One-step synthesis of Ni@Pd/NH2-Fe3O4 nanoparticles as affordable catalyst for formic acid dehydrogenation
2021-06-26MohammadRezaNabidYasaminBideMahsaJafari
Mohammad Reza Nabid,Yasamin Bide,Mahsa Jafari
Department of Polymer &Material Chemistry,Faculty of Chemistry &Petroleum Science,Shahid Beheshti University,Tehran 1983969411,Iran
Keywords:Core-shell Fe3O4 Catalysis Dehydrogenation Formic acid
ABSTRACT Currently,one of the critical issues in the world is finding an appropriate green alternative to fossil fuels due to the concerns about global warming.As a hydrogen source,formic acid has been given particular attention owing to the attractive features such as high-energy density,no toxicity,high stability at ambient temperature and high hydrogen content.Introducing an affordable and highly efficient catalyst with easy recovery from the reaction mixture for selective dehydrogenation of formic acid is still demanding.In this report,we used a simple one-step process to synthesize Ni@Pd core shell nanoparticles on 3-aminopropyltriethoxysilane modified Fe3O4 nanoparticles.The existence of Ni and Pd results in a synergic effect on the catalytic activity.The —NH2 groups play an important role for obtaining well-dispersed ultrafine particles with high surface area and active sites.In addition,Fe3O4 lead to convenient magnetic recovery of the catalyst from reaction mixture.Our results indicate that the as-prepared catalyst give the superb turnover frequency of 5367.8 h-1 with no additive,which is higher than most of the previously reported catalysts.
1.Introduction
By increasing the consumption of fossil energy,global warming has become a serious dilemma.In order to achieve a good alternative as a green fuel,hydrogen has attracted extensive attention,and many types of research have been done on hydrogen as a clean,renewable,and high-energy density material[1–3].So finding a suitable source of hydrogen is essential.Hydrogen traditionally stored in its pure form as a cryogenic liquid or a pressed gas[4].However,there are barriers to compressing or liquefying hydrogen gaseous.Both of these methods require significant amounts of energy,as well as hydrogen storage is also hazardous in liquid or gas forms [5].A convenient solution is the utilization of chemical storage of hydrogen in the form of liquid organic hydrogen carriers with high storage density[6].Among the various hydrogen carriers,formic acid has been considered by many researchers because of its unique properties including high energy density,no toxicity,high stability at ambient temperature and high hydrogen content(4.4 wt%)relative to other liquid hydrogen carriers [7–10].
Recently,several studies have been carried out on various homogeneous and heterogeneous catalysts for the selective decomposition of formic acid.Heterogeneous catalysts are more popular because of the inherent advantage of reusability [11–14].Noble metals such as Pt,Pd,and Au are commonly used in heterogeneous catalysis.Due to the high price of Au and Pt,Pd is much more economical.Although Pd based catalysts have high activity and stability for dehydrogenation of formic acid [15–17],until now,the activity and selectivity of non-noble metals Pd-based catalysts is negligible even at high temperature,particularly without extra additives [12].Accordingly,the expansion of facile and efficient strategy to synthesize extremely efficient catalysts with high content of non-noble metals is taken into consideration,hence the researchers synthesized bimetallic catalysts.Furthermore using a bimetallic catalyst can lead to lower cost of the catalyst due to the lower amount of noble metal [8].Moreover,the synergetic effect between metals can improve the performance of the catalyst.To increase the efficiency of the catalyst ultra-fine particles (UPs)catalyst can be synthesized with high surface area,numerous active sites and excellent performance.Due to the inherent aggregation of UPs,appropriate support for uniform dispersion of UPs is essential [18–20].Amine modified Fe3O4could be employed as an ideal support because of the magnetic recyclability,easy synthetic route,low cost and high stability.Use of efficient,economical and recyclable catalyst with low toxicity is indispensable according to green chemistry [21–24].In this study,we introduce a facile onestep synthesis of Ni@Pd/NH2-Fe3O4nanoparticles as an effective bimetallic catalyst in dehydrogenation of formic acid,with magnetic separation capability.
2.Experimental
2.1.Materials
Formic acid(FA),(3-Aminopropyl)triethoxysilane(APTES),palladium (II) chloride,sodium chloride,nickel (II) chloride hexahydrate,sodium borohydride,iron (III) chloride hexahydrate,trisodium citrate,sodium acetate and ethylene glycol were prepared from Merck company.
2.2.Instruments and characterization
High-resolution transmission electron microscopy (HR-TEM)and field emission scanning electron microscope (FE-SEM) images were taken with FEI Tecnai F20 system and Zeiss Sigma 500VP system,respectively.X-ray powder diffraction (XRD) data were collected on an XD-3A diffractometer using Cu Kα radiation.X-ray photoelectron spectroscopy (XPS) was carried out in a K-Alpha XPS System.The IR spectra were recorded on a BOMEM MBSeries FT-IR spectrophotometer.Ultrasonic bath (EUROSONICⓇ4D ultrasound cleaner with a frequency of 50 kHz and an output power of 350 W) was used to disperse catalyst in solvent.
2.3.Synthesis of Fe3O4 magnetic nanoparticles
Fe3O4was synthesized by dissolving 3.24 g iron (III) chloride hexahydrate and 0.6 g tri-sodium citrate in 60 ml ethylene glycol.Then,3.6 g Sodium acetate was added and the mixture was stirred vigorously for 30 min at room temperature,and then sealed in a Teflon-lined stainless-steel autoclave.The autoclave was heated at 200 °C for 10 h.The resulting sediment is then washed several times with water and ethanol with an external magnet,and then dried in a vacuum oven for 12 h at room temperature [24].
2.4.Synthesis of Ni0.4@Pd0.6/NH2-Fe3O4
Aqueous solution of Na2PdCl4(0.02 mol﹒L-1) was prepared by dissolving 1.2 mmol NaCl and 0.6 mmol PdCl2into 30.0 ml H2O with magnetic stirring for 3 h.
To prepare Ni0.4@Pd0.6/NH2-Fe3O4,Fe3O4aqueous solution(1.43 mg﹒ml-1,35 ml)was added into 0.2 ml APTES with sonicating at room temperature.Then,NiCl2(0.02 mol﹒L-1,3.0 ml) and Na2-PdCl4were added into the above mixture with magnetic stirring.Aqueous solution of NaBH4(100.0 mmol﹒L-1,1.0 ml) was then added.The resultant black Ni0.4@Pd0.6/NH2-Fe3O4product was separated and washed with water several times.The single metals supported on NH2-Fe3O4(Ni@NH2-Fe3O4and Pd@NH2-Fe3O4)were also prepared to study the possible synergetic effect of bimetallic nanoparticles.To investigate the effect of catalyst loading,different ratio of Ni to Pd including 0.6:0.4,1:1,and 0.4:0.6 on NH2-Fe3O4were synthesized through the same procedure [19].
2.5.Hydrogen generation from formic acid aqueous solution
An aqueous suspension containing the as-prepared catalyst was placed in a two-neck round-bottom flask,which was placed in a water bath with a temperature of 60°C.A calibrated cylinder filled with water was connected to the reaction flask to measure the volume of released gas.At first,0.003 g catalyst and 2 ml distilled water were placed in the round-bottomed flask.After 2 min sonicating the mixture,19 μl of formic acid was added and immediately by stirring the mixture with a magnet,the reaction starts.The volume of the evolved gas was monitored by recording the displacement of water in the Calibrated cylinder.Various parameters including temperature,the catalyst amount,the effect of each part of the catalyst were evaluated and optimized.To this purpose,the reaction was carried out at 25,40,and 60 °C in the presence of 0.003 g catalyst.The catalyst amount was optimized by using 0.002,0.003,and 0.004 g Ni@Pd/NH2-Fe3O4catalyst at 60 °C.To investigate the effect of each part of the catalyst,the Fe3O4,NH2-Fe3O4,Ni/NH2-Fe3O4,Pd/NH2-Fe3O4,Ni0.4@Pd0.6/NH2-Fe3O4,Ni0.5@-Pd0.5/NH2-Fe3O4,and Ni0.6@Pd0.4/NH2-Fe3O4were synthesized and their catalytic activity were investigated for formic acid dehydrogenation.
3.Results and Discussion
3.1.Synthesis and recognition of the catalyst
To synthesize the Ni@Pd/NH2-Fe3O4catalyst,the Fe3O4nanoparticles were first synthesized in a solvothermal process.Afterwards Na2PdCl4,APTES and NaBH4were mixed and Ni@Pd/NH2-Fe3O4was easily synthesized in one step at room temperature(Fig.1).
To study the morphology of the catalyst,FE-SEM and HRTEM analyses were used.SEM images of Fe3O4have been shown in Fig.2 which indicate that the magnetic nanoparticles were well dispersed with uniform size and nanosphere morphology.This analysis showed diameters of approximately 20–40 nm for Fe3O4magnetic nanoparticles.
SEM images of Ni@Pd/NH2-Fe3O4have been given in Fig.3.These images clearly showed that after the simultaneous functionalization of Fe3O4magnetic nanoparticles with amine groups and immobilization of Ni@Pd core shell nanoparticles,the size of the nanoparticles was increased to 25–45 nm.The Energy Dispersive Spectroscopy (EDS) analysis also confirmed the presence and amount of Fe,N,and Ni,Pd elements in the as-prepared catalyst.Furthermore,elemental mapping determines the uniformity of elements distribution in the catalyst (Fig.4).
For further investigations on the size and distribution of the catalyst,TEM test was also used (Fig.5).TEM images showed the attendance of well and uniformly dispersed spherical nanoparticles.The core-shell nanoparticles also perceived to have a polycrystalline structure according to the literature reports [25].The outer layer has a lattice fringe distance of 0.26 nm that may represent the (1 1 1) planes of face-centered cubic (fcc) Pd with a normative lattice spacing of approximately 0.223 nm.The interlayer in the core has a lattice spacing of 0.25 nm and demonstrate a variation of the crystal lattice parameters of Ni(1 1 1).In contrast,the standard lattice spacing of Ni(1 1 1) is on the order of 0.203 nm[26].The observed differences between the Pd (1 1 1) and Ni(1 1 1) lattice spacing and the standard values can be ascribed the interaction between the Ni and Pd in the NPs,as well as their interaction with Fe3O4nanoparticles.

Fig.1.Synthetic route of Ni@Pd/NH2-Fe3O4.

Fig.2.SEM images of Fe3O4.

Fig.3.SEM images of Ni@Pd/NH2-Fe3O4.

Fig.4.EDS analysis of Ni@Pd/NH2-Fe3O4 with elemental maps for N,Ni,Pd,and Fe.
To confirm the functional groups of synthesized catalyst,FT-IR has been used.FT-IR spectra of APTS,Fe3O4,and Ni@Pd/NH2-Fe3O4have been presented in Fig.6(a)–(c),respectively.FT-IR spectrum of Ni@Pd/NH2-Fe3O4catalyst shows a broad around 3400 cm-1representing hydroxyl stretching vibrations located on the surface of the Fe3O4nanoparticles.The absorption band at 1628 cm-1can be assigned to N—H bending vibrations indicating the presence of the amine group on magnetic the nanoparticles[19].
For phase recognition and study of crystalline structure,XRD analysis has been used.The XRD patterns of Fe3O4and Ni@Pd/NH2-Fe3O4have been shown in Fig.7(a) and (b),respectively.In the XRD pattern of Fe3O4,six diffraction peaks with 2θ values of 30.4°,35.8°,43.5°,54.1°,57.4°,and 61.2°are observed corresponding to the (2 2 0),(3 1 1),(4 0 0),(4 2 2),(5 1 1),and (4 4 0) crystal planes of Fe3O4nanoparticles,respectively[27].The XRD pattern of Ni@Pd/NH2-Fe3O4also showed peaks at 39.1°,44.1°,66.3°,and 73.3° which are ascribed to the characteristic (1 1 1),(2 0 0),(2 2 0),and(3 1 1)planes assigned to the face centered cubic structure(fcc)of palladium nanoparticles.Moreover,the XRD pattern of Ni@Pd/NH2-Fe3O4showed peaks at 44.3°,51.6°,and 76.0° corresponding to Ni (1 1 1),(2 0 0) and (2 2 0) planes,respectively[28].
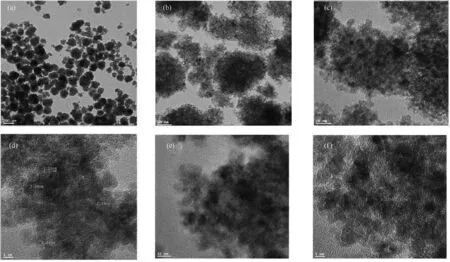
Fig.5.TEM images of Ni@Pd/NH2-Fe3O4.

Fig.6.FT-IR spectra of APTS (a),Fe3O4 (b),and Ni@Pd/NH2-Fe3O4 (c).
XPS analysis was employed to further investigation of elemental composition and accurate analysis of the Ni@Pd/NH2-Fe3O4catalyst structure.XPS survey confirms the existence of C,N,O,Fe,Ni and Pd(Fig.8(a)).According to Fig.8(b),the XPS peaks of Pd 3d5/2 and Pd 3d3/2 are observable at 338.2 and 344.1 eV,respectively,which shows that Pd metals are mostly metallic Pd0and electron transfer from Ni to Pd does not occur,and Ni is also metallic Ni0[29,30].The Fe 2p peak at binding energy of 710.22 eV is related to Fe2p3/2denoting the inverse cubic spinel phase of Fe3O4(Fig.8(c)) [31].XPS wide scan spectrum of N 1s clearly shows a peak in 400 eV,represent surface functionalization of nanoparticles with amine groups (Fig.8(d)) [32].

Fig.7.X-ray powder diffraction patterns of the Fe3O4(a)and Ni@Pd/NH2-Fe3O4(b).
3.2.Catalytic activity
An important issue with formic acid is that it can be decomposed in two different ways,dehydrogenation and dehydration.In hydrogen-based fuel cells,formic acid should be decomposed via dehydrogenation,since CO is toxic to fuel cell catalyst.Although according to Gibbs free energy,dehydrogenation is thermodynamically appropriate,dehydration is inevitable.So,selective decomposition of formic acid requires a suitable catalyst.After the successful synthesis and characterization of Ni@Pd/NH2-Fe3O4,the catalyst was used for dehydrogenation of formic acid.Various parameters have been modified to indicate optimal reaction conditions.
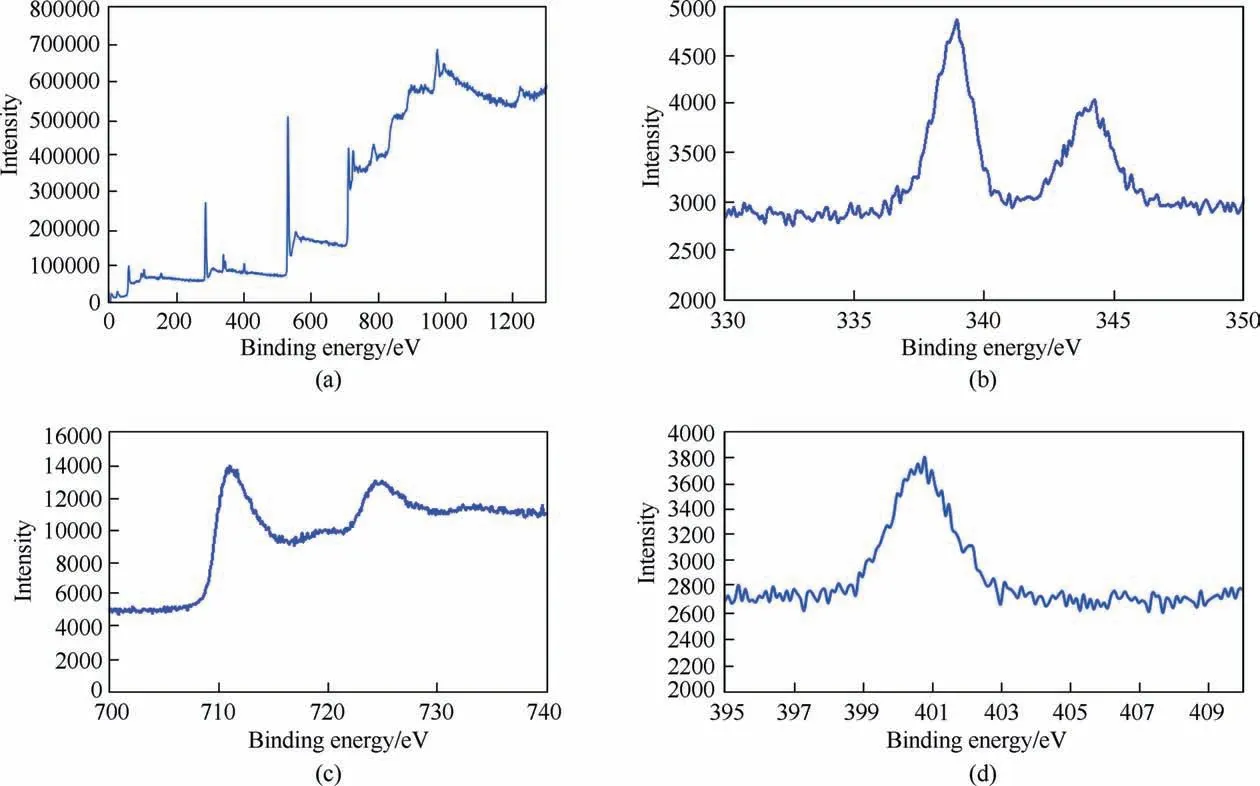
Fig.8.XPS survey spectrum of the Ni@Pd/NH2-Fe3O4 (a),XPS wide scan spectra of Pd 3d (b),Fe 2p (c),and N 1s (d).
The TOF is based on the number of (Ni+Pd) atoms in catalyst,which is calculated from the equation as follows:
where P0is the atmospheric pressure(101,325 Pa),V is the final generated volume of gas,R is the universal gas constant(8.3145 m3﹒Pa﹒mol-1﹒K-1),T is the temperature in K,nNiPdis the total molar number of(Ni+Pd)atoms in catalyst and t is the determined time of the reaction in hour.
TOF=P0V/2RTnNiPdt
The turnover frequency numbers (TOF) were calculated to be 4610.4,5367.8 and 4117.3 h-1with 0.002,0.003 and 0.004 g catalyst at 60 °C.The best result was obtained with 0.003 g of catalyst with respect to the amount of released gas and TOF (Fig.9).
As the reaction temperature clearly affect the catalytic performance,the dehydrogenation of formic acid was carried out at different temperatures in the presence of Ni0.4@Pd0.6/NH2-Fe3O4catalyst.The maximum amounts of gas release have been found to be 6,19,and 132 ml at 25,40,and 60°C,respectively.The results have been shown in Fig.10 which indicates the optimum temperature to be 60 °C.

Fig.9.Volume of generated gas with various catalyst amounts at 60 °C.
To find out the effect of each catalyst component on catalytic activity and their possible synergistic effect,various catalysts were synthesized and tested for the dehydrogenation of formic acid at 60 °C and 0.003 g catalyst,as shown in Fig.11.
According to the Fig.11,it can be easily understood that the most activity is related to the Ni@Pd/NH2-Fe3O4catalyst.To peruse the composition of generated gas,a trap containing 10 mol﹒L-1NaOH solution was used to complete absorption of CO2from the as formed gas of the catalytic reaction [33,34].The volume of gas reduced to half the original value suggesting a volume ratio of CO2and H2to be 1:1.Furthermore,the amount of CO was measured by GC spectrum which demonstrated no CO from the evolved gas.These results expressed the generation of only CO2and H2without releasing CO.

Fig.10.Volume of generated gas at different temperatures.

Fig.11.Volume of generated gas in the presence of various catalysts.
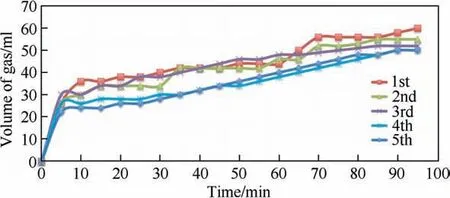
Fig.12.Recyclability test of Ni@Pd/NH2-Fe3O4 catalyst for dehydrogenation of formic acid under the optimum condition (0.003 g catalyst and 60 °C).
The stability and reusability of catalyst have a special importance to have economic justification.Therefore,the catalyst was utilized for four runs and no considerable loss of activity was observed (Fig.12).Also,given that this catalyst is magnetic,its recovery is simple and convenient using an external magnet.
Some similar heterogeneous catalysts used in the hydrogen generation from formic acid have been compared with the present work considering temperature,time,and TOF (Table 1).
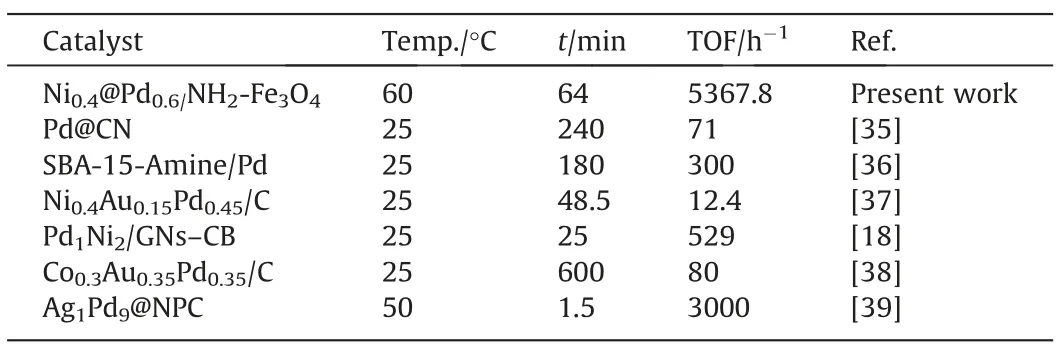
Table 1 Comparison of various catalysts for dehydrogenation of formic acid
According to the results,Ni@Pd/NH2-Fe3O4is one of the best reported catalysts in dehydrogenation of formic acid.So,by a simple and cost efficient synthesis route,a catalyst with high activity for formic acid dehydrogenation was obtained due to the presence of more active Pd nanoparticles on shell and less expensive Ni nanoparticles on core,and their well dispersion due to the NH2groups on the magnetic nanoparticles.
4.Conclusions
In this work,Ni@Pd/NH2-Fe3O4nanocatalyst was synthesized with a simple synthetic route.Several analyzes such as HRTEM,XPS and XRD were applied to confirm the formation of the catalyst and investigation of the structure.Compared to the most of similar previously reported catalysts for dehydrogenation of formic acid,the as-prepared hybrid material has much more activity based on the calculated TOF.This catalyst has very good durability with capability of simple magnetic recovery.These important features make Ni@Pd/NH2-Fe3O4an efficient and suitable catalyst for H2generation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The financial support provided by Shahid Beheshti university is gratefully acknowledged.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A comprehensive review of the effect of different kinetic promoters on methane hydrate formation
- An experimental study on the choked flow characteristics of CO2 pipelines in various phases
- Hydrothermal and entropy generation specifications of a hybrid ferronanofluid in microchannel heat sink embedded in CPUs
- Experimental and Numerical Study of Gas-Liquid Flow in a Sectionalized External-Loop Airlift Reactor
- Numerical simulation of heavy fuel oil atomization using a pulsed pressure-swirl injector
- Experimental research on steady-state operation characteristics of gas–solid flow in a 15.5 m dual circulating fluidized bed system
