Simultaneously spray-assisted assembling reversible superwetting coatings for oil–water separation
2021-06-26DexinChenZhixinKangWeiLiFenghuaSu
Dexin Chen ,Zhixin Kang ,Wei Li ,Fenghua Su
1 Institute of Advanced Wear &Corrosion Resistant and Functional Materials,Jinan University,Guangzhou 510632,China
2 Shaoguan Research Institute of Jinan University,Shaoguan 512027,China
3 School of Mechanical and Automotive Engineering,South China University of Technology,Guangzhou 510640,China
Keywords:Simultaneously spray deposition Superhydrophobicity Reversible superwetting Oil–water separation
ABSTRACT Here,superhydrophobic cuprous oxide(Cu2O)with hierarchical micro/nanosized structures was synthesized via spray-assisted layer by layer assembling.The as-prepared superhydrophobic meshes with high contact angle(159.6°)and low sliding angle(1°)are covered with Cu2O‘‘coral reef”-like micro/nanosized structures.Interestingly,the superhydrophobic mesh surfaces became superhydrophilic again due to the oxidization of Cu2O to CuO by annealing at a higher temperature (300°C).And the superhydrophobic properties would be recovered by heating at 120°C.Furthermore,the superwetting meshes were applied to design a miniature device to separate light or heavy oil from the water–oil mixtures with excellent separation efficiency.These superwetting surfaces by simultaneously spray-assisted layer by layer assembling technique show the potential application in universal oil–water separation.
1.Introduction
Smart surfaces with controllable superhydrophobicity or superhydrophilicity are of great attractive due to their potential industrial application [1,2].It is well established that the ability of superwetting is determined by chemical properties and geometrical structure of solid surfaces [3].Nevertheless,the switchable wettability is controlled by changing the chemical composition rather than surface structure,which is reacted to environmental stimuli involving electric fields [4,5],light irradiation [6],pH[7,8] and thermal treatment [9].Meanwhile,with the increasing concern of industrial grease dirt pollution,the rapid growth of research attention is directed toward separating oil from mixtures[10].Hierarchical structures with special wettability possess the ability to separate different liquids,indicating the potential applications of these materials for oil–water separation [11].
Recently,many techniques have been reported for the construction of a superhydrophobic surface for expanding the applications of alloys,such as self-assembly[12],chemical etching[13],sol–gel method [14],electrodeposition [15],and so on.For instance,Jiang reported a switchable method using UV and dark treatment[3].Liu described a reversible superhydrophobic-superhydrophilic surface via annealing and modifying low surface energy materials [16].However,the transitions of reversible wettability achieved by the above-mentioned methods require two kinds of external stimuli,which makes the transition less useful.Therefore,it would be desirable to realize switching under the same series of stimuli.Considering that the general fabrication methods are two-steps ways,it remains a big challenge to fabricate hierarchical structures on hydrophobic surfaces,due to the poisonousness of the low surface energy materials [3,16].
Cuprous oxide(Cu2O)has been widely reported in scientific and industrial fields owing to its potential applications in solar energy conversion,catalysis,battery,and so on [17,18].Spray deposition has been developed as a competitive one-step technique to construct superhydrophobic surfaces due to its advantages such as simplicity,low cost,nontoxicity and ability to make large-area surfaces on most of alloyed metals [19].Furthermore,the Cu2O/CuO films with micro/nanostructures possess the properties of superhydrophobicity and superhydrophilicity respectively,which can switch each other employing redox reaction [20].
One-step simultaneously spray-assisted layer by layer (LbL)assembling has been developed to prepare Ag coatings on several surfaces for plastic plating [19,21],flexible circuits [22] and solar reflector [23].In this paper,this technique was expanded to prepare cuprous oxide(Cu2O)hierarchical micro/nanosized structures with superwetting property.The surface feathers of microarchitectures related to wettability have been observed with the scanning electron microscope (SEM) as the Cu2O coatings were deposited layer by layer.The reversibility of superhydrophobic and superhydrophilic was explored with simple annealing at different temperatures.Finally,the superwetting brass meshes were used to set up a miniature device to separate different kinds of oils to find out the possibility of this technology for oil–water separation.
2.Experimental
2.1.Materials
Brass meshes(220 mesh),which contain 64 wt%Cu and 36 wt%Zn,were purchased from Shijiazhuang Yuanpeng Metal Manufacturing Works,(Hebei,China).Copper sulfate pentahydrate(CuSO4-﹒5H2O),seignette salt (C4O6H4KNa),sodium hydroxide (NaOH),sodium borohydride (NaBH4),HCl (37%),acetone and anhydrous ethanol were supplied by Guangzhou Chemical Reagent Factory(Guangdong,China).
2.2.Spray-assisted layer by layer deposition
The brass meshes were cut into 50×35 mm2slices.Then the achieved meshes were ultrasonically cleaned in 4 mol﹒L-1hydrochloric acid,acetone,anhydrous ethanol,and deionized water sequentially to remove the oxides and oil stains.0.15 mol﹒L-1CuSO4﹒5H2O,0.3 mol﹒L-1C4O6H4KNa,and 0.9 mol﹒L-1NaOH were successively added into deionized water to prepare mixed solution A as the saline solution.Meantime,solution B,which contained 0.67 mol﹒L-1NaBH4,was denoted as the reductant.As shown in Fig.1,the solutions A and B were simultaneously sprayed onto the sample surface to form a layer-by-layer coating.It is worth mentioning that the typical spray distance is about 15 cm in the experiments.After spraying,the meshes are dried thoroughly in a vacuum oven (DZF-6020D,Keelrein) at 120°C,which is called as dried mesh.To performing the revised wettability of these surfaces,the meshes are annealed with a muffle furnace at 300°C for 5 min,which is named as annealed mesh.And the meshes are heated at 120°C for two hours (heated mesh).
2.3.Characterization
The water contact angle (CA) and sliding angle (SA) were evaluated via an optical contact angle meter(OCA35,Dataphysics)with a 5 μl distilled water droplet.The average value was determined by measuring five different fresh spots on each sample.The surface morphological micro-nano structures were investigated by scanning electron microscopy(SEM,Quanta 200).The chemical composition of the surface was identified by X-ray photoelectron spectroscopy (XPS,PHI Quantera II) using the C 1 s peak energy(284.6 eV) as a calibrated energy standard and by Fouriertransform infrared spectroscopy (FT-IR,VERTEX 70,Bruker).Furthermore,the phase compositions of samples were analyzed with an X-ray diffractometer (XRD,D8 ADVANCE,Bruker) and Raman spectroscopy (LabRAM Aramis,H.J.Y.).
A sheet of as-prepared brass mesh was fastened to the end of a rubber hose by scotch tape,for a better sealing performance,which was used for the measurement of oil–water separation ability by means of a peristaltic pump (BT100L,Lead Fluid).The oil–water mixture was composed of 25 ml water and 25 ml oil.Turning on the self-priming pump,the oil–water penetrated the mesh and dropped into another beaker,while another couldn’t pass through the mesh.The oils which were used in the separation experiments were hexane,petroleum ether,toluene,kerosene,dichloromethane,and carbon tetrachloride.It is noteworthy that the oil was colored with oil red o,and the water was dyed with methylene blue.
3.Results and Discussion
Fig.2 shows the morphology of superhydrophobic coatings with different sprayed layers,showing that the morphologies of mesh varied apparently with the increase of sprayed layers.When the mesh was sprayed by 10 layers,nanoparticles uniformly grew on the mesh surface and made the mesh surface no longer smooth(Fig.2(a)).With the layers of spraying increasing,nanoparticles depositing on the mesh surface became denser and the mesh got rougher (Fig.2(b)).Increasing the sprayed layers to 50,the nanoparticles agglomerated together to produce ‘‘coral reef”-like hierarchical structures with the size of several micrometers on the surfaces (Fig.2(c)).The micro-coral reefs consisted of plenty of typical papillae nanoparticles (Fig.2(f)),resulting in superhydrophobic surface.Further increasing the sprayed layers to 70,the size and density of the micro-coral reef increased remarkably(Fig.2(d)).For sample with sprayed layers close to 150,the mesh surface was fully covered with ‘‘coral reef”,and the surface was so rough that the pore sizes of the mesh decreased obviously(Fig.2(e)).

Fig.1.Schematic illustration of the fabrication of Cu2O superhydrophobic surface by simultaneously spray-assisted layer by layer assembling.
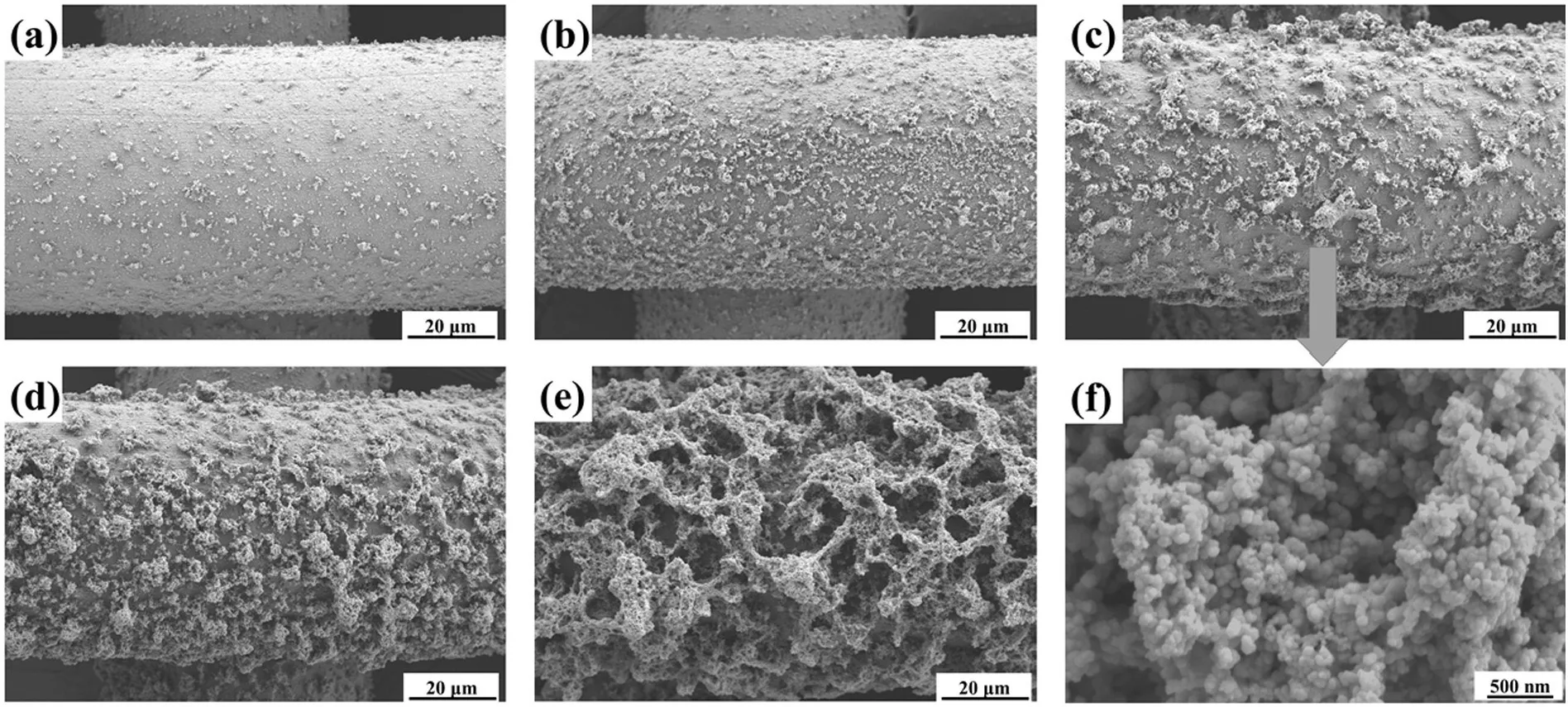
Fig.2.SEM images of the as-prepared superhydrophobic surface obtained under different layers of spraying:(a) 10 layers,(b) 30 layers,(c) 50 layers,(d) 70 layers,(e) 150 layers.(f) High magnification image of the superhydrophobic surface sprayed by 50 layers.

Fig.3.Variation in the water contact and sliding angles of the surfaces.
As illustrated in Fig.2,the morphology of the mesh varied with the layers of spraying.The corresponding distilled water contact angles were measured and the results were shown in Fig.3.The contact angle is up to (145.2±0.9)°,just spraying for 10 layers.If sprayed layers increase to 30,the CA increased to (155.6±0.6) °.Increasing the layers of spraying to 50 resulted in the largest CA(159.6±0.5)°,corresponding to the evenly distributed hierarchical micro/nanostructures on the mesh surface.But the CA began to gradually decrease,when layers of spraying were increasing once more.Fortunately,the CA was still (152.7±0.7)° even if the mesh was spayed for 150 layers.Moreover,all the sliding angles(SAs)of meshes were lower than 7°,as shown in Fig.3.When the mesh was spayed for 30 to 70 layers,the SAs are only 1°,indicating the low adhesion and excellent water repellency of the hydrophobicity.Hence the hierarchical micro/nanostructures possess a CA and smaller SA,due to the characteristics of trapping a great deal of air by the numerous gaps,which can be interpreted by the model presented by Cassie-Baxter equation [24]:

where θ and θ0are the CAs of the prepared and primary surfaces,respectively,and f is the area fraction of solid-water interface.As we known,the CA of Cu2O coatings on a smooth surface is approximately 110°.The f of surface with the largest CA of 159.6°was calculated as 0.096,showing that the contact area fraction of the airwater interfaces was 0.904.So that the as-prepared superhydrophobic meshes obtained under 50 layers of spraying was chosen for further investigation.

Fig.4.SEM images of(a)sprayed mesh,(b)dried mesh,(c)annealed mesh.The insets figures correspond to the water CAs on each surface;(d)the schematic of the wetting behavior transition.
To study the possibility of reversible feasibility,the brass meshes were placed in a vacuum oven for drying or annealing.The freshly sprayed meshes showed superhydrophilic surfaces with a CA of less than 1°,as shown in Fig.4a.After drying at 120°C for 1 h,the wettability of mesh surface could be tuned as superhydrophobic (Fig.4(b)).These surfaces covered with ‘‘coral reefs”like structures consisting of papillae nanoparticles,and not any obvious differences can be found between the SEM images of Fig.4(a) and (b).Annealing at 300°C for several minutes would result in superhydrophilic surfaces again with similar morphological structure (Fig.4(c)).In particular,the switchable wettability can be tuned and reversed under different temperatures for 20 cycles.This indicates that the change of wetting behavior induced by temperature is mainly achieved by changing the surface chemistry rather than the surface morphology,which can be explained that rough surface morphology plays an important role either in superhydrophobic or superhydrophilic surface.This is because Cu2O as a naturally hydrophobic material and its further oxide CuO as a hydrophilic material [20].Furthermore,appropriate surface morphology can make a hydrophobic surface more hydrophobic and a hydrophilic surface more hydrophilic.Hence,we concluded that the chemical components ought to make some changes during the heating at different temperatures.
Fig.5(a)shows the FT-IR spectra of the sprayed and dried mesh surface.In the high-frequency region of the two curves,the two series of adsorption peaks at about 3600 cm-1and 2125 cm-1are attributed to H2O,while the series of adsorption peaks at about 2350 cm-1corresponds to CO2.It can be seen that no other obvious peak exists in the high-frequency region of both the two curves,which demonstrates that no low surface energy materials were attached on the surfaces.What’s more,the peak for Cu2O at 618 cm-1emerges after drying for 2 h in the low-frequency region,which implies that the dried mesh is coated Cu2O [25].
Fig.5(b) shows the XRD patterns of sprayed mesh,dried mesh,annealed mesh,and heated mesh.The peaks at 42.34°,49.26° and 72.22° are indexed as the diffraction peaks of Cu0.64Zn0.36(JCPDS,NO.50–1333),which is from the substrate.The peaks at 43.30°,50.42°,and 74.142° indicate that Cu (JCPDS,NO.04–0836) corresponding to (111),(200) and (220) planes appear on the brass mesh(Fig.5(a)(1)).Fig.5(b)(2)shows the XRD patterns of the dried mesh,three peaks at 36.38°,61.52° and 73,40° in the patterns are assigned to (111),(220),and (311) planes of Cu2O (JCPDS,NO.65–3288).After annealing,three peaks appearing at 2θ=35.42°,38.70°and 61.50° in the patterns are (002),(111) and (11–3) planes of CuO (JCPDS,NO.48-1548) in Fig.5(b)(3),while the peaks of Cu2O disappeared,confirming that Cu2O was oxidized to CuO after annealing.Furthermore,peaks at 36.38°,61.52° and 73,40° corresponding to Cu2O(JCPDS,NO.65–3288)appeared again after heating in 120°C for 1 h.

Fig.5.FT-IR spectra (A),XRD patterns (B),Raman spectra (C) and Cu 2p XPS spectra (D) of (a) sprayed mesh,(b) dried mesh,(c) annealed mesh,(d) heated mesh.
Fig.5(c)shows the Raman spectra of different meshes.No peak can be found on the sprayed mesh surface,indicating that there is no compound existing on the surface.But the peaks at 151 cm-1,219 cm-1,407 cm-1,530 cm-1,and 625 cm-1indicates that Cu2O appearing on the dried mesh and heated mesh surfaces,and a peek at 298 cm-1corresponds to CuO on the annealed mesh surfaces[26,27].
Furthermore,XPS was applied to evaluate the surface chemistry of Cu in the various meshes at different temperatures.The highresolution XPS spectra of Cu 2p were shown in Fig.5(c).Two peaks at 952.0 and 932.3 eV,lower binding energy of Cu 2p,are detected on the freshly sprayed mesh in Fig.5(d)(1),which correspond to the Cu(0) peaks of Cu 2p1/2and Cu 2p3/2,indicating that the main element of the spraying surfaces is Cu.However,it is difficult to distinguish the metallic Cu and Cu2O by XPS since their Cu (2p)binding energies are too similar.Fortunately,three strong peaks centered at 952.2 eV,934.6 eV and 932.4 eV,corresponding to the Cu (I) are detected (Fig.5(d)(2)-(3)).Moreover,two weaker shakeup satellite peaks rather than intense satellite peaks showing only a little CuO on the surface,which could confirm the main composition of the dried and heated mesh are Cu2O.However,the annealed mesh (Fig.5(d)(3)) presents obvious peaks at 962.1 eV,953.6 eV,943.5 eV and 933.6 eV,attributing to Cu (II),which demonstrates that Cu2O was oxidized to CuO at a high temperature of 300°C,while the CuO was reduced to Cu2O again at the temperature of 120°C.
For the same why the chemical components change resulted in the superwetting transition? Based the description above,the formation mechanism of the‘‘coral reef”-like Cu2O superhydrophobic surface is estimated as follows:

Fig.1 shows the schematic illustration of the fabrication of Cu2O superhydrophobic surface.It’s worth noting that C4O6H4KNa possesses special properties of reducing ability,strong chelating and certain solubility.Once CuSO4was dissolved with C4O6H4KNa,the clathrate was formed and hydrolyzed to Cu(OH)2(Eqs.(2)–(3)).In brief,the C4O6H4KNa was used to tune amounts of Cu(OH)2.Owing to the strong reducing property of NaBH4,Cu(OH)2was reduced to Cu on the mesh surface (Eq.(4)).
Based on Fig.4 and the above discussion,the freshly sprayed mesh with micro/nanoscale Cu oxides is superhydrophilic and would convert to superhydrophobic.This indicates that heating would oxidize the Cu with hierarchical structures to Cu2O (Eq.(5)) [30].Notably,the surfaces can be superhydrophilic once the dried meshes were heated at 300°C for 5 min,and the oxidizing reaction processes should be:2Cu2O+O2→4CuO,indicating that intrinsic hydrophilic of CuO.However,the surfaces would return to be superhydrophobicity by heating at 120°C,as:4CuO →2Cu2O+O2[23,24].In a word,the switchable wettability of the sprayed brass meshes can be tuned by the temperature(Fig.4(d)).

Fig.7.(a) The corresponding efficiencies for different separating rates;the separation efficiencies for different kinds of oils with (b) superhydrophobic and (c)superhydrophilic mesh.
Owing to a combination of superoleophilicity and superhydrophobicity,the dried mesh obtains the ability to capture them from water.Interestingly,the brass mesh is superhydrophilic with WCA(water contact angle)of 158° after annealing and UOCA(under water–oil contact angle)of 0°.Therefore,both dried and heated brass mesh could efficiently separate oil/water mixtures.In consideration of the nonautomatic of the oil–water device with the aid of gravity [28],a miniature device was designed based on the asprepared mesh and used to separate oil from oil–water mixtures,as shown in Fig.6.The blue liquid is water,and the red liquid is oil.As shown in Fig.6(a),the as-prepared superhydrophobic meshes could continuously remove the light oil (Toluene) from the water,resulting in final oil–water separation.After the separation process,only residual oil was left in water–oil mixture.Meanwhile,the superhydrophilic brass meshes could separate water from oil–water mixtures (Fig.6(b)),coming out only light oil in the bottles.A typical heavy oil(dichloromethane)has been applied to verify the feasibility of oil–water separation.As shown in Fig.6(-c)-(d),the heavy oil or water could capture out from the oil–water mixtures successfully.
In order to investigate the oil–water separation properties of the as-prepared mesh,separating rates and separation efficiencies are displayed in Fig.7.The efficiencies of oil–water separation were calculated as the Eq.(6):

Where M and m were the oil mass before and after the separation using the superhydrophobic (superhydrophilic) meshes,respectively [25].Fig.7(a) displays the separation time and the corresponding efficiency under different rotate speeds.It is found that the separation abilities for hexane are all above 94%,even at high rotate speed (150 r﹒min-1),which can separate the oil/water mixture only in 11 s.What is nice that the separating rate can speed up with more hoses.As shown in Fig.7(b)-(c),different kinds of oils have been used to performing the separating process,and the separating efficiencies are larger than 97%,indicating only 3% residual oil was left in water–oil mixture.Comparing with the previous study [29,30],all the separation efficiencies of this work have been improved accordingly,even for the different kinds of oils with superhydrophobic and superhydrophilic mesh.The excellent tunable wetting behaviors and oil absorbency make the as-prepared meshes and the device promising in the oil–water separation.
4.Conclusions
In this work,we fabricated a reversible superwetting coatings by one-step simultaneously spray-assisted layer by layer assembling technique.The as-prepared superhydrophilic/ superhydrophobic meshes are covered with Cu2O ‘‘coral reef-like”micro/nanosized structures of various sprayed layers.The Cu2O superhydrophobic surface has a static water contact angle of 159.6 ° and a sliding angle as low as 1°.It is interesting that the superhydrophobic Cu2O surfaces became superhydrophilic again due to the oxidization of CuO after annealing at a higher temperature (300°C).And superhydrophobicity would recover by heating at 120°C for 2 h for reducing back to Cu2O.It can be concluded that the reversible wettability with Cu2O/CuO coatings is induced by temperature.Finally,the superwetting meshes were applied to design a miniature device to separate various oils,regardless of the light oil or heavy oil,from the water–oil mixtures with excellent separation efficiency.Present results elucidate that the superwetting meshes show the potential application in universal oil–water separation and the simultaneously spray-assisted layer by layer assembling technique can also be exploited to produce other kinds of superhydrophobic coatings.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge financial support from Guangdong Basic and Applied Basic Research Foundation,China(No.2019A15150101011282),Open Funds of National Engineering Research Center of Near-Net-Shape Forming for Metallic Materials(2019008) and the Fundamental Research Funds for the Central Universities (21619336).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A comprehensive review of the effect of different kinetic promoters on methane hydrate formation
- An experimental study on the choked flow characteristics of CO2 pipelines in various phases
- Hydrothermal and entropy generation specifications of a hybrid ferronanofluid in microchannel heat sink embedded in CPUs
- Experimental and Numerical Study of Gas-Liquid Flow in a Sectionalized External-Loop Airlift Reactor
- Numerical simulation of heavy fuel oil atomization using a pulsed pressure-swirl injector
- Experimental research on steady-state operation characteristics of gas–solid flow in a 15.5 m dual circulating fluidized bed system
