Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities,and Potential Applications
2021-06-25HUANGYiJIANGHongMAOXiangzhaoandCIFangfang
HUANG Yi, JIANG Hong, *, MAO Xiangzhao, 2), *, and CI Fangfang
Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities,and Potential Applications
HUANG Yi1), JIANG Hong1), *, MAO Xiangzhao1), 2), *, and CI Fangfang1)
1),,66003,2),266237,
Brown algae is one of the three major types of marine algae and includes approximately 2000 species. It is widely dis- tributed in various seas around the world. Brown algae contain a plethora of active substances, such as polysaccharides, polyphe- nols, omega-3 fatty acids, and carotenoids. Laminarin, a type of storage carbohydrate found abundantly in brown algae, is mainly formed by glucose monomers linked by β-1,3-glucosidic bonds and partial β-1,6-glucosidic bonds. Laminarin and laminarin oligo- saccharides, which contain 2–10 saccharide units, have extensive biological activities, such as antitumor, antioxidant, anti-inflam- matory, and prebiotic properties. Moreover, both laminarin and laminarin oligosaccharides can be considered as ideal substrates for bioethanol production because they are composed of abundant glucose residues. Therefore, brownalgae-derived laminarin and lami- narin oligosaccharides have various potential applications in the food, medicine, cosmetics, and bioenergy fields. This paper reviews the preparation methods of laminarin and laminarin oligosaccharides, as well as their biological activities and potential applications.
brown algae; laminarin; laminarin oligosaccharides; biological activities; potential applications
1 Introduction
Brown algae is widely distributed throughout various seas around the world.At present, there are approximate- ly 2000 species according to statistical reports (Thompson., 2019). It is well known for its richness in bioactive compounds, such as polysaccharides, polyphenols, omega- 3 fatty acids, and carotenoids, which have considerable economic values(Wijesekara., 2011).Laminarin, the storage carbohydrate in brown algae, is also known as brown algal starch and is mainly formed by glucose mo- nomers connected by β-1,3-glucosidic bonds on its back- bone and partial β-1,6-glucosidic bonds on its branches (Kadam., 2015). It belongs to the family of β-glu- cans because of its single structure and connection me- thod (Rioux., 2010). The laminarin content of brown algae is related to the species, growth environment, and harvest season. Generally,,, andspp. are the main sources of laminarin, and their laminarin content can be up to 62% of total dry weight at the highest. It has been reported that during the summer and autumn, laminarin accumulates and its content inincrease, while during the winter, the con-tent decreases due to the consumption of energy for new tissue growth (Adams., 2011). As a kind of func- tional marine algal polysaccharide, laminarin possesses a wide range of biological activities, including antitumor, antioxidant, anti-inflammatory, prebiotic, and other bio-functional activities (Kim., 2006;Kadam., 2015). In addition, it also plays an important role in the marine carbon cycle (Becker., 2020). As a relatively underexploited algal polysaccharide, laminarin also can be trans- formed into bioethanol through the bioprocess of fermen- tation (Lee and Lee, 2016). Therefore, laminarin possess an array of important applications in the fields of food, medicine, cosmetics, and energy.
Marine algal oligosaccharides can be obtained from marine algal polysaccharides through physical or chemi- cal and enzymatic methods. Currently, marine algal oli- gosaccharides, such as alginate oligosaccharides, agar oli- gosaccharides, and carrageenan oligosaccharides, have re- ceived increasing attention due to their excellent solubi- lity, bioavailability and prominent biological activities. Si- milarly, laminarin oligosaccharides, the degradation pro- ducts of laminarin, can also be prepared by these strate- gies of physical hydrolysis, chemical hydrolysis, and en- zymatic degradation. It has been reported that laminarin oligosaccharides have a significant effect on improving immunity and antitumor activity, and regulating the intes- tinal flora (Yvin., 1999; Kim., 2006; Kadam., 2015).
The present article reviews the preparation strategies, biofunctional activities, and potential applications of la- minarin and laminarin oligosaccharides.
2 Preparation of Laminarin
The process of laminarin preparation can be divided into pre-treatment of raw materials, extraction, and purifica- tion. Generally, the raw materials need to be washed se- veral times to remove epiphytes and sands, and dried thoroughly (Imbs., 2016). Traditionally, laminarin is extracted from dried brown algae with high temperature and mild acidic conditions. Nowadays, some environment- friendly green methods, including enzymatic extraction and microwave extraction, can also be exploited for lami- narin preparation.
2.1 Traditional Laminarin Extraction Strategy
During the traditional solvent extraction process, the ap- propriate concentration of ethanol can precipitate lamina- rin and separate some impurities. After ultrafiltration and dialysis, laminarin can be isolated(O’Shea., 2014). Most laminarin extraction methods use mild acidic or ba- sic solvents. The type of acid solution and extraction tem- perature can affect the extraction efficiency of laminarin. Devillé. (2004) investigated the extraction of lami- narin by different acid solutions. The results showed thatHCL produces a higher extraction yield than H2SO4. Mean- while, a high temperature can facilitate laminarin extrac- tion and improve the yield. In fact, a high temperature was beneficial to the extraction of laminarin. Zha.(2012) extracted laminarin fromat 4, 20, 40, 60, and 80℃ using water as the extraction solvent. The results showed that when the temperature was in- creased from 4 to 60℃, there was a corresponding in- crease in the quantity of laminarin. When the temperature was increased sequentially, the total sugar amount re- mained unchanged, and some extracted laminarin was de- graded into laminarin oligosaccharides. Following the ini- tial extraction, the obtained solution contained mixed poly- saccharides such as laminarin, fucoidan, and alginate. CaCl2or MnCl2can be added to the solution to eliminate thealginate because alginate exhibits gel properties in the Ca2+or Mn2+solution (Cong., 2016). Rioux. (2010)used CaCl2solution as a solvent to extract laminarin at 85℃ for 4h. The same volume of sodium chloride solution (2%) and twice the volume of anhydrous ethanol were added to the extraction solution to precipitate the laminarin for 1h at room temperature. Finally, laminarin can be further purified from the crude extract following the purification procedures described below (Cong., 2016).
2.2 Innovative Extraction Strategy of Laminarin
In addition to the traditional extraction methods, some environmentally friendly and efficient extraction methods, such as enzyme-assisted extraction (EAE), microwave- assisted extraction (MAE), and ultrasound-assisted ex- traction (UAE), have been used for the gradual extraction of laminarin (Chen., 2013).
EAE is a promising extraction strategy (Zhu., 2014). Charoensiddhi. (2016) used three commercial cell wall carbohydrate hydrolytic enzymes and three proteases to assist in the extraction of laminarin from the brown seaweedat 50℃ for 24h. During this process, the cell walls of the algae were broken up, and the polysaccharides containing laminarin were released. Subsequently, lyophilization and fractionation were em- ployed for the isolation and purification of laminarin, and the molecular weight (Mw) of the purified laminarin was less than 65kDa.
Microwaves have good penetrability and are effective for the extraction of active, heat sensitive substances (Pap., 2013). It was found that the amount of laminarin extracted increased with increasing microwave power du- ring the MAE process (Gao., 2006). However, when the microwave power exceeded 400W, the amount of la- minarin extracted gradually decreased. It was speculated that excessive power can cause the degradation of lami- narin. Therefore, it is necessary to control the power level in the MAE process to keep the yield and activity of la- minarin. Many algal polysaccharides have been extracted with MAE up to now, such as fucoidan from,, and(Quitain., 2013). Therefore, MAE shows good pros- pects for application in laminarin extraction.
UAE (ultrasound-assisted extraction) is another cost-ef- fective laminarin extraction technology with great poten- tial for large-scale industrialization (García-Vaquero., 2017). In addition to polysaccharide extraction, UAE has been used to extract functional components such as phy- coerythrin, amino acids, and lipids from a variety of algae sources (Adam., 2012). Kadam. (2015) used UAE with an ultrasonic power amplitude of 69% to ex- tract laminarin from. The extracts were treated with 0.1molL−1HCl for 15min followed by solid-liquid extraction. Finally, the amount of laminarin ob- tained was estimated to be 5.82% of the dry weight of. The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) ra- dical-scavenging activity of the extracted laminarin reach- ed as high as 93.23%, demonstrating a good antioxidant activity.
These new extraction strategies lay an industrial foundation for the efficient extraction of polysaccharides and other active substances from marine algae. A comparison of these extraction strategies is outlined in Table 1.
2.3 Purification of Laminarin
In order to remove impurities such as other polysaccha- rides, proteins, and phenolic compounds from the crude laminarin extract, it is necessary to further purify lami- narin (Ale., 2011). In fact, the procedures of lami- narin extraction and laminarin purification are sometimes not strictly distinguishable, because some extraction pro- cesses include certain purification steps. For example, the fractional precipitation of laminarin described in the extraction section can also be considered as a step of laminarin purification. The laminarin purification process de- scribed in this section mainly refers to the next step of separation and purification of pure laminarin to detect its biological activity. At present, the common methods ofpure laminarin purification include size-exclusion chro- matography (SEC) and affinity chromatography (AC).
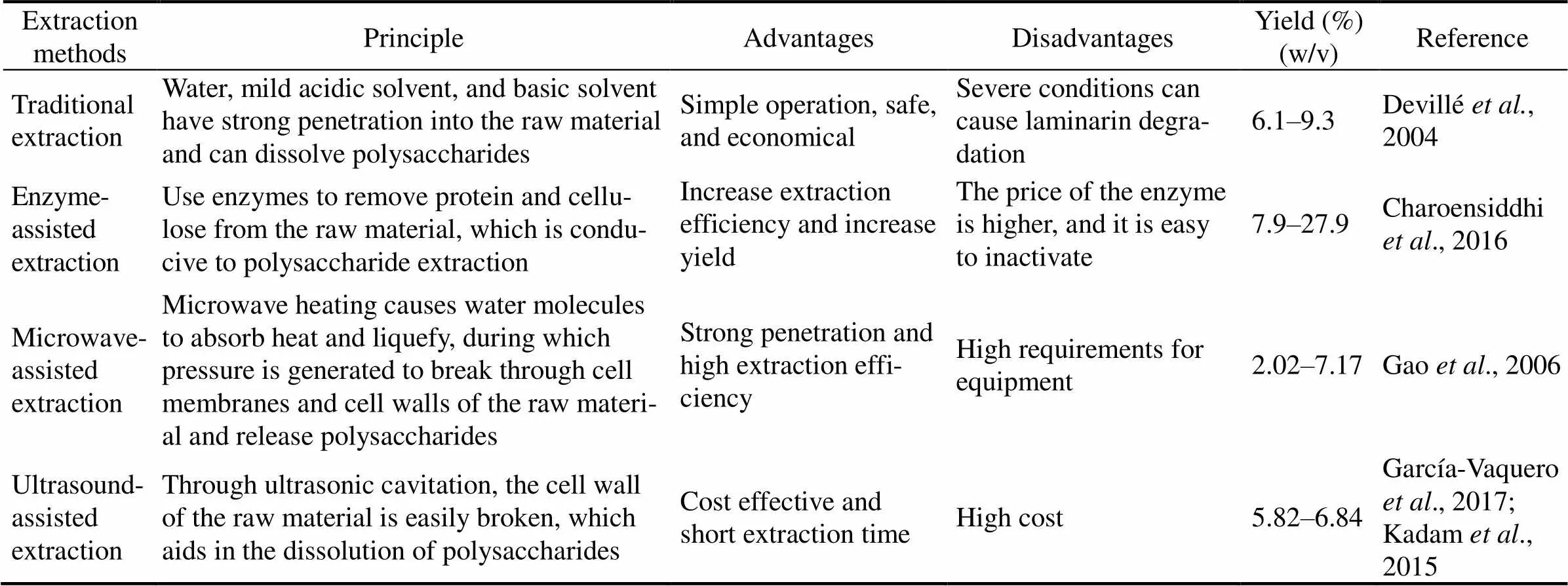
Table 1 Comparison of several strategies for extracting laminarin
SEC can be used to separate and purifypolysaccha- rides according to their molecular weights (Mw). Thus, SEC is an efficient and convenient method for separating and purifying laminarin. Zhang.(2015) used this me- thod with a Waters UltrahydrogelTM WATO 11530 size- exclusion column (300mm×7.8mm) to separate algal poly- saccharides and obtained 383.8mgg−1of laminarin, which showed a good purification effect. In addition, SEC can be used as an effective method to determine the Mw of polysaccharides (Gaborieau and Castignolles, 2011).
AC (affinity chromatography) is a powerful method to separate, purify, and analyze target compounds in a sam- ple (Pohleven., 2012). Affinity columns can be used alone or together with other purification methods to pu- rify laminarin (Hirabayashi., 2002). At present, AC has been used to successfully separate a variety of active substances, such as proteins, enzymes, antibodies, and poly- saccharides (Hahn., 2016). This method is common- ly used in the purification of sulfated polysaccharides (Mak., 2013). Labourel. (2015) combined both AC and SEC for the analysis of enzymatic products of laminarin and found that the laminarinase ZgLamCGH16 preferentially hydrolyzed branched laminarin.
SEC can be combined with many purification methods for further separation and purification of the target products. AC is mainly used to separate biomacromolecules such as proteins according to their different chemical struc- tures. SEC and AC have been applied successfully in the purification of many polysaccharides (García-Vaquero., 2017). However, high cost remains the main factor limit- ing their large-scale application. In addition to the above chromatography methods, there are other purification me- thods, such as ultrafiltration and membrane filtration(Oda., 2016). Ultrafiltration and membrane filtration can be used to separate diverse solutions and are suitable for polysaccharide purification on an industrial scale, but the product purity is relatively low. Therefore, the appropriate purification methods are usually selected according to ac- tual needs.
These methods can be chosen to further characterize the biological activity of laminarin. The processes of lami- narin extraction and purification are presented in Fig.1.
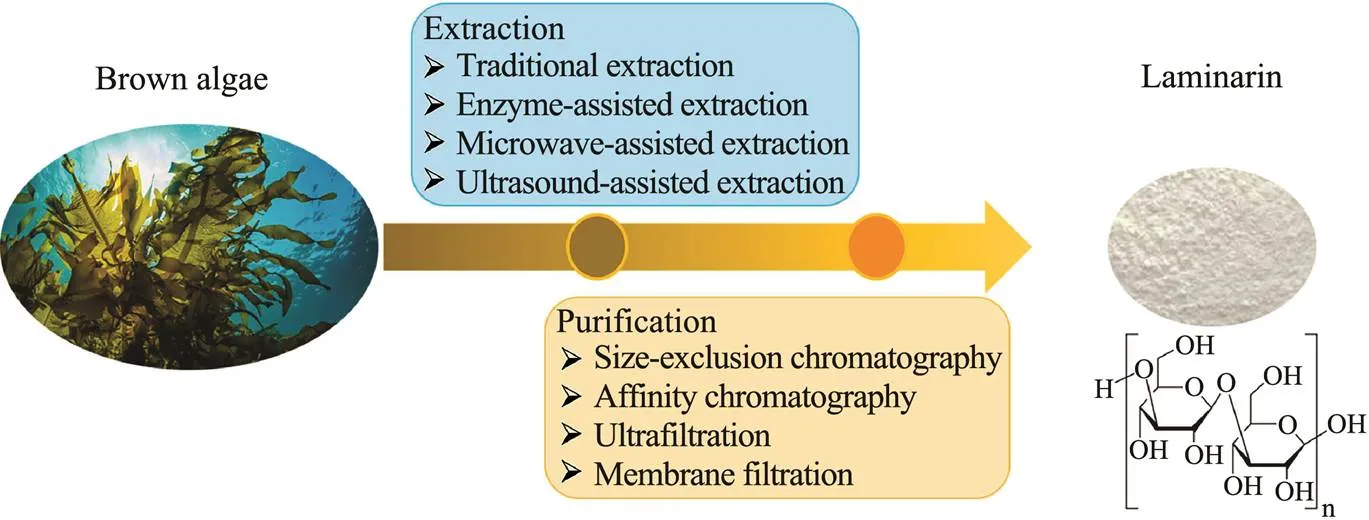
Fig.1 Laminarin extraction and purification processes.
3 Preparation of Laminarin Oligosaccharides
In general, laminarin oligosaccharides can be prepared by depolymerization of laminarin (chemical, physical, and enzymatic hydrolysis) (Iji and Tivey, 1998). Furthermore, laminarin oligosaccharides can also be produced by syn- thesis from disaccharide or monosaccharide substrates.
3.1 Laminarin Oligosaccharide Preparation by Chemical and Physical Hydrolysis
Many algal polysaccharides, such as fucoidan, carra- geenan, and agarose, can be degraded into oligosaccha- rides by acidic hydrolysis at high temperatures due to the cleavage of glycosidic bonds (Chen., 2005). Lamina- rin oligosaccharides can also be produced by partial acid hydrolysis and then separated by size-fractionation and re-versed-phase high-performance liquid chromatography (Na-tsuka., 2018). As an effective method of degradingpolysaccharides, acid hydrolysis can help us to understand the specific structure of polysaccharides. Graiff. (2016) explored the preparation of laminarin oligosaccharides by using 0.5molL−1H2SO4to hydrolyze commercial lami- narin. The hydrolysis temperature was 121℃, and the ion- exclusion chromatography results showed that when the hydrolysis time was 5min, there were oligosaccharides in the hydrolytic products. When the hydrolysis time was ex-tended to 180min, the chromatogram showed only glucose and sulfuric acid without oligosaccharides in the products (Renard., 1997).
Radiolysis, such as ultraviolet light and gamma rays, can induce the degradation of polysaccharides by breaking gly- cosidic bonds and is the main physical treatment for oligo- saccharide preparation (Ramani and Ranganathaiah, 2000).Gamma irradiation has been applied to the acquisition of oligosaccharides (Nagasawa., 2000). Choi. (2011) used gamma irradiation to obtain laminarin oligosaccha- rides, and the results of Nuclear Magnetic Resonance (NMR) analysis indicated that the glycosidic bonds of laminarin were randomly broken. The antioxidant activity of the pre- pared laminarin oligosaccharides was higher than that of laminarin. Unlike chemical methods, physical hydrolysis for laminarin oligosaccharide production is eco-friendly. However, laminarin oligosaccharides with the desired de- gree of polymerization (DP) cannot be produced by phy- sical irradiation of polysaccharides, and long exposure times to physical radiation may cause damage to the structures of oligosaccharides (Delattre., 2005).
3.2 Laminarin Oligosaccharide Preparation by Enzymatic Degradation
Some enzymes can efficiently and specifically hydrolyze laminarin into laminarin oligosaccharides under mild con- ditions. Enzymes that can specifically hydrolyze lamina- rin include endo-β-1,3-glucanase (laminarinase, EC 3.2.1.39)and exo-β-1,3-glucanase (EC 3.2.1.58) (Santos., 1979).Laminarinase can randomly cleave (1→3)-β-linkages with- in laminarin glycoside bonds to release oligosaccharides, while exo-β-1,3-glucanases can hydrolyze laminarin by se- quentially cleaving glucose residues from the non-redu- cing end and release glucose (Bara., 2003). There are many reports about laminarinases from bacteria, fungi, high-er plants, and archaea isolated from soil and marine envi- ronments (Tschiggerl., 2008). These glycoside hydro- lases can be used not only to prepare oligosaccharides,but also to accurately quantify laminarin in marine organic matter. Becker. (2017) used laminarinase to digest glycans selectively and quantify laminarin in particulate or- ganic matter.
The main sources of laminarinase include bacteria (such as,,sp.,, and), fungi (such as,, and), and algae (such as) (Sandini., 2007; Li., 2009; Kim., 2011; Kusaykin., 2017). According to sequence in- formation, endo-β-1,3-glucanases can be grouped into six glycoside hydrolase (GH) families including GH16, GH17, GH55, GH64, GH81, and GH128 in the CAZy database (http://www.cazy.org/) (Sakamoto., 2011). Similarly, exo-β-1,3-glucanases can be classified into GH3, GH5, GH17, GH55, and GH132 in the CAZy database (Kumar., 2018). Based on amino acid sequence divergence, most laminarinases derived from bacteria are categorized into GH16, while laminarinases originating from plants are all assigned to GH17 (Sandini., 2007). Diverse lami- narinases have been employed for the preparation of la- minarin oligosaccharides. The characteristics and hydro- lysis products of laminarinases from different GH fami- lies are listed in Table 2.
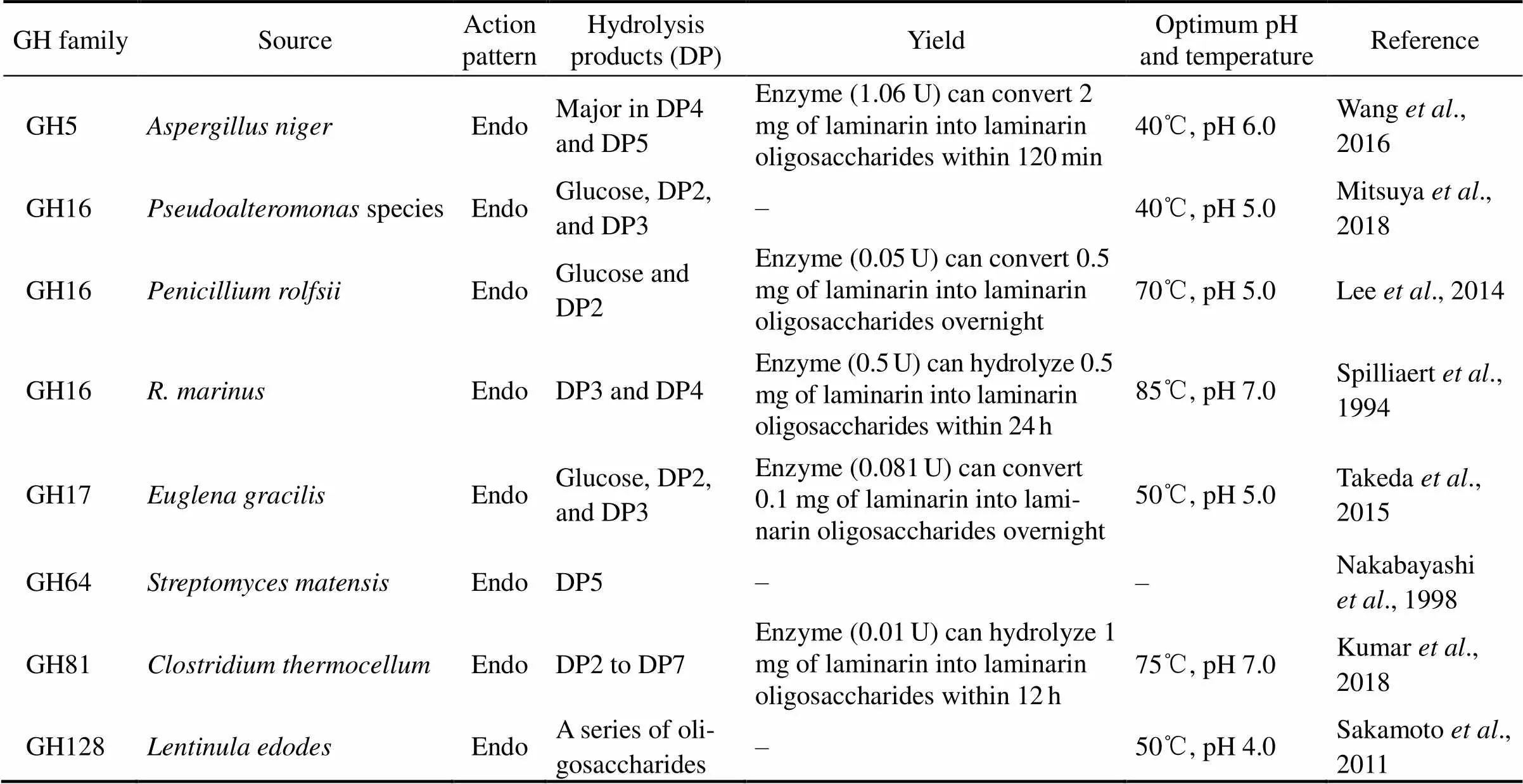
Table 2 Action patterns, hydrolysis products, optimum pH, and temperature of laminarinases
Most of the characterized laminarinasesbelonging to the GH16 family have conserved sequences. For example, the conserved sequences of the laminarinase from the ma- rine bacteriumKMM 3553 include the se- quences WPAXWXL (substrate binding site) and EIDXXE (catalytic active site) (Kusaykin., 2017). Until now, crystal structures of different laminarinases from the GH16 family have been analyzed, and their crystal structures share a β-jelly-roll fold, and the inner β-fold bends out- ward to form a long catalytic groove (Dong., 2015) (Fig.2A). The laminarinase fromc3-2(1) IBRL be- longs to the GH16 family, and it is a thermostable lamina- rinase that can hydrolyze laminarin to laminaribiose and glucose at 70℃ (Lee., 2014). The heat resistance of this laminarinase and its product characteristics indicate its application in the preparation of laminarin oligosaccha- rides.Recently, Badur. (2020) reported three lamina- rinases from1C10belong to the GH16 family. As a member of these laminarinases, laminarinase VbGH16C can hydrolyze laminarin to oligosaccharides of DP8 and DP9. This characteristic of the hydrolysate can be applied in the preparation of relatively large laminarin oligosaccharides.
The laminarinases derived from plants that have been reported so far belong to GH17 (Badur., 2020). Their crystal structures have a typical (roll) 8 TIM-barrel struc- ture, which consists of eight α-helices and eight β-sheets. Laminarinases of GH17 share many conserved sequences, which encode many α-helices, η-helices, β-sheets, and strict β-turns (Ezzine., 2016). Receveur-Bréchot.(2006)revealed a crystal structure at 1.45-Å resolution of the β-1,3-glucanase Ban-Gluc from banana.Its three-di- mensional structure consists of an internal crown of eight β-strands connected by extended loops to an outer crown of eight α-helices, which exhibit the typical (α/β) 8 TIM- barrel motif (Fig.2B). There are a few reports on these la- minarinases about the preparation of laminarin oligosac- charides, some of which even lack the degradation activi- ty of laminarin (Menu-Bouaouiche., 2003). Recently, the laminarinase VbGH17A belonging to GH17 family was identified from1C10 by Badur. (2020). It can hydrolyze laminarin into a series of laminarin oli- gosaccharides (DP4-DP9). In contrast, complex laminarin oligosaccharide products with different DP may cause dif- ficulties in the separation and purification of laminarin oligosaccharides at a later stage, which is not conducive to the production of laminarin oligosaccharides.
Compared with laminarinases belonging to GH16 and GH17, there have been fewer studies on laminarinases of GH55, GH64, and GH81. Until now, only the crystal struc- tures of laminarinases belonging to GH64 and GH81 have been reported (Ezzine., 2016; Badur., 2020).

Fig.2 Three-dimensional structures of laminarinases, including laminarinase ZgLamC of the GH16 family (PDB: 4CTE), laminarinase of the GH17 family (PDB: 2CYG), laminarinase LPHase of the GH64 family (PDB: 3GD9), and laminarinase ZgLamC of the GH81 family (PDB: 4K35).
Wu. (2009) revealed the essential amino acid re- sidues and crystal structure at 1.80Å resolution of the la- minarinase LPHase of GH64 fromDIC-108. Its conserved sequences include two strictly conserved car- boxylates (Glu154 and Asp170) and several saccharide- linked residues (Thr156, Thr167, Trp163, Asn165, andVal169). The LPHase structure consists of a barrel do- main and a mixed (α/β) domain, and its main hydrolysateis laminaripentaose (Fig.2C). The homogeneous product of enzymatic hydrolysis is conducive to the preparation of laminarin oligosaccharides.
Zhou.(2013) reported essential residues and a crys- tal structure to resolutions of 2.3 and 2.0Å of the lami- narinase as a member of GH81 from. The conserved amino acid residues (251–343 aa) may be involved in the stabilization of the whole structure. The overall structure of the laminarinase mainly consists of a β-sandwich domain and a C-terminal (α/α)6domain (as shown Fig.2D). Kumar. (2018) reported a thermosta- ble laminarinase belonging to GH81 from, and its hydrolysatesare a series of oligosaccharides (DP2 to DP7).
3.3 Laminarin Oligosaccharide Preparation by Synthesis Strategies
Several algal oligosaccharides, such as alginate oligo- saccharides and fucose oligosaccharides, can be produced by organic synthesis and biosynthesis(Mong., 2003). These synthesis strategies have also been applied in the preparation of laminarin oligosaccharides. He. (2003) reported an organic synthesis strategy. He used a 4,6-O- benzylidenated acceptor for β-(1→3) bond formation to avoid the predominant generation of α glycosides. Even- tually, a homogeneous laminarin oligosaccharide (DP4) with α-(1→3) and α-(1→6) bonds was prepared, and the yield was up to 43%. However, the operational steps were cumbersome and costly, and the synthesis of oligosaccha- rides was accompanied by the introduction of other gly- cosidic bonds. In contrast, the biosynthesis of laminarin oligosaccharides can be accomplished with other methods at a low cost. Sun. (2019) designed anmulti- enzyme catalytic system consisting of α-glucan phospho- rylase and laminaribiose phosphorylase. This catalytic sys- tem could convert low-value starch and glucose into high- value laminaribiose, which can be used to synthesize hy- aluronic acid.
In contrast to the degradation of polysaccharides, oli- gosaccharide synthesis is based on oligosaccharides withsmaller Mw (Boons, 1996). However, there are only a few studies on the biosynthesis of laminarin oligosaccharides, and only a few laminarin oligosaccharides with a specific DP can be synthesized. Table 3 presents strategies for la- minarin oligosaccharide preparation, specific methods, pro- duction, advantages, and disadvantages of each strategy.

Table 3 Strategies for laminarin oligosaccharides preparation, specific methods, production,advantages and disadvantages of each strategy
4 Health Beneficial Effects and Potential Applications of Laminarin and LaminarinOligosaccharides
Laminarin and laminarin oligosaccharides have been widely reported for their biological functions. Like other algal polysaccharides, laminarin has prebiotic, antioxidant, and anti-inflammatory activities (Kim., 2006; Kadam., 2015). In addition, due to its unique triple helical structure, laminarin also exhibits excellent antitumor and anticancer activities, which can be applied to drug deve- lopment (Novak and Vetvicka, 2008). The biological ac- tivities of laminarin and laminarin oligosaccharides are considered to depend on their molecular structure, such as the DP, the introduction of sulfate groups, and the side chain branches (Pang., 2005; Menshova., 2014; Zargarzadeh., 2020). Therefore, appropriate structu- ral modification of laminarin can significantly improve its biological activities.
4.1 Antitumor and Anticancer Activities
To date, many studies have shown that laminarin and la- minarin oligosaccharides have significant antitumor and an- ticancer activities (Park., 2012). The mechanisms of antitumor and anticancer activities of laminarin and lami- narin oligosaccharides include apoptosis and inhibition of cancer cell colony formation (Zargarzadeh., 2020). For instance, the capacity of laminarin to induce apoptosis in HT-29 colon cancer cells was investigated by Park.(2013). The results demonstrated that laminarin extracted fromcould not only induce apoptosis in HT-29 colon cancer cells through an apoptotic pathway involving growth factors, but also regulate the ErbB sig- naling pathway. Ji.(2012) used different concentra- tions of laminarin to treat human colon cancer LOVO cells at different times. The intracellular reactive oxygen spe- cies (ROS), pH, intracellular calcium ion concentration, mitochondrion permeability transition pore, mitochon- drial membrane potential, and expression levels of Cyt-C, Caspase-9, and Caspase-3 were detected and analyzed. It was found that laminarin could induce human colon can- cer LOVO cell apoptosis through a mitochondrial path- way. Subsequently, the relationship between laminarin-in- duced apoptosis and the death receptor-mediated (DR-me-diated) pathway in human colon cancer LOVO cells was il- luminated by Ji and Ji(2014). The structural characteristics and antitumor activity of laminarin fromwere determined and analyzed by Ermakova. (2013). Laminarin exhibited no direct cytotoxicity and showed sig- nificant antitumor activity against SK-MEL-28 human me- lanoma cells.
Similarly, Usoltseva.(2016) also found that lami- narin extracted fromandhad no cytotoxicityandcould effectively inhibit colony formation in HT-29 cells. Tian. (2020) elucidated the anticancer effect of laminarin fromon Human HCC cell lines (Bel-7404 and HepG2). The results reveal- ed that laminarin could inhibit the proliferation of Bel- 7404 and HepG2 cells in a dose-dependent manner, and the expression levels of SMP-30 in laminarin-treated cells were lower than those in the control LO2 cells. Thus, it is speculated that laminarincan inhibit cancer cells by regu- lating the expression levels of SMP-30.
Laminarin oligosaccharides also showed obvious anti- cancer activity (Pang., 2005; Menshova., 2014). The human tissue lymphoma cell line (U937 cells) was treated with the hydrolytic products of laminarin oligo- saccharides LB and LI, respectively (Pang., 2005). The results suggested that LB can inhibit the proliferation of U937 cells by stimulating monocytes to produce cyto- kines. Moreover, it was revealed that specific enzymatic products (laminarin oligosaccharides with DP 9-23) with a high content of 1,6-linked glucose residues showed sig- nificant anticancer activity, and they could inhibit colony formation in melanoma and colon cancer cells (Menshova., 2014). This also provides new ideas to produce la- minarin products with high antitumor activity through en- zymatic hydrolysis.
Additionally, laminarin can be modified to enhance its antitumor and anticancer activities. Ji. (2013)appli- ed the chlorosulfonic acid-pyridine method for laminarin- sulfated modification and obtained a sulfated laminarin (LAMS) with a sulfate content of 45.92%. The main sul- fate substitution site was at the hydroxyl groups of C2 and C6. The MTT assay (MTT) results showed that LAMS hadmore obvious inhibition effects than laminarin on LoVo cell growth, which suggested that LAMS had better antitumor activity. It was hypothesized that the introduction of sul- fate groups can not only change the molecular structure and spatial conformation of laminarin, but also enhance its an- ion repulsion to stretch the sugar chains and form hy- drogen bonds. These changes lead to the formation of a helical structure and adoption of an active conformation, which enhances the antitumor activity of laminarin (Zar- garzadeh., 2020). This means that some specific struc- tural modifications such as sulfated modification is an ef- fective method to enhance the antitumor activity of lami- narin. Nanoparticle modification of polysaccharide struc- ture is another strategy to improve its physical and che- mical properties. Laminarin decorated with selenium nano- particles (LP-SeNPs) was prepared by Cui.(2019). Laminarin with nano modification can induce mitochon- dria-mediated apoptosis of tumor cells (HepG2 cells) by promoting the expression of Bax and decreasing the ex- pression of Bcl-2.
4.2 Prebiotic Activity
It has been well documented that many algal polysac- charides and their functional oligosaccharides play an im- portant role in regulating human intestinal health, which is considered as a prebiotic activity (Ramnani., 2012).The prebiotic activities of laminarin and laminarin oligo- saccharides have also aroused the interest of many scho- lars. It was confirmed that neitherhydrochloric acid under physiological conditions norhomoge- nates of the human digestive system can hydrolyze lami- narin and laminarin oligosaccharides (Walsh., 2013; Kadam., 2015). Laminarin and laminarin oligosac- charides are resistant to hydrolytic enzymes in the human upper gastrointestinal tract (Devillé., 2004). How- ever, the intestinal microflora can utilize laminarin and la- minarin oligosaccharides (Devillé., 2007). Therefore, laminarin and laminarin oligosaccharides cannot be de- graded and absorbed by host.Thus they can become pre- bioticspotentially. This beneficial effects of laminarin on intestinal microorganisms were demonstrated by Nguyen.(2016). With the supplementation of laminarin in the diets of mice, a significant increase inand a significant decrease inwere observed. The re- sults suggested that laminarin could enhance the high en- ergy metabolism of the gut microbiota to reduce the side effects of a high-fat diet. Zaporozhets. (2014) pro- posed the prebiotic properties of laminarin. Moreover, it was shown that laminarin could regulate intestinal meta- bolismits effect on mucus composition, the intestinal pH, and short-chain fatty acids (SCFA) (Devillé., 2007). Leal. (2017) found that laminarin oligosaccharides were beneficial to the growth ofand, and could increase their produc- tion of SCFA, such as lactic acid and acetic acid.
4.3 Antioxidant Activity
Laminarin and laminarin oligosaccharides present good antioxidant activity (Zhou., 2009). Jia. (2010) separated and purified laminarin fromby gel chromatography and obtained two components with Mw of 5.5×104and 2.7×104Da. The results indicated that the laminarin with 5.5×104Da had a good scavenging effect on the hydroxyl free radical, superoxide anion free radical, and diphenyl generation of free radical.
When laminarin oligosaccharides were prepared by gam- ma irradiation of laminarin (Choi.,2012), they show- ed higher ferric reducing antioxidant potential values and greater DPPH radical-scavenging than that of nonirradi- ated laminarin. This means that proper reduction of the DPof laminarin can enhance its antioxidant activity. How- ever, the mechanism remains to be further explored.
4.4 Anti-Inflammatory Activity
Studies have confirmed that several algal polysaccha- rides and their oligosaccharides exhibited excellent anti- inflammatory activity (Dore., 2013). Dectin-1 is a re- ceptor related to extracellular pathogen recognition. Pre- vious studies found it was associated with anti-inflam- matory activity (Karsten., 2012). Smith. (2018)tested the biological activity of five different laminarin preparations and identified some of them as either Dectin- 1 antagonists or agonists. Kim. (2006) prepared lami- narin with Mw between 5 and 10kDa and laminarin oli- gosaccharides derived by enzymatic hydrolysis. Both lami- narin and laminarin oligosaccharides showed good results for suppression of apoptosis and extension of cell survival in culture. A mouse cDNA microarray indicated that the genes coding immune response proteins were induced in the process, which showed potential for application as new immunopotentiating substances of laminarin and lamina- rin oligosaccharides.
In addition, laminarin and laminarin oligosaccharides canalso be prepared as a drug for treating inflammatory diseases induced by non-specific inflammatory responses (Yvin., 2006).
4.5 Applications in Cosmetics
Skin protection and repair functions of laminarin have been proven (Yvin., 1999; Li., 2013). Yvin. (1999) found that laminarin and laminarin oligosaccha- rides had stimulating, regenerating, modulatory, and ener- gizing effects on human dermis fibroblasts and human epi- dermis keratinocytes, which is useful in cosmetics. Li.(2013) explored the effect of laminarin on matrix metal- loproteinase activity in photoaging skin. The results indi- cated that laminarin can regulate the metabolism of light- aged skin collagen by regulating matrix metalloproteinase activity. In addition, laminarin has a positive effect on wound healing (Choi., 2013).
4.6 Applications in Plant Disease Control and Growth Promotion
The fruit, leaves, and crowns of plants are vulnerable to pathogens such asand, causing huge economic losses. Chemical control of plant disease not only causes environmental pollution but also is harmful to human health (Hirooka and Ishii, 2013). Al- gal polysaccharides can increase basal metabolism and cell division as well as the level of essential oils or biomole- cules in plants, which can trigger protection against pa- thogens (González., 2013). Laminarin has been ap- plied in the control of strawberry leaf spot and powdery mildew under field conditions. The results showed that laminarin can reduce the incidences of leaf spot and pow- dery mildew by 50% and 70%–80%, respectively. Its ef- fectiveness at inhibitinginfection was also ob- vious, and the inhibition rate was as high as 80% (Meszka and Bielenin, 2011). Aflatoxin contamination has been a worldwide problem. Laminarin extracted fromhad inhibitory effects on growth and toxin production of(Hu., 2012). Laminarin also can be used as a plant growth-promoting agent. For instance, laminarin could stimulate the germination of seeds through interfer- ing with pathways that can lead to α-amylase formation(Yvin., 1998). In another study, transcriptome analy- sis indicated that laminarin can regulate the DEFL-me- diated pathway, thus affecting abiotic stress tolerance in plants (Wu., 2016).
4.7 Applications in Feed Additives
Laminarin can be used as a high-value feed additive, which can effectively improve growth performance, en- hance the immune response, and regulate the gut micro- biota of animals. Researchers found that the developmen-tal competence of early-stage porcine embryos could be dramatically improved when laminarin was used as a feed additive (Jiang., 2018). Some important physiologi- cal parameters, such as the blastocyst formation rate, hatch- ing rate, and total cell number in the blastocyst signifi- cantly increased when 20mgmL−1of laminarin was used as a food additive during theculture period of early-stage porcine embryos (Jiang., 2018). Walsh.(2013) confirmed that the supplementation of laminarin could suppress the secretion of pro-inflammatory cyto- kines, which could be helpful for the growth performance of pigs. After laminarin extracted fromwas add- ed to the diets of pigs, intestinal bacterial populations, volatile fatty acid concentrations, and the expression le- vels of cytokines and mucin genes in the ileum and colon were determined and analyzed by Smith.(2011). The results indicated that laminarin can improve gut health in the pig. In addition, dietary supplementation with lamina- rin and fucoidan could enhance pork meat quality through reducing saturated fatty acids and lowering lipid oxida- tion in longissimus thoracis and lumborum muscle of pig (Moroney., 2015).
Similarly, laminarin has been used as a feed additive in fish breeding. After growth performance, biochemical para- meters, and the expression levels of immune-related genesinfed with laminarin were measured, the results indicated that laminarin could regulate the im- mune response and promote the growth of fish (Yin., 2014).
4.8 Applications in Bioethanol Production
Bioethanol, a clean fuel, is considered as an important solution to the current fossil fuel energy crisis (Amin, 2009). Although technologies for ethanol production through yeast fermentation are relatively mature, production costs re- main the main barrier to bioethanol application. Due to its abundant resources, algal polysaccharide can be one of the ideal feedstocks for bioethanol production. Remarka- bly, laminarin is composed of glucose, which is the pre- ferable fermentable sugar in bioethanol producers such asand(Al Abdallah., 2016). Therefore, laminarin may emerge as a potential sub- strate for bioethanol production. For example, an optimizedcombination of laminarinase and β-glucosidase-display- ing yeasts could directly transform laminarin into bio- ethanol, and the bioethanol yield was 5.2gL−1(Motone.,2016). A two-stage bioprocess using an immobilized lami- narinase and a marine-derived yeast for bioethanol produc-tion from laminarin was established by Mitsuya. (2017).Finally, 0.51–0.58gL−1bioethanol was yielded from the sac- charified solution preparedthe immobilized laminari- nase. Fig.3 shows the biological activities and potential app- lications of laminarin and laminarin oligosaccharides.
5 Conclusions and Future Outlooks
As one of the important marine algal polysaccharides derived from brown algae, laminarin has many biofunc- tional activities. With the development and progress of the extraction process, EAE-, MAE-, and UAE-assisted ex- traction methods are becoming promising extraction stra- tegies for laminarin as they are environmentally friendly and efficient (Chen., 2013).
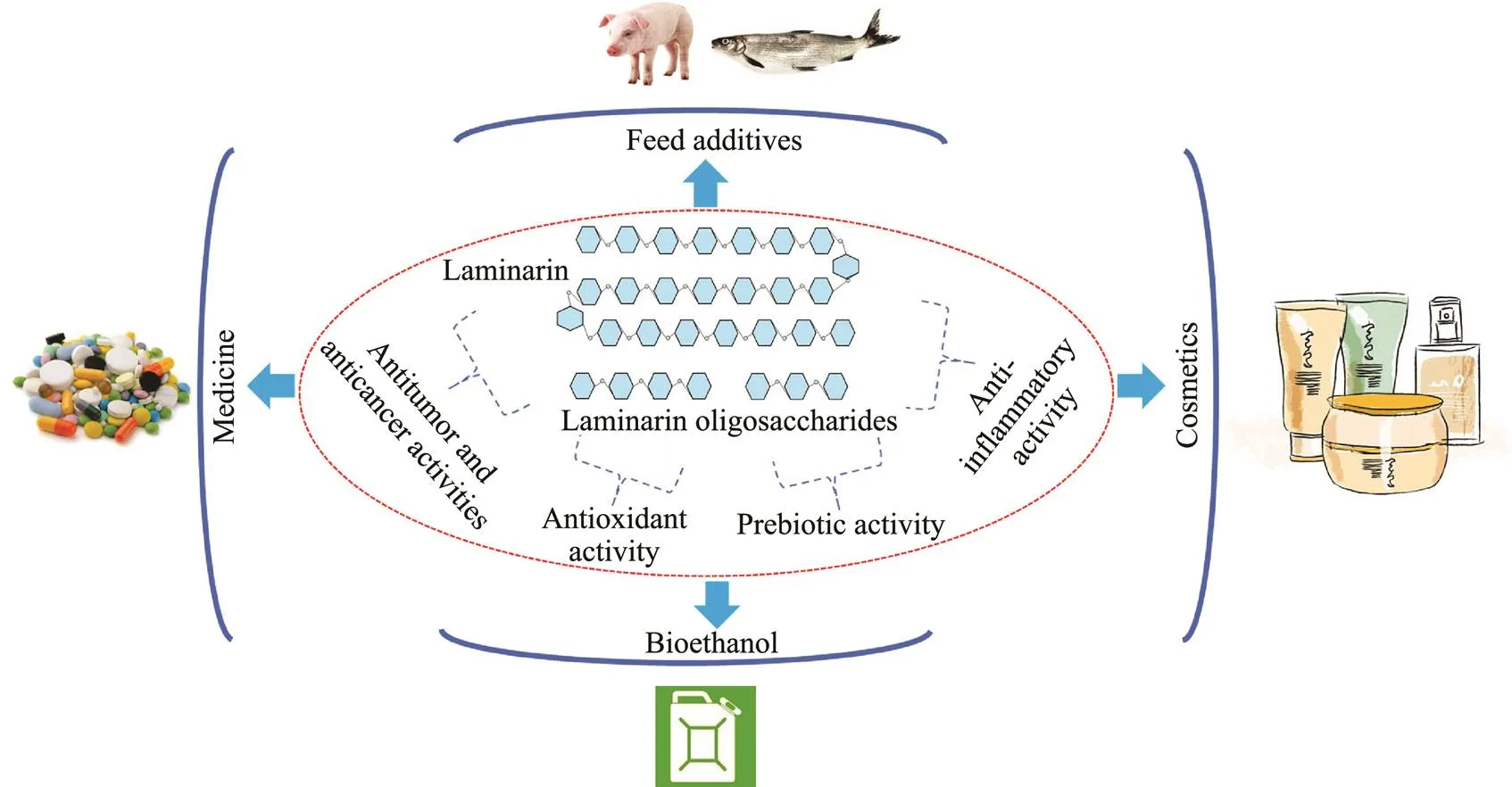
Fig.3 The biological activities and potential applications of laminarin and laminarin oligosaccharides.
Laminarin has many excellent health beneficial effects. The mechanisms of antitumor and anticancer activities of laminarin, such as apoptosis (ErbB signaling pathway, mi- tochondrial pathway, and DR-mediated pathway) and in- hibition of cancer cell colony formation (SMP-30), have been summarized and discussed in this review.Laminarin oligosaccharides possess higher antioxidant and antitumor activities than laminarin (Menshova., 2014), while thedifferences of other biological activities between lamina- rin oligosaccharides and laminarin, such as prebiotic ac- tivity and anti-inflammatory activity, have not been studi- ed in detail thus far. Therefore, large-scale preparation of laminarin oligosaccharides and evaluation of their more specific biological activities are a focus of research. En- zymatic degradation of laminarin by using laminarinases is one of the most promising approaches to produce lami- narin oligosaccharide because of the mild reaction condi- tions, low energy consumption, high output, and clear lami- narin oligosaccharide composition. In total, typical crystal structures of laminarinases from four GH families have been identified, which will benefit our understanding of the catalytic mechanisms and targeted production of lamina- rin oligosaccharides.
Moreover, modifications of laminarin such as sulfation, nanoparticle modification, and branched-chain modifica-tion can enhance some specific functional activities of la- minarin (Ji., 2013; Cui., 2019). For example, specific bit sulfated modification (C2 and C6) and increase the content of side chains are effective methods to enhance the antitumor activity of laminarin. However, the detailed mechanism requires further illustration. Thus, the production of laminarin with specific structural modifications and laminarin oligosaccharides with homogeneous DP and the illumination of their structure-activity relation- ship will become another research focus in the near future.
Acknowledgements
This work was supported by the National Natural Sci- ence Foundation of China (No. 31922072), the National Key Research and Development Program of China (Nos. 2019YFD0901902 and 2019YFD0901904), the Taishan Scholar Project of Shandong Province (No. tsqn2018120 20), and the Fundamental Research Funds for the Central Universities (No. 201941002).
Adam, F., Abert-Vian, M., Peltier, G., and Chemat, F., 2012. ‘Solvent-free’ ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process., 114: 457-465.
Adams, J., Ross, A., Anastasakis, K., and Hodgson, E., 2011. Seasonal variation in the chemical composition of the bio- energy feedstockfor thermochemical con- version., 102 (1): 226-234.
Al Abdallah, Q., Nixon, B. T., and Fortwendel, J. R., 2016. The enzymatic conversion of major algal and cyanobacterial car- bohydrates to bioethanol., 4: 36.
Ale, M. T., Mikkelsen, J. D., and Meyer, A. S., 2011. Important determinants for fucoidan bioactivity: A critical review of struc- ture-function relations and extraction methods for fucose-con- taining sulfated polysaccharides from brown seaweeds., 9 (10): 2106-2130.
Amin, S., 2009. Review on biofuel oil and gas production pro- cesses from microalgae., 50: (7): 1834-1840.
Badur, A. H., Ammar, E. M., Yalamanchili, G., and Hehemann, J. H., 2020. Characterization of the GH16 and GH17 laminari- nases from1C10., 104 (1): 161-171.
Bara, M. T. F., Lima, A. L., and Ulhoa, C. J., 2003. Purification and characterization of an exo-β-1,3-glucanase produced by., 219 (1): 81-85.
Becker, S., Scheffel, A., Polz, M. F., and Hehemann, J. H., 2017. Accurate quantification of laminarin in marine organic matter with enzymes from marine microbes., 83 (9): e03389-16.
Becker, S., Tebben, J., Coffinet, S., and Wiltshire, K., 2020. La- minarin is a major molecule in the marine carbon cycle., 117 (12): 6599- 6607.
Boons, G. J., 1996. Strategies in oligosaccharide synthesis., 52 (4): 1095-1121.
Charoensiddhi, S., Lorbeer, A. J., Lahnstein, J., and Bulone, V., 2016. Enzyme-assisted extraction of carbohydrates from the brown alga: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles., 51 (10): 1503-1510.
Chen, H. M., Zheng, L., and Yan, X. J., 2005. The preparationand bioactivity research of agaro-oligosaccharides., 43 (1): 29-36.
Chen, S. X., Shen, J. G., Chen, B., and Wang, D. Z., 2013. The optimization of polysaccharide extraction process fromin Fanjing mountain by microwave-assisted me- thod., 39(5):234-237 (in Chinese with English abstract).
Choi, J. A., Oh, T. H., Choi, J. S., and Chang, D. J., 2013. Im- pact of β-1,3-glucan isolated fromon cor- neal epithelial cell migration and on wound healing in a rat alkali burn model., 38 (12): 1207-1213.
Choi, J. I., Kim, H. J., and Lee, J. W., 2011. Structural feature andantioxidant activity of low molecular weight laminarin degrad- ed by gamma irradiation., 129 (2): 520-523.
Choi, J. I., Kim, H. J., Kim, J. H., and Lee, J. W., 2012. En- hanced biological activities of laminarin degraded by gamma- ray irradiation., 36 (4): 465- 469.
Cong, Q., Chen, H., Liao, W., and Xiao, F., 2016. Structural cha- racterization and effect on anti-angiogenic activity of a fucoi- dan from., 136: 899-907.
Cui, D., Ma, J., Liang, T., and Sun, L., 2019. Selenium nanopar- ticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis., 137: 829-835.
Delattre, C., Michaud, P., Courtois, B., and Courtois, J., 2005. Oligosaccharides engineering from plants and algae: Applica- tions in biotechnology and therapeutics., 17 (3): 107.
Devillé, C., Damas, J., Forget, P., and Dandrifosse, G., 2004. La- minarin in the dietary fibre concept., 84 (9): 1030-1038.
Devillé, C., Gharbi, M., Dandrifosse, G., and Peulen, O., 2007. Study on the effects of laminarin, a polysaccharide from sea- weed, on gut characteristics., 87 (9): 1717-1725.
Dong, W., Huang, J., Li, Y., and Tan, Y., 2015. Crystal structural basis for Rv0315, an immunostimulatory antigen and inactive beta-1,3-glucanase of., 5: 15073.
Dore, C. M. P. G., Alves, M. G. D. C. F., Will, L. S. E. P., and Costa, T. G., 2013. A sulfated polysaccharide, fucans, isolated from brown algaewith anticoagulant, an- tithrombotic, antioxidant and anti-inflammatory effects., 91 (1): 467-475.
Ermakova, S., Men’shova, R., Vishchuk, O., and Kim, S. M., 2013.Water-soluble polysaccharides from the brown alga: Structural characteristics and antitumor activity., 2 (1): 51-58.
Ezzine, A., Chahed, H., Hannachi, M., and Hardouin, J., 2016. Biochemical and molecular characterization of a new glyco- side hydrolase family 17 from., 28 (8): 1610-1621.
Gaborieau, M., and Castignolles, P., 2011. Size-exclusion chro- matography (SEC) of branched polymers and polysaccharides., 399 (4): 1413-1423.
Gao, M. X., Liu, H. W., and Zong, M. Y., 2006. Extracting poly- saccharides ofby microwave., 8: 69-72 (in Chinese with English abstract).
Garcia-Vaquero, M., Rajauria, G., O’doherty, J., and Sweeney, T., 2017. Polysaccharides from macroalgae: Recent advances, in- novative technologies and challenges in extraction and puri- fication., 99: 1011-1020.
González, A., Castro, J., Vera, J., and Moenne, A., 2013. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division., 32 (2): 443-448.
Graiff, A., Ruth, W., Kragl, U., and Karsten, U., 2016. Chemical characterization and quantification of the brown algal storage compound laminarin–A new methodological approach., 28 (1): 533-543.
Hahn, T., Zayed, A., Kovacheva, M., and Stadtmüller, R., 2016. Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae., 16 (1): 78-87.
He, H., Gu, G., and Du, Y., 2003. Synthesis of laminarin oligo- saccharide derivatives having D-arabinofuranosyl side-chains., 22 (5): 275-283.
Hirabayashi, J., Hashidate, T., Arata, Y., and Nishi, N., 2002. Oli- gosaccharide specificity of galectins: A search by frontal affi- nity chromatography.–, 1572 (2-3): 232-254.
Hirooka, T., and Ishii, H., 2013. Chemical control of plant di- seases., 79 (6): 390-401.
Hu, L. B., Li, H. B., Sun, J. L., and Zeng, J., 2012. Effect of la- minarin ongrowth and aflatoxin produc- tion., 343-344: 1168-1171.
Iji, P., and Tivey, D., 1998. Natural and synthetic oligosaccha- rides in broiler chicken diets., 54 (2): 129-143.
Imbs, T. I., Ermakova, S. P., Malyarenko, O. S., and Isakov, V. V., 2016. Structural elucidation of polysaccharide fractions from the brown algaandin- vestigation of their anticancer activity.,135: 162-168.
Ji, C. F., and Ji, Y. B., 2014. Laminarin-induced apoptosis in hu- man colon cancer LOVO cells., 7 (5): 1728- 1732.
Ji, C. F., Ji, Y. B., and Meng, D. Y., 2013. Sulfated modification and anti-tumor activity of laminarin., 6 (5): 1259-1264.
Ji, Y. B., Ji, C. F., and Zhang, H., 2012. Laminarin induces apop-tosis of human colon cancer LOVO cells through a mito- chondrial pathway., 17 (8): 9947-9960.
Jia, Y., and Min, W., 2010. Laminarin purification and extra- organ antioxidation., 8: 26-29 (in Chinese with English abstract).
Jiang, H., Liang, S., Yao, X. R., and Jin, Y. X., 2018. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress., 115: 38-44.
Kadam, S. U., O’Donnell, C. P., Rai, D. K., and Hossain, M. B., 2015. Laminarin from Irish brown seaweedsand: Ultrasound assisted extrac- tion, characterization and bioactivity., 13 (7): 4270-4280.
Kadam, S. U., Tiwari, B. K., and O’Donnell, C. P., 2015. Extrac- tion, structure and biofunctional activities of laminarin from brown algae., 50 (1): 24-31.
Karsten, C. M., Pandey, M. K., Figge, J., and Kilchenstein, R., 2012. Anti-inflammatory activity of IgG1 mediated by Fc ga- lactosylation and association of FcγRIIB and dectin-1., 18 (9): 1401.
Kim, K. H., Kim, Y. W., Kim, H. B., and Lee, B. J., 2006. Anti- apoptotic activity of laminarin polysaccharides and their en- zymatically hydrolyzed oligosaccharides from., 28 (6): 439-446.
Kim, M. J., Nam, S. W., Tamano, K., and Machida, M., 2011. Opti- mization for production of exo-β-1,3-glucanase (laminarinase) fromin., 26 (5): 427-432.
Kumar, K., Correia, M. A., Pires, V. M., and Dhillon, A., 2018. Novel insights into the degradation of β-1,3-glucans by the cellulosome ofrevealed by struc- ture and function studies of a family 81 glycoside hydrolase., 117: 890- 901.
Kusaykin, M. I., Belik, A. A., Kovalchuk, S. N., and Dmitrenok, P. S., 2017. A new recombinant-1,3-β-D-glucanase from the marine bacteriumKMM 3553: Enzyme characteristics and transglycosylation products analysis., 33 (2): 40.
Labourel, A., Jam, M., Legentil, L., and Sylla, B., 2015. Struc- tural and biochemical characterization of the laminarinase ZgLamCGH16 fromsuggests pre- ferred recognition of branched laminarin., 71 (2): 173-184.
Leal, B. E. S., Prado, M. R., Grzybowski, A., and Tiboni, M., 2017. Potential prebiotic oligosaccharides from aqueous ther- mopressurized phosphoric acid hydrolysates of microalgae used in treatment of gaseous steakhouse waste., 24: 138-147.
Lee, K. C., Arai, T., Ibrahim, D., and Kosugi, A., 2014. Purification and characterization of a thermostable laminarinase fromc3-2 (1) IBRL., 9 (1): 1072- 1084.
Lee, O. K., and Lee, E. Y., 2016. Sustainable production of bio- ethanol from renewable brown algae biomass., 92: 70-75.
Li, J., Xie, L., Qin, Y., and Liang, W., 2013. Effect of laminarin polysaccharide on activity of matrix metalloproteinase in pho-toaging skin., 38 (14): 2370-2373.
Li, W., Huan, X., Zhou, Y., and Ma, Q., 2009. Simultaneous clo- ning and expression of two cellulase genes fromnewly isolated from Golden Takin (Bed-fordi)., 383 (4): 397-400.
Mak, W., Hamid, N., Liu, T., and Lu, J., 2013. Fucoidan from New Zealand: Monthly variations and de- termination of antioxidant activities., 95 (1): 606-614.
Menshova, R. V., Ermakova, S. P., Anastyuk, S. D., and Isakov, V. V., 2014. Structure, enzymatic transformation and antican- cer activity of branched high molecular weight laminaran frombrown alga., 99: 101- 109.
Menu-Bouaouiche, L., Vriet, C., Peumans, W. J., and Barre, A., 2003. A molecular basis for the endo-β1,3-glucanase activity of the thaumatin-like proteins from edible fruits., 85 (1-2): 123-131.
Meszka, B., and Bielenin, A., 2011. Activity of laminarin in con- trol of strawberry diseases., 62: 15-23.
Mitsuya, D., Sugiyama, T., Zhang, S., and Takeuchi, Y., 2018. En- zymatic properties and the gene structure of a cold-adapted laminarinase fromspecies LA., 126 (2): 169-175.
Mitsuya, D., Yamamoto, M., Okai, M., and Inoue, A., 2017. Con- tinuous saccharification of laminarin by immobilized lamina- rinase ulam111 followed by ethanol fermentation with a ma- rine-derived yeast., 7 (5): 387-403.
Mong, T. K. K., Lee, H. K., Durón, S. G., and Wong, C. H., 2003. Reactivity-based one-pot total synthesis of fucose GM1 oligo- saccharide: A sialylated antigenic epitope of small-cell lung can- cer., 100 (3): 797-802.
Moroney, N., O’Grady, M., Robertson, R., and Stanton, C., 2015. Influence of level and duration of feeding polysaccharide (la- minarin and fucoidan) extracts from brown seaweed () on quality indices of fresh pork., 99: 132-141.
Motone, K., Takagi, T., Sasaki, Y., and Kuroda, K., 2016. Direct ethanol fermentation of the algal storage polysaccharide lami- narin with an optimized combination of engineered yeasts., 231: 129-135.
Nagasawa, N., Mitomo, H., Yoshii, F., and Kume, T., 2000. Ra- diation-induced degradation of sodium alginate., 69 (3): 279-285.
Nakabayashi, M., Nishijima, T., Ehara, G., and Nikaidou, N., 1998. Structure of the gene encoding laminaripentaose-producing β- 1,3-glucanase (LPHase) ofDIC-108., 85 (5): 459- 464.
Natsuka, S., Tachibana, A., Sumiyoshi, W., and Nakakita, S. I., 2018. Preparation of a molecular library of branched β-glucan oligosaccharides derived from laminarin., 65 (4): 45-49.
Nguyen, S. G., Kim, J., Guevarra, R. B., and Lee, J. H., 2016. La-minarin favorably modulates gut microbiota in mice fed a high- fat diet., 7 (10): 4193-4201.
Novak, M., and Vetvicka, V., 2008. β-glucans, history, and the present: Immunomodulatory aspects and mechanisms of ac- tion., 5 (1): 47-57.
Oda, M., Tanabe, Y., Noda, M., and Inaba, S., 2016. Structural and binding properties of laminarin revealed by analytical ul- tracentrifugation and calorimetric analyses., 431: 33-38.
O’Shea, C., McAlpine, P., Sweeney, T., and Varley, P., 2014. Ef- fect of the interaction of seaweed extracts containing lami- narin and fucoidan with zinc oxide on the growth perfor- mance, digestibility and faecal characteristics of growing pig- lets., 111 (5): 798-807.
Pang, Z., Otaka, K., Maoka, T., and Hidaka, K., 2005. Structure of β-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells., 69 (3): 553-558.
Pap, N., Beszédes, S., Pongrácz, E., and Myllykoski, L., 2013. Microwave-assisted extraction of anthocyanins from black cur-rant marc., 6 (10): 2666- 2674.
Park, H. K., Kim, I. H., Kim, J., and Nam, T. J., 2012. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells., 30 (4): 734-738.
Park, H. K., Kim, I. H., Kim, J., and Nam, T. J., 2013. Induction of apoptosis and the regulation of ErbB signaling by lami- narin in HT-29 human colon cancer cells., 32 (2): 291-295.
Pohleven, J., Štrukelj, B., and Kos, J., 2012.. InTech, Janeza Trdine 9, 51000 Rijeka, Croatia, 49-74.
Quitain, A. T., Kai, T., Sasaki, M., and Goto, M., 2013. Micro- wave-hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled., 52 (23): 7940- 7946.
Ramani, R., and Ranganathaiah, C., 2000. Degradation of acry- lonitrile-butadiene-styrene and polycarbonate by UV irradia- tion., 69 (3): 347-354.
Ramnani, P., Chitarrari, R., Tuohy, K., and Grant, J., 2012.fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate sea- weeds., 18 (1): 1-6.
Receveur-Bréchot, V., Czjzek, M., Barre, A., and Roussel, A., 2006. Crystal structure at 1.45-Å resolution of the major al- lergen endo-β-1,3-glucanase of banana as a molecular basis for the latex-fruit syndrome., 63 (1): 235-242.
Renard, C. M., Lahaye, M., Mutter, M., and Voragen, F. G., 1997. Isolation and structural characterisation of rhamnogalacturo- nan oligomers generated by controlled acid hydrolysis of su- gar-beet pulp., 305 (2): 271-280.
Rioux, L. E., Turgeon, S. L., and Beaulieu, M., 2010. Structural characterization of laminaran and galactofucan extracted from the brown seaweed., 71 (13): 1586-1595.
Sakamoto, Y., Nakade, K., and Konno, N., 2011. Endo-β-1,3- glucanase GLU1, from the fruiting body of, belongs to a new glycoside hydrolase family., 77 (23): 8350-8354.
Sandini, S., La Valle, R., De Bernardis, F., and Macrì, C., 2007. The 65kDa mannoprotein gene ofencodes a putative β-glucanase adhesin required for hyphal morpho- genesis and experimental pathogenicity., 9 (5): 1223-1238.
Santos, T., Del Rey, F., Conde, J., and Villanueva, J., 1979. Sac- charomyces cerevisiae mutant defective in exo-1,3-beta-glu- canase production., 139 (2): 333-338.
Smith, A. J., Graves, B., Child, R., and Rice, P. J., 2018. Immu- noregulatory activity of the natural product laminarin varies widely as a result of its physical properties., 200 (2): 788-799.
Smith, A., O’Doherty, J., Reilly, P., and Ryan, M., 2011. The ef- fects of laminarin derived fromon mea- surements of gut health: Selected bacterial populations, intes- tinal fermentation, mucin gene expression and cytokine gene expression in the pig., 105 (5): 669-677.
Spilliaert, R., Hreggvidsson, G. O., Kristjansson, J. K., and Eg- gertsson, G., 1994. Cloning and sequencing of agene, bglA, coding for a thermostable β-Gluca- nase and its expression in., 224 (3): 923-930.
Sun, S., Wei, X., and You, C., 2019. The construction of ansynthetic enzymatic biosystem that facilitates laminari- biose biosynthesis from maltodextrin and glucose., 14 (4): 1800493.
Takeda, T., Nakano, Y., Takahashi, M., and Konno, N., 2015. Iden-tification and enzymatic characterization of an endo-1,3-β- glucanase from., 116: 21-27.
Thompson, T. M., Young, B. R., and Baroutian, S., 2019. Ad- vances in the pretreatment of brown macroalgae for biogas production., 195: 106151.
Tian, L., Li, C. M., Li, Y. F., and Huang, T. M., 2020. Laminarin from seaweed () inhibits hepatocellular car- cinoma through upregulating senescence marker protein-30., 35 (4): 277-283.
Tschiggerl, H., Breitwieser, A., de Roo, G., and Verwoerd, T., 2008. Exploitation of the S-layer self-assembly system for site directed immobilization of enzymes demonstrated for an extremophilic laminarinase from., 133 (3): 403-411.
Usoltseva, R. V., Anastyuk, S. D., Shevchenko, N. M., and Zv- yagintseva, T. N., 2016. The comparison of structure and anti- cancer activityof polysaccharides from brown algaeand., 153: 258-265.
Walsh, A., Sweeney, T., O’Shea, C., and Doyle, D., 2013. Effect of dietary laminarin and fucoidan on selected microbiota, in- testinal morphology and immune status of the newly weaned pig., 110 (9): 1630-1638.
Walsh, A., Sweeney, T., O’Shea, C., and Doyle, D., 2013. Effect of supplementing varying inclusion levels of laminarin and fu-coidan on growth performance, digestibility of diet compo- nents, selected faecal microbial populations and volatile fatty acid concentrations in weaned pigs.,183(3-4): 151-159.
Wang, D., Kim, D. H., Seo, N., and Yun, E. J., 2016. A novel glycoside hydrolase family 5 β-1,3-1,6-endoglucanase from2-40T and its transglycosylase ac- tivity., 82 (14): 4340- 4349.
Wijesekara, I., Pangestuti, R., and Kim, S. K., 2011. Biological activities and potential health benefits of sulfated polysaccha- rides derived from marine algae., 84 (1): 14-21.
Wu, H. M., Liu, S. W., Hsu, M. T., and Hung, C. L., 2009. Struc-ture, mechanistic action, and essential residues of a GH-64 enzyme, laminaripentaose-producing β-1,3-glucanase., 284 (39): 26708-26715.
Wu, Y. R., Lin, Y. C., and Chuang, H., 2016. Laminarin modu- lates the chloroplast antioxidant system to enhance abiotic stress tolerance partially through the regulation of the defen- sin-like gene expression., 247: 83-92.
Yin, G., Li, W., Lin, Q., and Lin, X., 2014. Dietary administra- tion of laminarin improves the growth performance and im- mune responses in., 41 (2): 402-406.
Yvin, J. C., Alban, S., and Franz, G., 2006. Anti-inflammatory and healing medicine based on laminarin sulphate. United States, 7 March 2006, US7008931B2.
Yvin, J. C., Levasseur, F., Amin-Gendy, C., and Tran Thanh, K. N., 1998. Laminarin as a seed germination and plant growth accelerator. United States, 12 May 1998, US5750472A.
Yvin, J. C., Levasseur, F., and Hud’Homme, F., 1999. Use of laminarin and oligosaccharides derived therefrom in cosme- tics and for preparing a skin treatment drug. United States, 9 November 1999, US5980916A.
Zaporozhets, T., Besednova, N., Kuznetsova, T., and Zvyagint- seva, T., 2014. The prebiotic potential of polysaccharides and extracts of seaweeds., 40 (1): 1-9.
Zargarzadeh, M., Amaral, A. J., Custódio, C. A., and Mano, J. F., 2020. Biomedical applications of laminarin., 232: 115774.
Zha, X. Q., Xiao, J. J., Zhang, H. N., and Wang, J. H., 2012. Poly-saccharides in(LP): Extraction, physi- cochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis., 134 (1): 244-252.
Zhang, H., and Row, K. H., 2015. Extraction and separation of polysaccharides fromby size-exclusion chromatography., 53 (4): 498-502.
Zhou, J., Wu, H., Liu, Q., and Jie, M., 2009. Study on antioxida- tive activity of laminarin., 25 (4): 397-400 (in Chinese with English abstract).
Zhou, P., Chen, Z., Yan, Q., and Yang, S., 2013. The structure of a glycoside hydrolase family 81 endo-β-1,3-glucanase., 69 (10): 2027-2038.
Zhu, Y., Li, Q., Mao, G., and Zou, Y., 2014. Optimization of en- zyme-assisted extraction and characterization of polysaccha- rides from., 101: 606-613.
May 9, 2020;
July 10, 2020;
December 22, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
E-mail: jh@ouc.edu.cn E-mail: xzhmao@ouc.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Case Study of a Short-Term Wave Energy Forecasting Scheme:North Indian Ocean
- Temporal and Spatial Characteristics of Wave Energy Resources in Sri Lankan Waters over the Past 30 Years
- Vibration Deformation Monitoring of Offshore Wind Turbines Based on GBIR
- Dependence of Estimating Whitecap Coverage on Currents and Swells
- The Variation of Microbial (Methanotroph) Communities in Marine Sediments Due to Aerobic Oxidation of Hydrocarbons
- 3-Aminopropyltriethoxysilane Complexation with Iron Ion Modified Anode in Marine Sediment Microbial Fuel Cells with Enhanced Electrochemical Performance
