PI3K/Akt/mTOR Signaling as Targets for Developing Anticancer Agents from Marine Organisms
2021-06-25GUOMingyueZUOLingQIAOGanLIUMinghuaCAOShousongandLINXiukun
GUO Mingyue, ZUO Ling, QIAO Gan, LIU Minghua, CAO Shousong,and LIN Xiukun, *
PI3K/Akt/mTOR Signaling as Targets for Developing Anticancer Agents from Marine Organisms
GUO Mingyue1), #, ZUO Ling1), #, QIAO Gan1), 2), LIU Minghua1), CAO Shousong1),and LIN Xiukun1), *
1),,,646000,2),646000,
The PI3K/Akt/mTOR signaling pathway is one of the most frequently dysregulated pathways in cancer. Targeting the PI3K-mediated pathway has been an important strategy for developing novel anticancer agents. In the past decades, more than 40 inhibitors of the PI3K/Akt/mTOR pathway have been developed at different clinical stages. Temsirolimus, everolimus, idelalisib, and copanlisib have been approved for clinical use by the Food and Drug Administration of the United States (FDA). However, the toxicity and drug resistance limit their efficiency in the treatment. Novel compounds with greater potency and selectivity, as well as improved therapeutic indices with reduced toxicity, are clearly required. Over the past three decades, a lot of bioactive ingredients with anticancer effects by affecting the PI3K-mediated pathways have been found from marine organisms. In the present mini-review, anticancer compounds from marine source that target the PI3K/Akt/mTOR signaling were reviewed. The molecular entities and their modes of action were presented. The marine compounds targeting special factors of the PI3K/Akt/mTOR were highlighted.
marine organisms; PI3K/Akt/mTOR pathway; anticancer activity
1 Introduction
The PI3K/Akt/mTOR pathway is one of the most frequently dysregulated pathways in cancer and, consequent- ly, more than 40 compounds that target key components of this signaling network have been tested in clinical trials involving patients with a range of different cancers (Janku, 2018). The clinical developments of many of these agents, however, have not advanced to late-phase rando- mized trials due to toxicity (Song, 2010). Presently, the mTOR inhibitors temsirolimus and everolimus and the PI3K inhibitors idelalisib and copanlisib have been app- roved by the FDA for clinical use in the treatment of a number of different cancers (Willis, 2020). Novel compounds with greater potency and selectivity, as well as improved therapeutic indices with reduced toxicity, are clearly required. In addition, biomarkers that are predictive of a response, such as PIK3CA mutations for inhibitors of the PI3K catalytic subunit α isoform, must be iden- tified and analytically and clinically validated (Wu, 2020). Finally, considering that oncogenic activation of the PI3K/Akt/mTOR pathway often occurs alongside pro-tu- morigenic aberrations in other signaling networks, rational combinations are also needed to optimize the effective-ness of treatment.
Marine organisms are rich sources for finding natural anticancer compounds. Due to their particular lifestyles in the sea with a special environment, some of the compounds such as bromophenols and depsipeptides are mostly found in marine sources. The biodiversity of marine organisms provides a rich source for the discovery and development of novel anticancer agents in the treatment of human malignancies (Zheng, 2018). Organisms living in the sea synthesize a wide variety of chemicals used as defense for predators (Wang, 2020). Compared with other biological components, some of the components frommarine organisms display more powerful cytotoxicity. Over the years, an increasing number of novel compounds with an- ticancer effects have been isolated from marine organisms, and many of them have been reported to possess promising anticancer activityinhibition of PI3K-mediated sig- naling. In this mini-review, we presented those compounds isolated from marine organisms with inhibitory effects on the PI3K/Akt/mTOR signaling. The challenges and prospects for developing anticancer agents using the PI3K/ Akt/mTOR signaling as target were also discussed.
2 Components Isolated from Marine Organisms Targeting the PI3K/-Akt/mTOR Pathway
As a natural carotenoid, fucoxanthin (Fig.1) is isolated from seaweed, and the compound exhibits a broad spectrum of biological activities, including anti-inflammatory (Heo, 2010), anti-mutagenic (Tanaka, 2012), and anticancer activities (Nishino, 1995; Satomi, 2017). Fu- coxanthin is capable of inhibiting the growth of a number of cancer cells, including liver cancer (Liu, 2009, 2013;Satomi and Nishino, 2009), colon cancer (Hosokawa, 2004; Liu, 2012), leukemia (Ishikawa, 2008; Yamamoto, 2011), prostate cancer (Satomi, 2012) and bladder cancer (Wang, 2012). It has been demonstrated that inactivation of the PI3K/Akt signaling is an important event in fucoxanthin-induced cancer cell apoptosis; treatment with fucoxanthin (1μmolL−1) led to down regulation of the p-PI3K, p-Akt significantly in HeLa can- cer cells (Ye, 2014). Additionally, exposure of hu- man glioma U251 and U87 cancer cells with fucoxanthin (50μmolL−1) resulted in an inhibitory effect of the Akt/ mTOR signaling and the compound also inhibited themigration and invasion of the cancer cells (Liu, 2016).ROS production is an important event in fucoxanthin- induced inhibitory effect on the invasion and migration of glioma cells; the inhibitory effect of the compound on can- cer cell growth was diminished in the presence of antioxidant glutathione (Wu, 2019). Recent study also found that fucoxanthin was capable of increasing the cytotoxicity of cisplatin; in the presence of fucoxanthin, the cell viability was markedly suppressed compared with that of cisplatin alone and the combination of fucoxanthin and cisplatin also increased the ratio of Bax/Bcl2 (Liu, 2013).
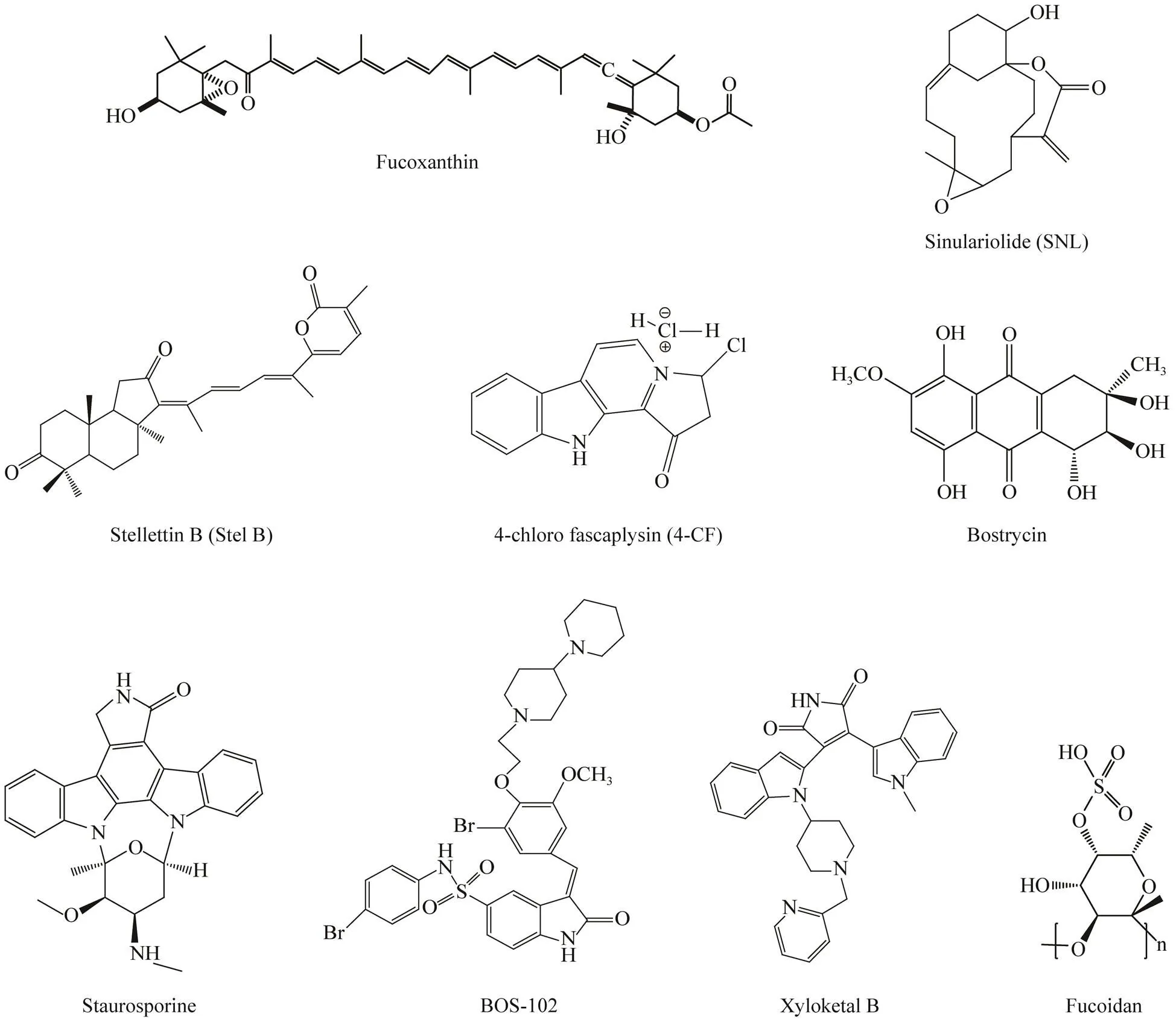
Fig.1 Chemical structures of compounds, isolated from marine organisms, targeting PI3K/Akt/mTOR signaling pathway.
Marine sponges are recognized as one of the most pro- ductive sources of anticancer components, and a lot of bio- active compounds with anticancer activity have been foundfrom marine sponges (Bader, 2005). Stellettin B (Stel B, Fig.1) belongs to an isomalabaricane triterpene. It has been extracted from marine spongePrevious study indicated that the compound was capable of inducing apoptosisthe PI3K/Akt/mTOR signaling pathway in cancer cells. Stel B treatment induced apoptosisenhancing the production of ROS, the cleavage of PARP, as well as inhibiting the activity of caspase 3/7 in human glioblastoma SF295 cells (Tang, 2014). Recent study showed that Stel B is able to inhibit the phosphorylation of PI3K and Akt in SF295 cells (1μmolL−1) and human chronic myeloid leukemia K562 cells (0.036μmolL−1) (Chen, 2017). Moreover, Stel B also leads to the ROS generation and increased expression of PARP in NSCLC cells (Hsiao, 2016). It can also induce G1 arrestinhibiting the expression of cyclin D1 and increasing the expression of p27 (Cheng, 2019). The PI3K/Akt/mTOR signaling pathway also plays a role in Stel B-induced (1μmolL−1) apoptosis in A549 cells. Additionally, Stel B treatment results in down-ex- pression of PI3K-p110 and inhibitory of the phosphorylation of PDK1, Akt, mTOR, and p70S6K (Hsiao, 2016).
In 1988, Rollisolated an alkaloid fascapysin from marine spongesp. (Roll, 1988). Re- cent study showed that its synthesized derivative 4-chloro fascaplysin (4-CF, 3μmolL−1, Fig.1) exhibited potent an- tiangiogenetic activityblocking the PI3K/Atk/mTOR pathway (Sharma, 2017). Treatment with 4-CF resulted in downregulation of VEGF and Akt in HUVEC cells, while in the presence of the inhibitors of VEGF and Akt (sunitinib and perifosine), the cytotoxic effect of 4- CF was significantly inhibited in HUVEC cells.
Sinulariolide (SNL, Fig.1), a cembrane-based diterpenoid, was isolated from cultured-type soft coral(Neoh, 2012). Previous studies have shown that SNL possesses anticancer effects by inducing apoptosis in several cancer cells; SNL treatment led to activation of caspase cascade in A375 melanoma cells (Li, 2013). Recent studies showed that the PI3K/Akt signaling played an important role in SNL-induced (10μgmL−1) cancer cell apoptosis in cancer cell invasion and migrationinhibiting the phosphorylation of PI3K, Akt and mTOR (Wu, 2015). In order to increase the efficiency of the compound, SNL was modified by conjugating with HA (hyaluronan) nanoparticles to form a novel complex compound HA/SNL aggregates. Since hyaluro- nan nanoparticles possesses high hydrophilicity, the bio- availability of the compound was greatly increased, and HA/SNL displays more powerful anticancer effect than SNL (Hsiao, 2016).
As an anticancer alkaloid, staurosporine (Fig.1) was ori- ginally isolated from a terrestrialsp. (Omu- ra, 1977). The compound was also isolated from several other marinesp. (Cartuche, 2019; Pimentel-Elardo, 2010). Staurosporine was capable of inducing apoptosis in human pancreatic carcinoma PaTu 8988t and Panc-1 cells. Staurosporine treatment resulted in decreased expression of Bcl-2 and Bad. The PI3K/Akt signaling plays an important role in stau- rosporine-induced cancer cell apoptosis. Treatment with staurosporine (20nmolL−1) can inhibite the Akt phosphoralytion significantly in HepG2 cells (Ding, 2017). To increase the anticancer activity, several derivatives of staurosporine were synthesized. Enzastaurin (Fig.1) is one of the derivatives. It can inhibit the cancer cell growth withpotent activity and higher water solubility (Faul, 2003). Enzastaurin (3.56μmolL−1) can inhibit the proliferation of gastric cancer bothandaffecting the Akt signaling cascade (Lee, 2008). Preclinical study showed that enzastaurin inhibited the growthof a panel of small-cell lung cancer lines. Enzastaurin treat- ment led to G1 arrest and apoptosis by suppressing the PKC/ERK 1/2 pathway in uveal melanoma cells carrying GNAQ mutation (Öztaşkın, 2015), and resulted in downregulation of the phosphorylation of GSK3βSer9, ri- bosomal protein S6Ser240/244, and AktThr308 significant- ly in xenogeneic glioblastoma cells. Enzastaurin also displays antiangiogenesis activity by downregulating VEGF (vascular endothelial growth factor) and suppressing the microvessel density in human tumor xenograft-bearing nude mice (Keyes, 2004). The safety of enzastaurin was confirmed by phase I clinical study for patients with refractory solid tumors and lymphoma. Oral administration of 500 mg of enzastaurin once daily were well tolerated (Li, 2016). Phase I clinical trial of the compound showed promise as minimal toxicity was demonstrated.However, phase II and phase III clinical trials is disappointing for patients with diffuse B cell lymphoma (Ro- bertson, 2007), relapsed and refractory mantle cell NSCLC (Oh, 2008) and lymphoma (Morschhauser, 2008). Therapy of enzastaurin with other chemotherapeutic agents were developed; the combination of en- zastaurin with bevacizumab for the treatment of recurrent malignant gliomas was well-tolerated, with similar results to bevacizumab monotherapy (Odia, 2016). Synergistic effects were also found using the combination of en- zastaurin and Ibrutinib in the treatment of large B cell lymphoma (He, 2019).
Bromophenols, a large class of marine natural products, are widely distributed in various marine organisms inclu- ding marine sponges, algae, cyanobacteria and marine fun- gi (Wang, 2013; Blunt, 2018). Bromophenols exhibits broad biological activities such as antibacterial activities, antivirus and anticancer activity (Liu, 2011; Öztaşkın, 2015; Wang, 2015). Shi’s laboratory developed a series of bromophenol derivatives and one of the bromophenol derivatives BOS-102 (Fig.1) displayed potent anticancer effect toward several human cancer cell lines especially for human lung cancer cells. Treatment with BOS-102 resulted in apoptosis and cell cycle arrest in lung cancer A549 cells. BOS-102 was able to activate caspase- 3 and PARP, enhance the Bax/Bcl-2 ratio, increase the ROS generation, and decrease ΔΨm, leading cytochrome release from mitochondria. Recent study revealed that the PI3K/Akt pathway was involved in the cancer cell apoptosis induced by BOS-102 (10μmolL−1). Treatment with the compound resulted in downregulation of the phospho- rylation of PI3K and Akt significantly in A549 cells (Guo, 2018).
Bostrycin (Fig.1) found in marine fungisp. in the South China Sea is able to inhibit the growth ofprostate and gastric cancer (Yang,2012; Fan, 2018). Studies have shown that bostrycin (10μmolL−1)can suppress the proliferation of human lung carcinoma A549 cellsdownregulation of the PI3K/Akt signaling. Treatment with bostrycin downregulated the levels of p- Akt significantly, while the levels of p27 were up-regu- lated, leading to cell cycle arrest at G1 phase (Chen, 2011).
As a sulfated polysaccharide compound, fucoidan (Fig.1) was originally found in the cell wall matrix of many kinds of brown seaweeds (Li, 2008). Fucoidan possesses a broad spectrum of bioactivities including antivirus, anti- bacterial and anti-cancer activities. In recent years, in- creasing attention is focused on the anti-cancer effect of fucoidan since the compound exhibits potent anticancer effect with low toxicity (Lee, 2004; Aisa, 2005; Gideon and Rengasamy, 2008; Yamasaki-Miyamoto, 2009; Yang, 2013). The PI3K/Akt signaling is invo- lved in fucoidan-induced cancer cell apoptosis in a num- ber of cancer cells. Treatment with fucoidan (200μgmL−1) suppressed the cell migration and viability significantly in HT-29 cells (Han, 2015). Fucoidan can also inhibit the growth of human breast cancer MDA-MB-231 cells by downregulating the expressions of p-PI3K, p-Akt, and p-GSK3β (Xue, 2017). Recent study also found that fucoidan was capable of suppressing the cell viability and inducing the apoptosis in human prostate PC-3 cancer cellsinhibiting the PI3K/Akt signaling pathway (Boo, 2013). Fucoidan-induced cancer cell apoptosis and cell cy- cle arrest are not associated with p53 expression. Treatments of the p53 positive and negative HCT-116 cells with fucoidan result in similar apoptosis and DNA damage (Park, 2017).
Xyloketal B (Fig.1) is obtained from mangrove fungussp. in the South China Sea (Lin, 2001). The compound exhibits antioxidant activity and protective ef- fects on endothelial and neuronal oxidative injuries. Xyloketal B (300μmolL−1) is capable of inhibiting the growth of glioma cells. The PI3K/Akt signaling is involved in the regulation of proliferation and migration of glioblastoma cells (Yajima, 2012). Treatment with xyloketal B leads to the downregulation of p-Akt and p-ERK1/2 sig-nificantly in human glioma U251 cancer cells (Chen, 2015).
3 Discussion
In the present mini-review, we discussed the compounds isolated from marine organisms and their main targets arethe PI3K/Akt/mTOR signalling (Table 1). However, it should be emphasized that some of the compounds can target multiple pathways. Except for targeting the PI3K/ Akt signaling, fucoxanthin can also suppress the p38 MAPK and NF-κB pathways; treatment with fucoxanthin can in- hibit the phosphorylation of MAPKs, and increase the expression of NF-κB-regulated Bax/Bcl-2 ratio (Kumar, 2013). ROS-mediated oxidative damage plays arole in fucoxian-induced cancer cell apoptosis (Jang, 2018). The PI3K/Akt/mTOR pathway is also involved in inflammatory event. The fucoxanthin can decrease pro-in- flammatory cytokines, such as IL-1b, IL-6, and TNF-α, by suppressing NO production and iNOS expression (Heo, 2010). Moreover, fucoxanthin can significantly in- hibit the inflammation of mice with paw edema by suppressing the levels of NO and Akt in the plasma. It can protect catalase (CAT) and superoxide dismutase (SOD) against disruption in mice with paw edema (Choi, 2016). More studies are needed to address if there is correlation between the anticancer activity and anti-inflam- matory effects of fucoxanthin.

Table 1 The compounds isolated from marine organisms and their main targets on the PI3K/Akt/mTOR signaling pathway
In the past decades, significant progress has been made in developing anticancer agents targeting the PI3K signalling. However, the toxicity and the drug resistance hinder the clinical application of these drugs. Several approaches overcoming the drug resistance are developing. It is important to find novel agents targeting alternative binding sites on the signalling factors or targeting the other pathways that are required for activating the PI3K signaling (Gumireddy, 2005). Future studies should focus on finding novel compounds from marine organisms with low toxicity and targeting special sites on the PI3K/Akt signaling. As the special marine environment provides the diversity of marine natural products to offer unique anticancer agents with promising clinical value, it is worthy to identify more anticancer agents that can be used clinically.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (Nos. 81573457 and 81773776). We are also grateful to the support from the Taishan Talents Project of Shandong Province and the Department of Science and Technology in Shandong Province of China (Nos. ZR2017MH117, 2018YYSP025, and ZR2017MH 027), and Department of Science and Technology of Si- chuan Province, China (Nos. 2017HH0104 and 2019YFS 0116).
Aisa, Y., Miyakawa, Y., Nakazato, T., Shibata, H., Saito, K., Ike- da, Y., and Kizaki, M., 2005. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase- 3 and down-regulation of ERK pathways., 78 (1): 7-14.
Bader, A. G., Kang, S., Zhao, L., and Vogt, P. K., 2005. Oncoge- nic PI3K deregulates transcription and translation. Nature re- views., 5 (12): 921-929.
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R., 2018. Marine natural products., 35 (1): 8-53.
Boo, H. J., Hong, J. Y., Kim, S. C., Kang, J. I., Kim, M. K., Kim, E. J., Hyun, J. W., Koh, Y. S., Yoo, E. S., Kwon, J. M., and Kang, H. K., 2013. The anticancer effect of fucoidan in PC-3 prostate cancer cells., 11 (8): 2982-2999.
Ishikawa, C., Tafuku, S., Kadekaru, T., Sawada, S., Tomita, M., Okudaira, T., Nakazato, T., Toda, T., Uchihara, J. N., Taira, N., Ohshiro, K., Yasumoto, T., Ohta, T., and Mori, N., 2008. Anti- adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol., 123 (2008): 2702-2712.
Cartuche, L., Sifaoui, I., Cruz, D., Reyes-Batlle, M., López- Arencibia, A., Javier Fernández, J., Díaz-Marrero, A. R., Piñero, J. E., and Lorenzo-Morales, J., 2019. Staurosporine fromactivates programmed cell death invia the mitochondrial pathway and presents lowcytotoxicity levels in a macrophage cell line., 9 (1): 1-12.
Chen, W. L., Turlova, E., Sun, C. L. F., Kim, J. S., Huang, S., Zhong, X., Guan, Y. Y., Wang, G. L., Rutka, J. T., Feng, Z. P., and Sun, H. S., 2015. Xyloketal B suppresses glioblastoma cell proliferation and migrationthrough inhibiting TRPM7- regulated PI3K/Akt and MEK/ERK signaling pathways., 13 (4): 2505-2525.
Chen, W. S., Hou, J. N., Guo, Y. B., Yang, H. L., Xie, C. M., Lin, Y. C., and She, Z. G., 2011. Bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway., 30 (1): 17.
Chen, Y., Zhou, Q., Zhang, L., Zhong, Y., Fan, G., Zhang, Z., Wang, R., Jin, M., Qiu, Y., and Kong, D., 2017. Stellettin B in- duces apoptosis in human chronic myeloid leukemia cells via targeting PI3K and Stat5., 8 (17): 28906-28921.
Cheng, S. Y., Chen, N. F., Lin, P. Y., Su, J. H., Chen, B. H., Kuo, H. M., Sung, C. S., Sung, P. J., Wen, Z. H., and Chen, W. F., 2019. Anti-invasion and antiangiogenic effects of Stellettin B through inhibition of the Akt/Girdin signaling pathway and VEGF in glioblastoma cells., 11 (2): 220.
Choi, J. H., Kim, N. H., Kim, S. J., Lee, H. J., and Kim, S., 2016. Fucoxanthin inhibits the inflammation response in paw ede- ma model through suppressing MAPKs, Akt, and NFκB., 30 (3): 111-119.
Ding, Y., Wang, B., Chen, X., Zhou, Y., and Ge, J., 2017. Stau- rosporine suppresses survival of HepG2 cancer cells through Omi/HtrA2-mediated inhibition of PI3K/Akt signaling pathway., 39 (3): 10 1042831769431.
Fan, M., Nath, A. K., Tang, Y., Choi, Y. J., Debnath, T., Choi, E. J., and Kim, E. K., 2018. Investigation of the anti-prostate can- cer properties of marine-derived compounds., 16 (5): 160.
Faul, M. M., Gillig, J. R., Jirousek, M. R., Ballas, L. M., Schotten, T., Kahl, A., and Mohr, M., 2003. Acyclic N-(azacycloalkyl) bisindolylmaleimides: Isozyme selective inhibitors of PKCbeta., 13 (11): 1857-1859.
Gideon, T. P., and Rengasamy, R., 2008. Toxicological evaluation of fucoidan from., 11 (4): 638-642.
Gumireddy, K., Reddy, M. V. R., Cosenza, S. C., Boominathan, R., Boomi Nathan, R., Baker, S. J., Papathi, N., Jiang, J., Hol- land, J., and Reddy, E. P., 2005. ON01910, a non-ATP-compe- titive small molecule inhibitor of Plk1, is a potent anticancer agent., 7 (3): 275-286.
Guo, C. L., Wang, L. J., Zhao, Y., Liu, H., Li, X. Q., Jiang, B., Luo, J., Guo, S. J., Wu, N., and Shi, D. Y., 2018. A novel bro- mophenol derivative BOS-102 induces cell cycle arrest and apoptosis in human A549 lung cancer cells via ROS-mediated PI3K/Akt and the MAPK signaling pathway., 16 (2): 43.
Han, Y. S., Lee, J. H., and Lee, S. H., 2015. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of ra-pamycin pathways., 12 (3): 3446- 3452.
He, Y., Li, J., Ding, N., Wang, X., Deng, L., Xie, Y., Ying, Z., Liu, W., Ping, L., Zhang, C., Song, Y., and Zhu, J., 2019. Com- bination of Enzastaurin and Ibrutinib synergistically induces anti-tumor effects in diffuse large B cell lymphoma., 38 (1): 86.
Heo, S. J., Yoon, W. J., Kim, K. N., Ahn, G. N., Kang, S. M., Kang, D. H., Affan, A., Oh, C., Jung, W. K., and Jeon, Y. J., 2010. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages., 48 (8-9): 2045-2051.
Hosokawa, M., Kudo, M., Maeda, H., Kohno, H., Tanaka, T., and Miyashita, K., 2004. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells., 1675 (1-3): 113-119.
Hsiao, K. Y., Wu, Y. J., Liu, Z. N., Chuang, C. W., Huang, H. H., and Kuo, S. M., 2016. Anticancer effects of sinulariolide-con- jugated hyaluronan nanoparticles on lung adenocarcinoma cells., 21 (3): 297.
Jang, E. J., Kim, S. C., Lee, J. H., Lee, J. R., Kim, I. K., Baek, S. Y., and Kim, Y. W., 2018. Fucoxanthin, the constituent of, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress., 18 (1): 97.
Janku, F., Yap, T. A., and Meric-Bernstam, F., 2018. Targeting the PI3K pathway in cancer: Are we making headway?, 15 (5): 273-291.
Keyes, K. A., Mann, L., Sherman, M., Galbreath, E., Schirtzin- ger, L., Ballard, D., Chen, Y. F., Iversen, P., and Teicher, B. A., 2004. LY317615 decreases plasma VEGF levels in human tu- mor xenograft-bearing mice., 53 (2): 133-140.
Kumar, S. R., Hosokawa, M., and Miyashita, K., 2013. Fuco-xanthin: A marine carotenoid exerting anti-cancer effects by af- fecting multiple mechanisms., 11 (12): 5130- 5147.
Lee, J. B., Hayashi, K., Hashimoto, M., Nakano, T., and Hayashi, T., 2004. Novel antiviral fucoidan from sporophyll of(Mekabu)., 52 (9): 1091-1094.
Lee, K. W., Kim, S. G., Kim, H. P., Kwon, E., You, J., Choi, H. J., Park, J. H., Kang, B. C., Im, S. A., Kim, T. Y., Kim, W. H., and Bang, Y. J., 2008. Enzastaurin, a protein kinase C beta in- hibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric can- cer cells., 68 (6): 1916-1926.
Li, B., Lu, F., Wei, X., and Zhao, R., 2008. Fucoidan: Structure and bioactivity., 13 (8): 1671- 1695.
Li, H. H., Su, J. H., Chiu, C. C., Lin, J. J., Yang, Z. Y., Hwang, W. I., Chen, Y. K., Lo, Y. H., and Wu, Y. J., 2013. Proteomic in- vestigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade., 11 (7): 2625-2642.
Li, X., Fang, X., Li, S., Zhang, W., Yang, N., Cui, Y., Huang, H., Cai, R., Lin, X., Fu, X., Hong, H., and Lin, T., 2016. A pharmacokinetic and safety study of a fixed oral dose of enza- staurin HCl in native Chinese patients with refractory solid tumors and lymphoma., 7 (14): 18585-18593.
Lin, Y., Wu, X., Feng, S., Jiang, G., Luo, J., Zhou, S., Vrijmoed, L. L. P., Jones, E. B. G., Krohn, K., Steingröver, K., and Zsila, F., 2001. Five unique compounds: Xyloketals from mangrove fungussp. from the South China Sea coast., 66 (19): 6252-6256.
Liu, C. L., Huang, Y. S., Hosokawa, M., Miyashita, K., and Hu, M. L., 2009. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication., 182 (2-3): 165-172.
Liu, C. L., Lim, Y. P., and Hu, M. L., 2012. Fucoxanthin attenuates rifampin-induced cytochrome P450 3A4 (CYP3A4) and multiple drug resistance 1 (MDR1) gene expression through pregnane X receptor (PXR)-mediated pathways in human he- patoma HepG2 and colon adenocarcinoma LS174T cells., 10 (1): 242-257.
Liu, C. L., Lim, Y. P., and Hu, M. L., 2013. Fucoxanthin enhancescisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepa- toma HepG2 cells., 11 (1): 50-66.
Liu, M., Hansen, P. E., and Lin, X., 2011. Bromophenols in ma- rine algae and their bioactivities., 9 (7): 1273- 1292.
Liu, Y., Zheng, J., Zhang, Y., Wang, Z., Yang, Y., Bai, M., and Dai,Y., 2016. Fucoxanthin activates apoptosis via inhibition ofPI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human gli- oblastoma cells., 41 (10): 2728-2751.
Malsy, M., Bitzinger, D., Graf, B., and Bundscherer, A., 2019. Stau- rosporine induces apoptosis in pancreatic carcinoma cells PaTu8988t and Panc-1 via the intrinsic signaling pathway., 24 (1): 5.
Morschhauser, F., Seymour, J. F., Kluin-Nelemans, H. C., Grigg, A., Wolf, M., Pfreundschuh, M., Tilly, H., Raemaekers, J., van’t Veer, M. B., Milpied, N., Cartron, G., Pezzutto, A., Spencer, A., Reyes, F., and Dreyling, M., 2008. A phase II study of enza- staurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma., 19 (2): 247-253.
Neoh, C. A., Wang, R. Y. L., Din, Z. H., Su, J. H., Chen, Y. K., Tsai, F. J., Weng, S. H., and Wu, Y. J., 2012. Induction of apo- ptosis by sinulariolide from soft coral through mitochondrial- related and p38MAPK pathways on human bladder carcino- ma cells., 10 (12): 2893-2911.
Nishino, H., 1995. Cancer chemoprevention by natural carotenoids and their related compounds., 22: 231-235.
Odia, Y., Iwamoto, F. M., Moustakas, A., Fraum, T. J., Salgado, C. A., Li, A., Kreisl, T. N., Sul, J., Butman, J. A., and Fine, H. A., 2016. A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas., 127 (1): 127-135.
Oh, Y., Herbst, R. S., Burris, H., Cleverly, A., Musib, L., Lahn, M., and Bepler, G., 2008. Enzastaurin, an oral serine/threonine ki- nase inhibitor, as second- or third-line therapy of non-small- cell lung cancer., 26 (7): 1135- 1141.
Omura, S., Iwai, Y., Hirano, A., Nakagawa, A., Awaya, J., Tsu- chya, H., Takahashi, Y., and Masuma, R., 1977. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization., 30 (4): 275-282.
Öztaşkın, N., Çetinkaya, Y., Taslimi, P., Göksu, S., and Gülçin, İ., 2015. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives., 60: 49-57.
Park, H. Y., Park, S. H., Jeong, J. W., Yoon, D., Han, M. H., Lee, D. S., Choi, G., Yim, M. J., Lee, J. M., Kim, D. H., Kim, G. Y., Choi, I. W., Kim, S., Kim, H. S., Cha, H. J., and Choi, Y. H., 2017. Induction of p53-independent apoptosis and G1 cell cy- cle arrest by fucoidan in HCT116 human colorectal carcino- ma cells., 15 (6): 154.
Pimentel-Elardo, S. M., Kozytska, S., Bugni, T. S., Ireland, C. M., Moll, H., and Hentschel, U., 2010. Anti-parasitic compounds fromsp. strains isolated from mediterranean sponges., 8 (2): 373-380.
Pollet, M., Krutmann, J., and Haarmann-Stemmann, T., 2018. Commentary: Usage of mitogen-activated protein kinase small molecule inhibitors: More than just inhibition!, 9: 935, https://doi.org/10.3389/fphar.2018.00098.
Robertson, M. J., Kahl, B. S., Vose, J. M., de Vos, S., Laughlin, M., Flynn, P. J., Rowland, K., Cruz, J. C., Goldberg, S. L., Mu- sib, L., Darstein, C., Enas, N., Kutok, J. L., Aster, J. C., Neuberg, D., Savage, K. J., LaCasce, A., Thornton, D., Slapak, C. A., and Shipp, M. A., 2007. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or re- fractory diffuse large B-cell lymphoma., 25 (13): 1741-1746.
Roll, D. M., Ireland, C. M., Lu, H. S. M., and Clardy, J., 1988. Fascaplysin, an unusual antimicrobial pigment from the marine spongesp., 53 (14): 3276-3278.
Satomi, Y., 2012. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human pro- state cancer cells., 32 (3): 807-813.
Satomi, Y., 2017. Antitumor and cancer-preventative function of fucoxanthin: A marine carotenoid., 37 (4): 1557-1562.
Satomi, Y., and Nishino, H., 2009. Implication of mitogen-ac- tivated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells., 1790 (4): 260- 266.
Sharma, S., Guru, S. K., Manda, S., Kumar, A., Mintoo, M. J.,Prasad, V. D., Sharma, P. R., Mondhe, D. M., Bharate, S. B., and Bhushan, S., 2017. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade., 275: 47-60.
Song, J. W., and Chung, K. C., 2010. Observational studies: Co- hort and case-control studies., 126 (6): 2234-2242.
Tanai, C., Yamamoto, N., Ohe, Y., Takahashi, T., Kunitoh, H., Mu- rakami, H., Yamamoto, N., Nakamura, Y., Nokihara, H., Shu- kuya, T., Baldwin, J. R., Koshiji, M., and Tamura, T., 2010. A phase I study of enzastaurin combined with pemetrexed in advanced non-small cell lung cancer., 5 (7): 1068-1074.
Tanaka, T., Shnimizu, M., and Moriwaki, H., 2012. Cancer che- moprevention by carotenoids., 17 (3): 3202-3242.
Tang, S. A., Zhou, Q., Guo, W. Z., Qiu, Y., Wang, R., Jin, M., Zhang, W., Li, K., Yamori, T., Dan, S., and Kong, D., 2014.antitumor activity of stellettin B, a triterpene from marine sponge, on human glioblastoma cancer SF295 cells., 12 (7): 4200-4213.
Wang, B. G., Gloer, J. B., Ji, N. Y., and Zhao, J. C., 2013. Halogenated organic molecules of rhodomelaceae origin: Chemistry and biology., 113 (5): 3632-3685.
Wang, E., Sorolla, M. A., Gopal Krishnan, P. D., and Sorolla, A., 2020. From seabed to bedside: A review on promising marine anticancer compounds., 10 (2): 248.
Wang, J., Chen, S., Xu, S., Yu, X., Ma, D., Hu, X., and Cao, X., 2012.induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR sig- naling in sarcoma 180 (S180) xenografts-bearing mice., 10 (9): 2055-2068.
Wang, L. J., Wang, S. Y., Jiang, B., Wu, N., Li, X. Q., Wang, B. C., Luo, J., Yang, M., Jin, S. H., and Shi, D. Y., 2015. Design, synthesis and biological evaluation of novel bromophenol derivatives incorporating indolin-2-one moiety as potential anti- cancer agents., 13 (2): 806-823.
Willis, O., Choucair, K., Alloghbi, A., Stanbery, L., Mowat, R., Charles Brunicardi, F., Dworkin, L., and Nemunaitis, J., 2020.gene aberrancy and role in targeted therapy of solid malignancies., 27: 634-644.
Wu, H. L., Fu, X. Y., Cao, W. Q., Xiang, W. Z., Hou, Y. J., Ma, J. K., Wang, Y., and Fan, C. D., 2019. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-me- diated oxidative damage and regulation of MAPKs and PI3K- AKT pathways., 67 (8): 2212-2219.
Wu, J., Cang, S., Liu, C., Ochiai, W., and Chiao, J. W., 2020. Development of human prostate cancer stem cells involves epigenomic alteration and PI3K/AKT pathway activation., 9: DOI: 10.1186/s40164- 020-00168-0.
Wu, Y. J., Neoh, C. A., Tsao, C. Y., Su, J. H., and Li, H. H., 2015. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloprotei- nase-2/-9 through MAPKs and PI3K/Akt signaling pathways., 16 (7): 16469- 16482.
Xue, M., Ji, X., Xue, C., Liang, H., Ge, Y., He, X., Zhang, L., Bian, K., and Zhang, L., 2017. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathwayand., 94: 898-908.
Yajima, I., Kumasaka, M. Y., Thang, N. D., Goto, Y., Takeda, K., Yamanoshita, O., Iida, M., Ohgami, N., Tamura, H., Kawamoto, Y., and Kato, M., 2012. RAS/RAF/MEK/ERK and PI3K/ PTEN/AKT signaling in malignant melanoma progression and therapy., 2012 (1687- 6015): 354191.
Yamamoto, K., Ishikawa, C., Katano, H., Yasumoto, T., and Mo- ri, N., 2011. Fucoxanthin and its deacetylated product, fuco- xanthinol, induce apoptosis of primary effusion lymphomas., 300 (2): 225-234.
Yamasaki-Miyamoto, Y., Yamasaki, M., Tachibana, H., and Yamada, K., 2009. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells., 57 (18): 8677-8682.
Yang, L., Wang, P., Wang, H., Li, Q., Teng, H., Liu, Z., Yang, W., Hou, L., and Zou, X., 2013. Fucoidan derived frominduces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway., 11 (6): 1961-1976.
Yang, W. J., Yang, C. S., Huang, C. J., Chen, K. S., and Lin, S. F., 2012. Bostrycin, a novel coupling agent for protein immobilization and prevention of biomaterial-centered infection produced bysp. No. 407., 50 (6-7): 287-292.
Ye, G., Lu, Q., Zhao, W., Du, D., Jin, L., and Liu, Y., 2014. Fu- coxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway., 35 (11): 11261-11267.
Zheng, L., Xu, Y., Lin, X., Yuan, Z., Liu, M., Cao, S., Zhang, F., and Linhardt, R. J., 2018. Recent progress of marine polypeptides as anticancer agents., 13 (4): 445-454.
June 16, 2020;
July 3, 2020;
December 25, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
# The two authors contributed equally to this work.
. E-mail: xiukunlin@126.com
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Case Study of a Short-Term Wave Energy Forecasting Scheme:North Indian Ocean
- Temporal and Spatial Characteristics of Wave Energy Resources in Sri Lankan Waters over the Past 30 Years
- Vibration Deformation Monitoring of Offshore Wind Turbines Based on GBIR
- Dependence of Estimating Whitecap Coverage on Currents and Swells
- The Variation of Microbial (Methanotroph) Communities in Marine Sediments Due to Aerobic Oxidation of Hydrocarbons
- 3-Aminopropyltriethoxysilane Complexation with Iron Ion Modified Anode in Marine Sediment Microbial Fuel Cells with Enhanced Electrochemical Performance
