2-chloropropionic acid from L-alanine via diazotization and chlorination reactions;and(2)synthesis of R-PPA from S-2-chloropropionic acid and phenol via etherification reaction.The optimal reaction conditions were as follows:HCl:NaNO2:KI:L-Ala=2.0:1.2:0.7:1.0(in molar),125°C reflux for 1.5 h,with KI as catalyst,and KI:S-2-chloropropionic acid:phenol=0.075:1.2:1.0 (in molar).Under these conditions,an improved molar conversion rate (74.9%,calculated in phenol) was achieved.After extraction using anionic exchange resin Amberlite IRA-400(CI),R-PPA product with a purity of 95.08%was obtained.The purified R-PPA was identified and evaluated in the application of the biotransformative production of R-HPPA.The results indicated that the synthesized R-PPA supported the R-HPPA biosynthesis with a comparable yield as that of the standard R-PPA.The R-PPA synthesis method provided herein exhibited the advantages of low price and easy availability of raw materials,less toxicity of reagents,simple manipulations,and low equipment/instrument requirements.
1.Introduction
R-2-(4-hydroxyphenoxy)propionic acid(R-HPPA)is a key intermediate for the synthesis of enantiomers of pure benzene-methyl propionate herbicides,a class of classic herbicides with high selectivity against grassy weed [1].At present,the synthesis of R-HPPA is mainly through chemical methods with the raw materials such as S-chloropropionic acid and S-2-substituted sulfonyloxypropionate [2,3],hydroquinone/alpha-halogenated propionic acid or its ester derivative,4-acetylphenol/methyl α-chloropropionate[4],and ethyl (R)-lactate/thionyl chloride [5].However,there are many disadvantages in chemical reactions,including high-cost raw materials,low yield,strict reaction condition,complicated separation process [6,7],and environmental unfriendliness.Alternatively,the biocatalysis method for synthesizing R-HPPA under the mild reaction conditions has attracted more attentions,due to the high product yield,high stereoselectivity,ease of product separation and so on [8,9].
The main route for R-HPPA biosynthesis is to hydroxylate the substrate R-2-phenoxypropionic acid(R-PPA)at C-4 position using the oxidases in microbes [10–13].Therefore,the sufficient supply of the substrate R-PPA plays an important role in the industrial production of R-HPPA.At present,some studies on the synthesis of R-PPA have been reported.As reported,R-PPA could be efficiently synthesized from the main raw materials 2-chloropropionic acid and phenate (or halogen and/or methyl substituted 2-chloropropionic acid and phenol)in an inert organic solvent with high boiling point under reflux [14,15] or in the water solution under the protection of inert gas [16].However,the high-cost raw materials and high energy consumption limited the industrial application of these methods.
The purpose of this work is to develop a more practical R-PPA synthesis approach for effective biotransformation of R-HPPA.With a low-cost L-alanine (L-Ala) as the starting material,diazotization and chlorination reactions proceeded to form S-2-sodium propionate which was then etherified with phenol.The configuration was reversed during the etherification reaction and the R-2-phenoxypropionate sodium was synthesized.Subsequently,RPPA was obtained through acidification of the R-2-phenoxypropionate sodium with hydrochloric acid.The obtained product was extracted,characterized,and applied for R-HPPA biosynthesis.The method established in this work has the advantages of low price and easy availability of raw materials,simple process,and high purity of the product.
2.Materials and Methods
2.1.Chemicals
L-Ala was provided by TCI (Shanghai) Development Co.,Ltd.(Shanghai,China).Phenol,potassium chloride(KCl),and potassium iodide(KI)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).The standard R-HPPA and acetonitrile(HPLC grade) were purchased from Aladdin Reagents Co.,Ltd.(Shanghai,China).The standard R-PPA was provided by Rainbow Chemical Co.,Ltd.(Weifang,China).Anionic exchange resin Amberlite IRA-400 (CI) was provided by Alfa aesar (China) Chemical Co.,Ltd.(Shanghai,China).All other chemicals were analytical grade and purchased from commercial sources.
2.2.Microorganism,medium,and cultivation methods
Beauveria bassiana C-7,a mutant of B.bassiana ZJB16001 [13]was used for hydroxylating R-PPA to R-HPPA in this study.
The potato dextrose broth (PDB) medium containing 200 g﹒L-1potato and 20 g﹒L-1glucose with natural pH was used for seed preparation.The biotransformation (fermentation) medium was composed of 20 g﹒L-1glucose,5 g﹒L-1yeast extract,5 g﹒L-1(NH4)2-SO4,0.5 g﹒L-1MgSO4﹒7H2O,0.05 g﹒L-1MnSO4﹒H2O,1.5 g﹒L-1KH2-PO4,3.6 g﹒L-1K2HPO4﹒3H2O,1 ml﹒L-1microelement solution [13],and pH 6.8.
For the biotransformative production of R-HPPA from R-PPA by the B.bassiana C-7 cells,the individual colonies grown on a potato dextrose agar(PDA)plate were inoculated into 250 ml Erlenmeyer flasks containing 30 ml PDB medium and incubated at 28°C,200 r﹒min-1for 3 d on a shaking incubator (HZQ-X300C,Bluepard Instruments Co.,Ltd.,Shanghai,China).1.0 ml seed broth of B.bassiana C-7 was inoculated into 30 ml fermentation medium prepared in advance with the supplementation of 20 g﹒L-1R-PPA.The biosynthesis of R-HPPA was conducted at 28°C for 7–9 d under static state (without shaking) on the incubator.
2.3.The synthesis route of R-PPA
The overall route towards R-PPA synthesis consisted of two main processes (Fig.1),the synthesis of intermediate product S-2-chloropropionic acid and the formation of R-PPA via etherification of S-2-chloropropionic acid with phenol.
2.3.1.Synthesis of S-2-chloropropionic acid from L-Ala via diazotization and chlorination reactions
The synthesis of S-2-chloropropionic acid was achieved through diazotization and chlorination of L-Ala,one of the major starting materials.The initial process is described as follows:1.0 mol LAla (89.1 g) and 312.5 ml HCl solution (8.0 mol﹒L-1) were added into a 1-L three-necked flask.And then 0.7 mol KCl (52.185 g)was added to the reaction solution with stirring of 500 r﹒min-1.When the solution was naturally cooled down to 0°C,200 ml NaNO2solution(6.0 mol﹒L-1)was added dropwise into the reaction mixture at an average rate of 20–30 drops﹒min-1;during the process,the solution gradually turned into yellow and yellow-brown gas was generated.After the addition was finished,stirring was continued for 1 h at 0°C in order to ensure a complete reaction.The reaction solution was naturally warmed up to room temperature by removing the circulatory cooling system.Then 0.5 mol NaCO3(53.0 g) was added in batches and stirring was allowed for another 1.0 h.CO2gas formed in the reaction between NaCO3and excess acid existing in the reaction system would destroy the binding of nitrogen oxides and the resultant of reaction,leading to an easy discharge of the nitrogen oxides from the reaction system.After the reaction,the mixture is extracted for 4 times with 100 ml ethyl acetate in each run.The organic layer was collected together and condensed by rotary evaporator;after most of the ethyl acetate was removed,a condensed liquid with pale green color was obtained.The conversion of S-2-chloropropionic acid was determined by gas chromatography (GC).
2.3.2.Synthesis of R-PPA from S-2-chloropropionic acid and phenol via etherification reaction
R-PPA was synthesized by etherification of S-2-chloropropionic acid and phenol.1.0 mol phenol(73.4 g)and 120 ml NaOH solution(7.5 mol﹒L-1) were added into a 1-L reactor.The solution was stirred for 0.5 h at 300 r﹒min-1and room temperature;and then S-2-chloropropionic acid solution prepared in the procedure described above,90 ml NaOH solution (7.5 mol﹒L-1),and 9.71 g KI(0.059 mol) were added in sequence.The solution was heated at 125°C to reflux with stirring for 1.5 h.After that,it was acidified to pH 4.0 with 6.0 mol﹒L-1HCl.Some yellow oily substance was precipitated and was subsequently extracted with 100 ml ethyl acetate for 4 times.All the organic layers were combined together and rotary evaporated.Finally,a yellow viscous liquid was yielded.The resultant liquid was passed through an ion exchange chromatography column to purify the product R-PPA.After purification and dryness,white powder of R-PPA was obtained.
2.4.Separation and purification of R-PPA
R-PPA is a weak acid with pKavalue of 3.22±0.20[17].In order to obtain high purity of R-PPA,the yellow viscous solution was diluted and adjusted to pH 8.0–9.0 with NaOH,and then the reactants were separated and purified on a 15 mm×200 mm glass column (Bio-Rad,USA) wet-packed with anionic exchange resin Amberlite IRA-400(CI).The uploading flow rate was 1.0 ml﹒min-1.R-PPA adsorbed on the resins was eluted by 4.0 mol﹒L-1HCl solution.The effluent in the 40–100 ml interval was collected and the purity was determined by HPLC assay.
2.5.Analysis methods

Fig.1.R-PPA synthesis route.
S-2-chloropropionic acid was detected by GC equipped with a capillary column (30 m×0.25 mm) and FID detector.Nitrogen flow rate was 30.0 ml﹒min-1;hydrogen flow rate was 40.0 ml﹒min-1;oxygen flow rate was 400.0 ml﹒min-1;column temperature was 100°C;vaporization chamber temperature was 220°C;detector temperature was 250°C;injection volume was 5 μl;programmed temperature was 100°C for 4 min,and then raised to 200°C at the rate of 20°C﹒min-1[18].R-PPA and RHPPA were assayed with HPLC equipped with C18 column(250 mm×4.6 mm,5 μm) and DAD detector;mobile phase was phosphoric acid solution (pH 2.0) and acetonitrile (3:2,v/v) at a flow rate of 1.0 ml﹒min-1.Detection wavelength was 220 nm.Column temperature was 30°C.The biomass was gravimetrically expressed as dry cell weight (DCW).
The synthesized product was confirmed by Proton Nuclear Magnetic Resonance(1H NMR),Carbon-13 Nuclear Magnetic Resonance (13C NMR),and Fourier Transform Infrared Spectroscopy(FTIR).NMR experiments were performed on a Bruker AVANCE III 500 MHz NMR spectrometer(Faellanden,Switzerland).FTIR analysis was conducted on a Thermo Scientific NicoletTM6700 spectrometer (Waltham,MA,USA).The optical rotations of optically active compounds were measured by the Automatic Polarimeter Autopol IV-T (Rudolph,Germany) at 589 nm and 20°C.Prior to measurement,the chiral compound was dissolved in methanol to prepare sample solution at the concentration of 0.01 g﹒ml-1.The specific optical rotation was the angle of rotation calculated with reference to a 1-dm thick layer of the sample solution and divided by the solution concentration (g﹒ml-1).
3.Results and Discussion
3.1.The mechanism analysis of chemical reaction for R-PPA synthesis
The detailed reaction mechanism was shown in Fig.2.L-Ala is diazotized with sodium nitrite to form 2-azidopropionic acid in an acidic environment.2-Azidopropionic acid is attacked by chloride ions in solution,and a substitution reaction occurs to form S-2-chloropropionic acid.The resulting S-2-chloropropionic acid is subjected to Williamson reaction with sodium phenolate in an alkaline environment to generate R-PPA.
Nitrous acid undergoes a reversible reaction(2)to form nitrosyl cations.As diazotization reagents,nitrosyl cations and protonated nitrous acid combine with a lone pair of electrons of the ammonia N atom in L-Ala to form N-nitroso-active intermediate;this intermediate is then subjected to a series of proton transfer reactions((4),(5),(6),and (7)) to form 2-azidopropionic acid.And 2-azidopropionic acid is then subjected to a single-molecule nucleophilic substitution (SN1) at α-C by Cl-in the reaction system(8),eventually forming S-2-chloropropionic acid.α-C of S-2-chloropropionic acid is positively charged,which can be attacked by phenoxy anion for bimolecular nucleophilic substitution(SN2).Due to the large steric hindrance of benzene ring,the nucleophilic attack proceeds from the backside of Cl-.After the phenoxy anion is combined with α-C of S-2-chloropropionic acid,Cl-is removed to complete the substitution reaction (10),during which configuration turning occurs to form R-PPA [19–21].
3.2.Optimization of reaction conditions for S-2-chloropropionic acid synthesis
The supplement amounts of raw materials during the diazotization reaction performed at low temperature were directly correlated with the molar conversion of the target product.In order to obtain high conversion of S-2-chloropropionic acid,the diazotization reaction conditions mainly including the usage amounts of HCl,NaNO2,and KCl were optimized,respectively.The amount of L-Ala (1.0 mol) was used as a fixed reference.
The effect of different amounts of HCl in the range of 1.0–3.0 mol﹒(mol L-Ala)-1on the S-2-chloropropionic acid synthesis was examined.As shown in Fig.3(a),increasing the amount of HCl could increase the molar conversion of S-2-chloropropionic acid.When 2.0–2.5 mol HCl﹒(mol L-Ala)-1was used,the conversion of S-2-chloropropionic acid reached the highest value (60.5%–63.5%).When the amount of HCl was further increased to 3.0 mol﹒(mol L-Ala)-1,the synthesis of S-2-chloropropionic acid was reduced compared with that at 2.0 or 2.5 mol﹒(mol L-Ala)-1.The possible reason was that excessive HCl converted L-Ala into ammonium salt,which was further hydrolyzed to form free amine.Furthermore,nitrous acid was liable to dissociate in an acidic environment,adverse to the diazotization reaction.Therefore,the optimum amount of HCl was 2.0 mol﹒mol-1.
As shown in Fig.3(b),the usage amount of NaNO2had significant effects on S-2-chloropropionic acid production.Increasing the NaNO2amount from 1.0 to 1.2 (mol﹒(mol L-Ala)-1was beneficial to the synthesis of S-2-chloropropionic acid.The highest molar conversion was achieved at 1.2 mol﹒mol-1L-Ala) of NaNO2.While more NaNO2was unfavorable to the diazotization reaction,might because nitration,oxidation,or other reaction of the coupling component was caused [22].Therefore,1.2 mol NaNO2﹒(mol L-Ala)-1was used in the following experiments.
The effect of different amounts of KCl(0.5–0.9 mol﹒mol-1)on S-2-chloropropionic acid synthesis was also examined.As shown in Fig.3(c),increasing the amount of KCl from 0.5 to 0.8 mol﹒(mol L-Ala)-1was beneficial to S-2-chloropropionic acid synthesis.When KCl was 0.7–0.8 mol﹒(mol L-Ala)-1,the highest molar conversion of S-2-chloropropionic acid was obtained.While,higher amount of KCl (0.9 mol﹒mol-1) was not conducive to S-2-chloropropionic acid formation,possibly because too high concentration of KCl in the reaction system lowered the solubility of other reagents,resulting in an adverse impact on diazotization reaction.Thus,the amount of KCl was suggested as 0.7 mol﹒(mol L-Ala)-1.
3.3.Optimization of reaction conditions for enhanced R-PPA synthesis
3.3.1.Effect of the reaction temperature on R-PPA molar conversion rate
The effect of different temperatures(25,50,75,100,and 125°C)on the synthesis of R-PPA was investigated and the results were shown in Fig.4(a).The conversion rate of R-PPA was increased as temperature rose.When temperature was 125°C,the concentration of R-PPA arrived the highest level,because the reflux enhanced the activation of the molecules and mixing of the reactants in the reaction system.There was possibility that the conversion rate would be further increased when the temperature was further raised.In fact,when the temperature was higher than 125°C,the reaction system reached a boiling state and the reflux was accelerated.As a result,the real temperature of the reaction system could not be further raised in practice.Taking into account the equipment cost and manipulation feasibility,a higher reaction temperature (>125°C) was not investigated in this work.At 125°C reflux for 1.5 h,the R-PPA molar conversion rate reached 64.72% (calculated in phenol).After 1.5 h,the conversion rate of R-PPA decreased slightly.The optimum temperature was thus selected at 125°C,and the reflux time was 1.5 h.
3.3.2.Effect of the catalyst type and KI dosage on R-PPA molar conversion rate
In organic reaction,the catalyst plays a great role in promoting the reaction process.It was reported that KI and ethylene glycol had positive effects on the etherification of phenol [23,24].These two catalysts were selected and tested herein both at an addition dosage of 0.075 mol﹒mol-1phenol.The reaction without catalyst was used as control.
As shown in Fig.4(b),both KI and ethylene glycol proved to facilitate the etherification reaction.At 125°C reflux,when ethylene glycol was used as catalyst,the molar conversion rate of RPPA increased from 51.95%to 61.72%(calculated in phenol),while when KI was used,the molar conversion rate arrived to 68.41%(calculated in phenol),31.68% higher than the control.Therefore,KI was chosen as the catalyst for the etherification reaction.

Fig.2.The process and mechanism of chemical synthesis of R-PPA.
Subsequently,the conversion rate of R-PPA with different dosage of KI(0.025,0.05,0.06,0.075,and 0.10 mol﹒(mol phenol)-1)was examined.As shown in Fig.4(c),a dosage of 0.075–0.10 mol﹒(mol phenol)-1was most effective in promotion of R-PPA synthesis,indicating that 0.075 mol﹒(mol phenol)-1of KI is sufficient.Therefore,the optimum amount of KI is suggested as 0.075 (mol﹒(mol phenol)-1).
3.3.3.Optimization of the molar ratio of S-2-chloropropionic acid to phenol
In the process of etherification reaction,phenol is easy to be oxidized and generate by-products at alkaline condition,which will influence the conversion rate and increase the difficulty of separation and purification.Herein,the molar ratio of S-2-chloropropionic acid to phenol was optimized for a high conversion rate of R-PPA.As can be seen from Fig.4(d),increasing the amount of S-2-chloropropionic acid from 0.9 to 1.2 mol﹒mol-1phenol contributed to the increase of the conversion rate of R-PPA.When the ratio was 1.2:1.0 (mol﹒mol-1) and the reaction time was 1.5 h,the R-PPA conversion rate reached the maximum value 74.9% (calculated in phenol).However,it was decreased when the ratio was 1.3 (mol﹒mol-1),possibly because excessive S-2-chloropropionic acid was prone to be hydrolyzed under alkaline condition to generate lactic acid,reducing the pH of the reaction system which was not conducive to the substitution reaction of phenol.Accordingly,the optimum molar ratio of S-2-chloropropionic acid to phenol was 1.2:1.0 (mol﹒mol-1).
3.4.Identification of the synthesized product
3.4.1.1H NMR spectrum analysis of the synthesized product

Fig.3.Effect of different amounts of HCl (a),NaNO2 (b),and KCl (c) on the conversion rates of S-2-chloropropionic acid.

Fig.4.Effect of reaction temperature (a),catalyst type (b),amount of KI (c),and the ratio of S-2-chloropropionic acid to phenol (d) on the synthesis of R-PPA.SPA:S-2-chloropropionic acid.
After purification by the anionic exchange resin Amberlite IRA-400 (CI),white powder of synthesized R-PPA was obtained with a purity of 95.08% determined by HPLC.The1H NMR spectrum of the synthesized product was shown in Fig.5(b).There were 6 kinds of protons in the synthesized compound,with a proton ratio of 3:1:2:1:2:1 and a total proton number of 10.At chemical shift of δ=1–5,an obvious CH3-CH-peak was observed.Methyl peak in CH3-CH-was at δ=1.5,containing three protons and one methyl,splitted into two peaks by the adjacent tert-base.Tert-base peak in CH3-CH-was at δ=4.8,containing one proton and one tert-base;might due to a connection with oxygen atom,formant was formed in the lower field and was divided into four peaks by the adjacent methyl.At δ=6–9,an obvious benzene ring peak was observed;and there were 3 kinds of protons with a total number of 5,indicating a substitution on the benzene ring.At δ=6.8,there were two peaks,indicating that the carbon on the benzene ring only had one proton,and the number of protons was 2,that was,the orthoproton existed on the benzene ring.At δ=6.9,there were three peaks,indicating two identical protons on the carbon connected to it,and the number of protons was 1,that was,para-proton existed on the benzene ring.At δ=7.2 there were four peaks,indicating that there were two different protons on the carbon connected to it,and the number of protons was 2,that was,the metaproton was present on the benzene ring.At δ=13 there was an obvious —COOH peak and was a group of multiplets,indicating that there were no protons on the carboxyl carbon.In summary,the compound consisted of methyl protons,tert-base protons,carboxyl protons,and benzene ring protons,consistent with the1H NMR spectrum of the standard R-PPA (Fig.5(a)).

Fig.5.1H NMR spectrum of the standard R-PPA (a) and the synthesized product (b).
3.4.2.13C NMR spectrum analysis of the synthesized product
The13C NMR spectrum of the synthesized product was shown in Fig.6(b).There was a total of 8 sets of peaks in the synthesized compound,of which the peaks at chemical shift of δ=39–41 were the characteristic peaks of carbon in the solvent deuterondimethyl sulfoxide,and the other 7 groups of peaks indicated the presence of the 7 carbon groups at different environments in the compound.The peaks at δ=173.149 and 157.477 represented the carboxyl carbon and the substituted carbon on the benzene ring,respectively.The peaks at δ=129.515,120.946,and 114.749 represented the benzene ring that were not replaced by carbon.The total13C spectral line of the benzene ring was 4,less than the C numbers in the benzene ring formula,which indicated that there were two groups of carbon-containing groups with the same environment on the benzene ring,that is,the benzene ring had a single replacement.The peaks at δ=71.416 and 18.340 represented the carbon in C—O and methyl group,respectively.In summary,the compound had a total of 9 carbon atoms,including methyl carbon,tertiary carbon,carboxyl carbon,and benzene ring carbon,which was consistent with13C NMR spectrum of the standard R-PPA(Fig.6(a)).

Fig.6.13C NMR spectrum of the standard R-PPA (a) and synthesized product (b).
3.4.3.Analysis of synthesized product with Fourier-transform infrared spectroscopy (FTIR)
The FTIR spectrum of the synthesized product was shown in Fig.7(b).The synthesized compound possessed the characteristic absorption peaks of an aromatic ring (3064.1 cm-1for C—H stretching vibration,and 1600–1450 cm-1for C=C skeleton vibration),an aromatic ether structure (1227 and 1043.7 cm-1for Ar—O and R—O stretching absorption,respectively),a carboxyl group (1713 and 1291.4 cm-1were the stretching absorption frequency of C=O and C—O,respectively;and 915 cm-1was the O—H bending vibration absorption frequency),a methyl(2939.6 cm-1for saturated C—H stretching vibration),and a single replacement of H atom on the benzene ring (peak at 691 cm-1suggested only one H atom was replaced).These were consistent with those of standard R-PPA (Fig.7(a)).Furthermore,the specific rotation of the product(=+38.22(C=1,CH3OH)and standard R-PPA (=+37.68) indicated that the synthesized product was in R configuration.In combination with the analysis results of the1H NMR and13C NMR spectra,the compound was identified as R-PPA.
According to our best knowledge,there are few reports about the R-PPA synthesis with L-alanine and phenol as the starting materials.As shown in Table 1,in previous literatures,R-PPA or its derivatives were mainly synthesized from the raw materials 2-chloropropionic acid and phenate (or halogen and/or methyl substituted 2-chloropropionic acid and phenol) [14–16].The market price of L-alanine and S-2-chloropropionic were 2800 USD per ton and 21,900 USD per ton,respectively.Obviously,this route utilizing L-alanine as the starting material developed herein was lower-cost.Furthermore,the total reaction time (2.5 h) of this route developed in this study,including 1.0 h for the first step(synthesis of S-2-chloropropionic acid from L-Ala via diazotization and chlorination reactions) and 1.5 h for the second step (synthesis of R-PPA from S-2-chloropropionic acid and phenol via etherification reaction),was shorter than that of other methods.Thus,this route was relatively more economical and efficient.
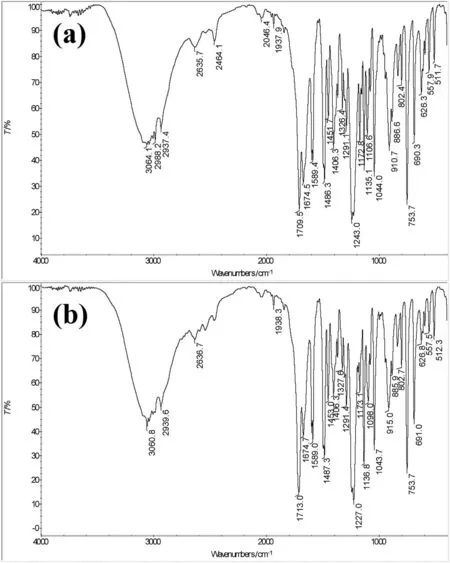
Fig.7.FTIR spectrum of the standard R-PPA (a) and the synthesized product (b).
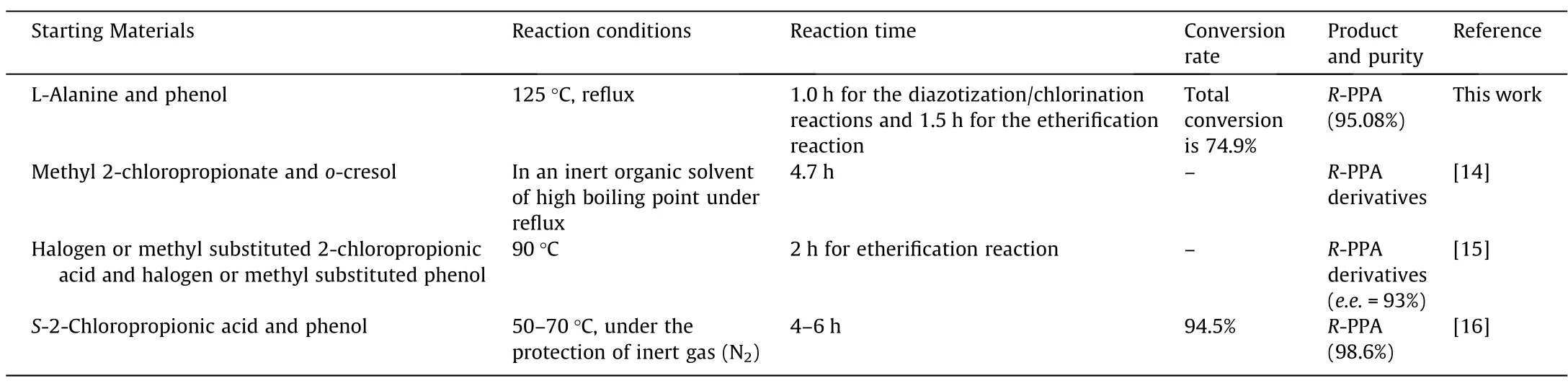
Table 1 The comparison of different synthesis methods of R-PPA and its derivatives
3.5.Application of the synthesized product in the R-HPPA biosynthesis

Fig.8.Application of the synthesized R-PPA in the R-HPPA biosynthesis reaction.
The synthesized R-PPA was utilized as substrate for biotransformative production of R-HPPA by the B.bassiana C-7 at 28°C for 9 d under static cultivation.The standard R-PPA was used as control.As shown in Fig.8,during the biocatalytic reaction,the growth of B.bassiana C-7 with two sources of R-PPA showed similar patterns.With the synthesized R-PPA,the substrate consumption rate and RHPPA biosynthesis rate were relatively lower than those of the control in the early-and mid-stage of the biotransformation process;However,there was no significant difference in the final conversion rates (about 38% in both) between these two substrates.It was speculated that trace amount of impurities contained in the synthesized product interfered with the catalytic efficiency of the cells in the early-and mid-stage of the biotransformation(fermentation),and thus reduced the biosynthesis of the R-HPPA.Nevertheless,this did not affect the cell growth of the strain B.bassiana C-7 and the R-PPA conversion in the late-stage of the biotransformation.It was believed that,the purities of the R-PPA could be greatly improved after optimization of the separation and purification conditions and the synthesized product R-PPA could be applied for the efficient biosynthesis of R-HPPA.
The product (R-HPPA) formation by B.bassiana C-7 from the synthesized R-PPA was proved via the structural characteristics analysis based on the1H NMR,13C NMR,and FTIR spectra(Figs.S1–S3),which also revealed that the hydroxylation reaction took place exclusively on the para site (C4’) of the aromatic ring.Furthermore,the specific optical rotation of the hydroxylated product (=+34.41 (C=1,CH3OH)) was consistent with that of the standard R-HPPA (=+34.86).
4.Conclusions
An effective R-PPA synthesis method was established with a low price and easy availability of raw materials and less toxicity of reagents.The synthesis process mainly consisted of two steps.The diazotization reaction between L-Ala and sodium nitrite in acidic environment was first carried out and an unstable 2-azide propionic acid was formed.Then α-C of the 2-azide propionic acid was attacked by the chloride ion and a substitution reaction occurred,during which the azide group was removed and S-2-chloropropionic acid was thus generated.The optimal material molar ratio was 2.0:1.2:0.7:1.0 (HCl:NaNO2:KI:L-Ala).Under the conditions,the molar conversion rate of intermediate S-2-propionic acid reached 75.2%.Then,Williamson etherification reaction between S-2-chloropropionic acid and phenol was conducted in the alkaline environment to synthesize R-PPA.The optimal reaction temperature and time was 125°C reflux for 1.5 h,the catalyst was KI,and the material molar ratio was 0.075:1.2:1.0(KI:S-2-chloropropionic acid:phenol).Under these conditions,an improved molar conversion rate was achieved (74.9%,calculated in phenol).After extraction using resin Amberlite IRA-400 (CI),a high purity (95.08%) of R-PPA product was obtained.The purified R-PPA supported the R-HPPA biosynthesis with a comparable yield as that of the standard R-PPA.The R-PPA synthesis method provided by this work exhibited the advantages of high efficiency,simple manipulations,and low equipment/instrument requirements.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.06.013.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A comprehensive review of the effect of different kinetic promoters on methane hydrate formation
- An experimental study on the choked flow characteristics of CO2 pipelines in various phases
- Hydrothermal and entropy generation specifications of a hybrid ferronanofluid in microchannel heat sink embedded in CPUs
- Experimental and Numerical Study of Gas-Liquid Flow in a Sectionalized External-Loop Airlift Reactor
- Numerical simulation of heavy fuel oil atomization using a pulsed pressure-swirl injector
- Experimental research on steady-state operation characteristics of gas–solid flow in a 15.5 m dual circulating fluidized bed system
