Dietary Supplementation with Sea Cucumber Saponins and Exercise Can Significantly Suppress Adipose Accumulation in Mice Fed with High-Fat Diet
2021-06-25LIRongMENGJingSHIHaohaoWANGChengchengLIZhaojieXUEChanghuWANGYumingandZHANGTiantian
LI Rong, MENG Jing, 3), SHI Haohao, WANG Chengcheng, LI Zhaojie,XUE Changhu, 2), WANG Yuming, 2), *, and ZHANG Tiantian, *
Dietary Supplementation with Sea Cucumber Saponins and Exercise Can Significantly Suppress Adipose Accumulation in Mice Fed with High-Fat Diet
LI Rong1), MENG Jing1), 3), SHI Haohao1), WANG Chengcheng1), LI Zhaojie1),XUE Changhu1), 2), WANG Yuming1), 2), *, and ZHANG Tiantian1), *
1),,266003,2),,266237,3),272025,
Dietary supplementation with sea cucumber saponins (SCS) and exercise have been confirmed to be effective in preventing the development of obesity and its related diseases. However, the combined effectiveness of these interventions has not been explored. Here, we studied whether the beneficial influences of exercise could be further enhanced by dietary supplementation with SCS in high-fat diet-fed KM (Kunming) mice.Mice were randomly divided into four groups, including the high-fat diet group (HF), the SCS group (HF-S), the exercise group (HF-E), and the combination of dietary SCS and exercise group (HF-S+E). There were eight mice in every group. The results demonstrated that the combination of dietary SCS and exercise could synergistically reduce fat accumulation.In particular, white adipose tissue decreased by 63% in the HF-S+E group compared with that in the HF group. SCS supplementation with exercise also improved peripheral markers, such as serum parameters and hepatic TG levels. Further mechanical testing indicated that the combined effects of dietary SCS and exercise on inhibiting fat accumulation might be attributed to the inhibition of lipid synthesis in the liver and the activation of lipolysis in white adipose tissue to increase energy consumption.
exercise; sea cucumber saponins; fat accumulation; lipogenesis; lipolysis
1 Introduction
Obesity has recently been recognized as a significant public health issue because it is a known risk factor for chronic diseases such as type II diabetes, hypertension, and coronary heart disease (Wu., 2013). Obesity is the result of an imbalance between energy intake and me- tabolic expenditure and causes marked increases in the weight of adipose tissue (Galani., 2007). Many re- searchers have demonstrated that the current approaches for treating obesity, including pharmacotherapy and bariatric surgery, feature a number of important limitations, such as adverse reactions and high rebound rates (Kush- ner, 2014).
Control of energy intake through the diet and increased metabolic expenditure through exercise are two major pro- grams that can suppress body fat accumulation(Adegboye and Linne, 2013). Many food ingredients, such as sapon- ins, can effectively alleviate the development of obesity (Wang., 2018). Sea cucumber is a traditional Asian food, and sea cucumber saponins (SCS) are important se- condary metabolites. Holothurin A (HA) and echinoside A (EA) are two major saponins in sea cucumber(Fig.1) (Wang., 2014). Previous studies revealed that dietary supplementation with SCS significantly suppresses adi- pose tissue accumulation and ameliorates obesity-induced inflammation (Zhao., 2018). Additionally, a number of researchers have indicated that exercise can normalize adipose tissue weight, body weight, and inflammation in diet-induced obesity mice; however,some Chinese sports and health surveys have suggested that the participation rate of Chinese adults in regular physical exercise is only 10% (Ye., 2016). It is important to encourage Chinese adults to attend more physical exercises in addition to paying attention to nutrition. Thus exploring the combined effects of dietary supplementation with SCS and exer- cise on obesity is a worthwhile endeavor.
In the present study, the body weight and fat accumulation of high-fat diet-fed mice were analyzed following treatment to evaluate the combined effects of dietary supplementation with SCS and exercise on obesity. The possible underlying mechanism was also described.
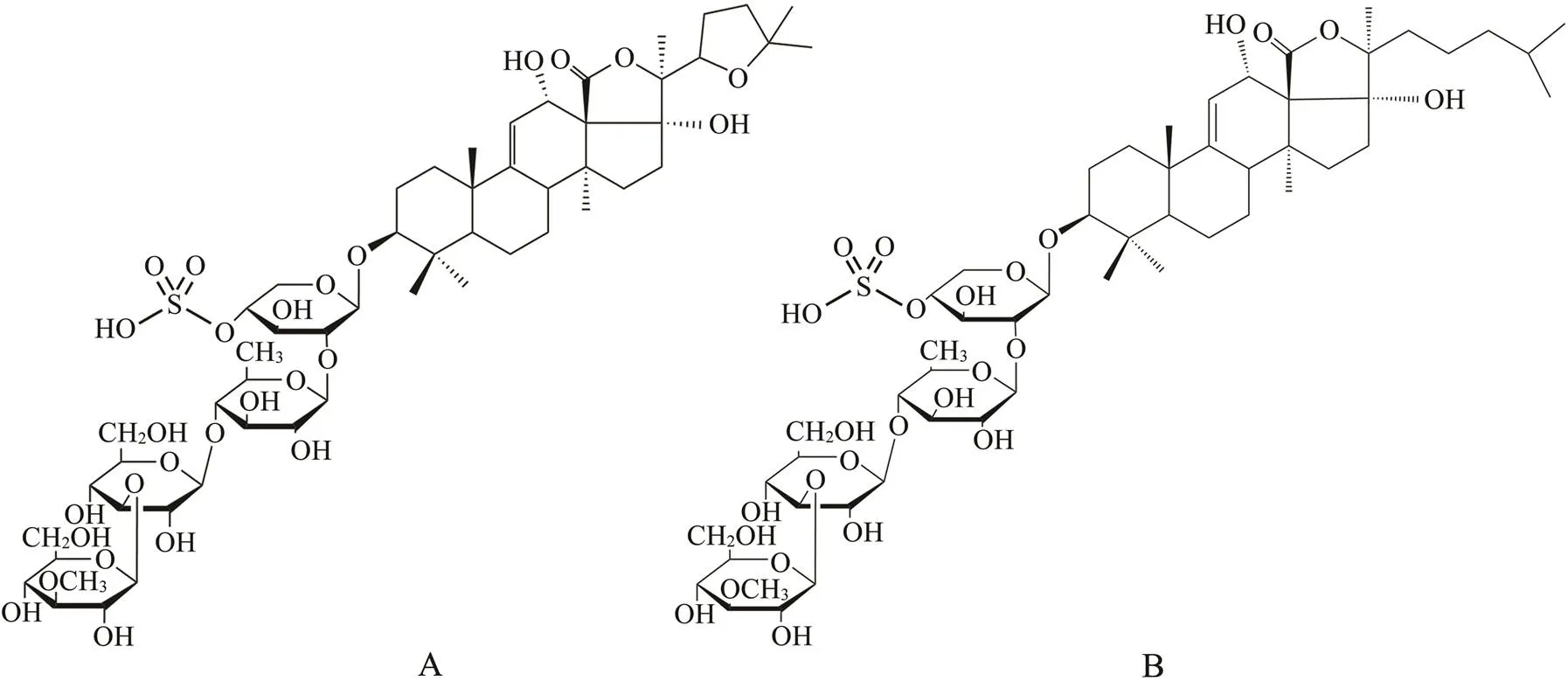
Fig.1 Structures of sea cucumber saponins: Holothurin A (A) and echinoside A (B).
2 Materials and Methods
2.1 Materials
Acetonitrile and ammonium bicarbonate were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). Deio- nized water was obtained from a Milli-Q gradient system (Millipore, Bedford, MA, USA). Sea cucumber () was purchased from Nanshan Aquatic Market (Shandong, China). All other reagents used in this work were of analytical grade.
2.2 Preparation and Analysis of Sea Cucumber Saponins
Saponins were extracted from sea cucumbers according to a reported method (Hu., 2010). Briefly, the body walls of air-dried sea cucumbers were pulverized and then extracted four times with 60% ethanol (v/v) at room temperature. The solvent-to-material ratio was 4:1. The ethanol extracts were subsequently extracted in water and chlo- roform. The aqueous layer was extracted with-butanol. The obtained-butanol extract was dissolved in water,placed on a HP-20 resin column, and eluted with water and 80% ethanol. The obtained 80% ethanol extract was loaded onto a normal-phase silica gel column and eluted with chloroform:methanol:water (10:1:0.1–7:3:0.3, v/v/v). Finally, the eluate (7:2.5:0.2, v/v/v) was mixed and dried to obtain the SCS sample. Column chromatography with an ODS silica gel column (YMC-Pack, Japan) was used to separate the pure EA and HA standard substances by gradual elution using methanol and H2O.
HPLC-UV (Agilent 1260 Infinity system) analysis with an Eclipse XDB-C18 column (150mm×4.6mm, 5μm; Agi- lent, Palo Alto, CA, USA)was employed to evaluate the contents of EA and HAat 205nm. The column temperature was maintained at 30℃, and the mobile phase consisted of acetonitrile (A) and 0.1% ammonium bicarbonate (B). Gradient elution was performed as follows: 0–5min, 30% A; 5–30min, 60% A; 30–35min, 30% A. The flow rate was 1mLmin−1, and the injection volume was 20μL. The purity of the obtained SCS sample was 90.2%, including 21.6% HA and 68.6% EA.
2.3 Animals and Treatments
The experiment was conducted according to the guidelines provided by the Ethical Committee of Experimental Animal Care at Ocean University of China (Approval No. SPXY2015012, Qingdao, China). KM male mice (8 weeks old, 18–20g) were purchased from Vital River (Beijing, China). All mice were cultured in individual cages with a constant temperature of 24℃, relative humidity of 65%±15%, and an alternating light/dark cycle of 12h/12h. The mice acclimated for 1 week and then randomly divided into four groups as follows: HF (high-fat diet group), HF-S (SCSgroup), HF-E (exercise group), HF-S+E (the combination of dietary SCS and exercise group). There are eight mice in every group. All mice in these four groups were fed a high-fat diet with 20% lard and 5% corn oil. The diets of mice in the HF-S and HF-S+E groups were supplementedwith 0.06% SCS. The HF-E and HF-S+E groups were task- ed to run 1h per day using a motor-driven wheel-treadmill (YLS-10B, Shandong Academy of Medical Sciences, Jinan, China) rotating at a speed of 21rmin−1(moderate difficul- ty). The mouse diet was regulated on the basis of AIN-93G. After 3 weeks, the mice were fasted 10h before sacrifice. Blood was collected and serum was obtained by centrifugation at 3500rmin−1for 15min at 4℃. Tissues and viscera were quickly removed and stored at −80℃ until bioanalysis.
2.4 Biochemical Analyses of Serum
The corresponding enzymatic reagent kits (Biosino, Bei- jing, China) were used to determine serum total cholesterol (TC), triacylglycerol (TG), free fatty acid (FFA), and glycerin concentrations. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL- C) were respectively measured using the phosphotungs- tic acid-Mg2+precipitation and polyvinyl sulfate sedimen- tation methods.
2.5 Hepatic Lipid and Fatty Acid Composition Analysis
Hepatic lipids were extracted using a reported method (Folch., 1957). The concentrations of TC and TG were detected using the kits according to the manufacturer’s instructions (Biosino). Fatty acid composition ana- lysis was performed using an Agilent 6890 gas chromato- graphy equipped with a flame-ionization detector and anHP-INNOWAX capillary column (30m×0.32mm×0.25μm) (Liu., 2016). The detector temperature was maintain- ed at 250℃, and the injector temperature was kept at 240℃. The column oven was heated from 170℃ to 240℃ at arate of 3℃min−1and then held at this temperature for 15min. Nitrogen was used as the carrier gas and supplied at a flow rate of 1.2mLmin−1.
2.6 Histological Analysis of Liver and Epididymal White Adiposes
The collected livers and epididymal white adipose tissues were stained with hematoxylin and eosin (H&E) and viewed under a light microscope equipped with a camera (Ni-E, Nikon, Japan).
2.7 RNA Extraction and Real Time (RT)-PCR
Trizol reagent (Invitrogen, USA) was used to extract the total RNA from liver and epididymal white adipose tis- sues (Liu., 2014). A total of 2μg RNA was reverse- transcribed into cDNA using random primers (TOYOBO, Osaka, Japan). SYBR Green I Master Mix (Riche, Mann- heim, Germany) and an iCycler IQ5 system (Bio-Rad La- boratories, Hercules, CA, USA) were used to amplify the target genes with forward and reverse primers. The procedure of real time PCR was as follows: 1 cycle of 95℃ for 10min and 45 cycles of 95℃ for 15s, 55℃–60℃ for 20s, and 72℃ for 30s. The primers were synthesized by the Shanghai Sangon Gene Company (Shanghai, China). Changes in relative gene expression were calculated using the 2–ΔΔCtmethod, and the GADPH gene was used as the reference gene. Results are reported as changes relative to the control group.
2.8 Statistical Analysis
All results were expressed as mean±standard error of the mean (SEM). Statistical analysis was performed by one- way analysis of variance and multiple comparisons (Dun- can’s post hoc test) using SPSS.20 software with a confi- dence level of 95%. Different letters indicate significant dif- ferences among groups when<0.05.
3 Results
3.1 Influence of Dietary SCS and Exercise on Food Intake and Body Weight
The body weight of the mice was measured every day to determine whether dietary SCS and exercise exhibits synergistic effects on alleviating obesity. After 3 weeks, the liver weight in the HF-S+E group decreased by 20% in comparison with that in the HF group. Interestingly, no significant differences in the visceral index of the liver, heart, kidney, and spleen (Table 1) were observed among the four treatment groups. Moreover, no significant diffe- rence in daily food intake was observed among the four groups throughout the 3-week experiment (Fig.2A). Com- pared with the HF group, the dietary SCS and exercise groups showed reductions in body weight gain, but the dif- ferences observed were not statistically significant. Dietary SCS combined with exercise significantly reduced bo- dy weight gain from the 7th day to the 20th day of treatment compared with that in the HF group (<0.05, Fig.2B). This result suggests the synergistic effects of SCS and ex- ercise on mitigating obesity without changing food intake and visceral index.
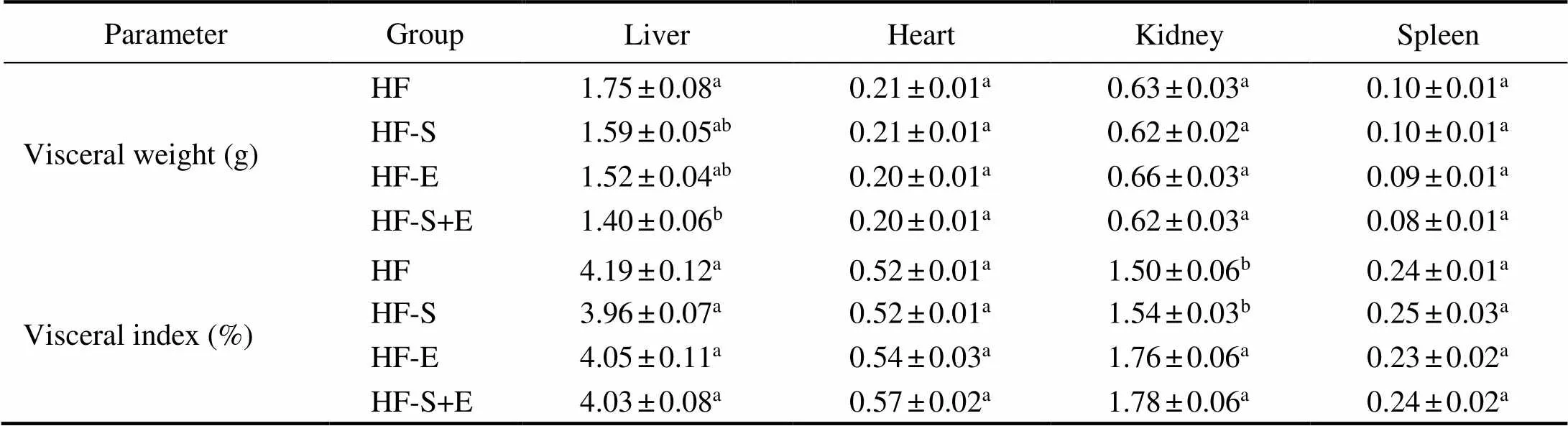
Table 1 Effect of saponins and exercise on the visceral weight (g) and visceral index (%) of KM mice
Notes: Values reflect the mean±SEM of eight mice. Different letters indicate significant differences at<0.05. HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.

Fig.2 Average food intake per day of KM mice (A). Changes in the body weight of high-fat diet-fed KM mice over time (B). Different letters represent significant differences (P<0.05). HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.
3.2 Influence of Dietary SCS and Exercise on Serum Parameters
Table 2 shows the influence of SCS supplementationand exercise on serum parameters, including TG, TC, HDL- C, LDL-C, glycerin, and FFA. Because serum TG is hy- drolyzed into glycerol and fatty acids under the action of lipoprotein lipase (LPL), serum triglycerides were analy- zed with glycerol phosphate oxidase-p-aminophenazone method (Sullivan., 1985). In fact, the measured gly-cerol concentrations reflect the contents of glycerol and triglycerides in serum and are listed as TG+glycerol in Table 2. Therefore, TG concentrations were calculated fromthe difference between the measured TG+glycerol level and the measured glycerol content. Compared with that in the HF group, serum triglyceride concentrations in the HF- S, HF-E, and HF-S+E groups significantly decreased (<0.05). Only dietary SCS combined with exercise signi- ficantly reduced LDL-C levels by 85% compared with that in the HF group. Dietary supplementation of SCS or exercise alone decreased serum cholesterol concentrations, but the difference was not significant. Interestingly, no significant changes in HDL-C level were observed among the four treatment groups. Additionally, the levels of TG hydrolysates, glycerin, and FFA increased to varying de- grees among the four treatment groups; however, the exercise group exhibited the highest levels of glycerin and FFA.

Table 2 Influence of sea cucumber saponins and exercise on serum parameters
Notes: Values reflect the mean±SEM of eight mice. Different letters indicate significant differences at<0.05. HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise. TG, triacylglycerol; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FFA, free fatty acid.
3.3 Influence of Dietary SCS and Exercise on Liver Lipids and Morphology
Consumption of a high-fat diet has been associated with steatosis and metabolic liver disorders. Thus, the contents of TC and TG in the liver were determined in this study (Figs.3A and B). Dietary SCS and exercise alone could re- duce TG content in the liver by 8% and 34%, respectively, whilethe combination of them significantly decreased the content of TG by 55%. Compared with that in the HF group, the contents of TC in the HF-S, HF-E, and HF-S+E groups showed a downward trend, although no significant difference was observed among these groups. Because HF diets could induce fatty liver, we assayed the impact of dietary SCS and exercise on lipid accumulation and hepa- tic steatosis by H&E staining. Results showed intense li- pid accumulation in the livers of mice in the HF group (Fig.3C). Among the four groups, the HF-S+E group show- ed the least lipid accumulation (Figs.3C–F). These results demonstrate that the combination of dietary SCS and ex- ercise remarkably prevents HF-induced lipid accumulation and blocks the development of hepatic steatosis compared with either intervention alone.
The fatty acid composition of lipids in the mouse liver was detected using gas chromatography (GC), and the re- sults are shown in Table 3. The combination of SCS and exercise could significantly reduce the proportion of mono- unsaturated fatty acids (C16:1, C18:1) and increase the proportion of saturated fatty acids (C18:0) compared with those in the other groups. Compared with those of the HF group, the liver samples of the HF-S+E group showed sig- nificant increases in arachidonic acid (C20:4) and docosa- hexaenoic acid (C22:6). The desaturation indices (16:1– 16:0 and 18:1–18:0) of the different groups were calculated (Sjögren., 2008), and the data demonstrated that the desaturation indices of the HF-E and HF-S+E groups were significantly reduced compared with those of the HF group (<0.05). Interestingly, the HF-S+E group show- ed reductions in desaturation indices of 16:1–16:0 and 18:1– 18:0 of 50% and 44%, respectively, (Table 3), which was superior to the HF-E groupin reducing desaturation indices.
3.4 Influence of Dietary SCS and Exercise on Adipose Mass and Morphology
As shown in Figs.4A and B, supplementation with SCS and exercise alone significantly decreased high-fat diet- induced fat accumulation in white adipose tissue, including epididymal fat, perirenal fat, mesenteric fat, visceral white fat, and subcutaneous white fat. The decrease was accompanied by a parallel reduction in body weight (<0.05). Interestingly, the combination of dietary SCS and exercise demonstrated excellent effects on reducing fat weight and index compared with those obtained from die- tary SCS or exercise alone; no significant difference in adipose weight and index was observed between the HF- S and HF-E groups. Compared with the HF group, the HF- S+E group showed decreases of 60%, 66%, 65%, 63%, 69%, and 63% in epididymis fat, perirenal fat, mesenteric fat, visceral white fat, subcutaneous white fat, and whiteadipose tissue weight, respectively. Adipose tissue cells are closely associated with lipid metabolism. The morpholo- gy of the adipose tissues from the different treatment groupswas analyzed by H&E staining. Compared with the HF group, the HF-S+E group showed significant reductions in the size of adipocytes in the epididymal white adipose tissue. These reductions were greater than those observed in the HF-S and HF-E groups.
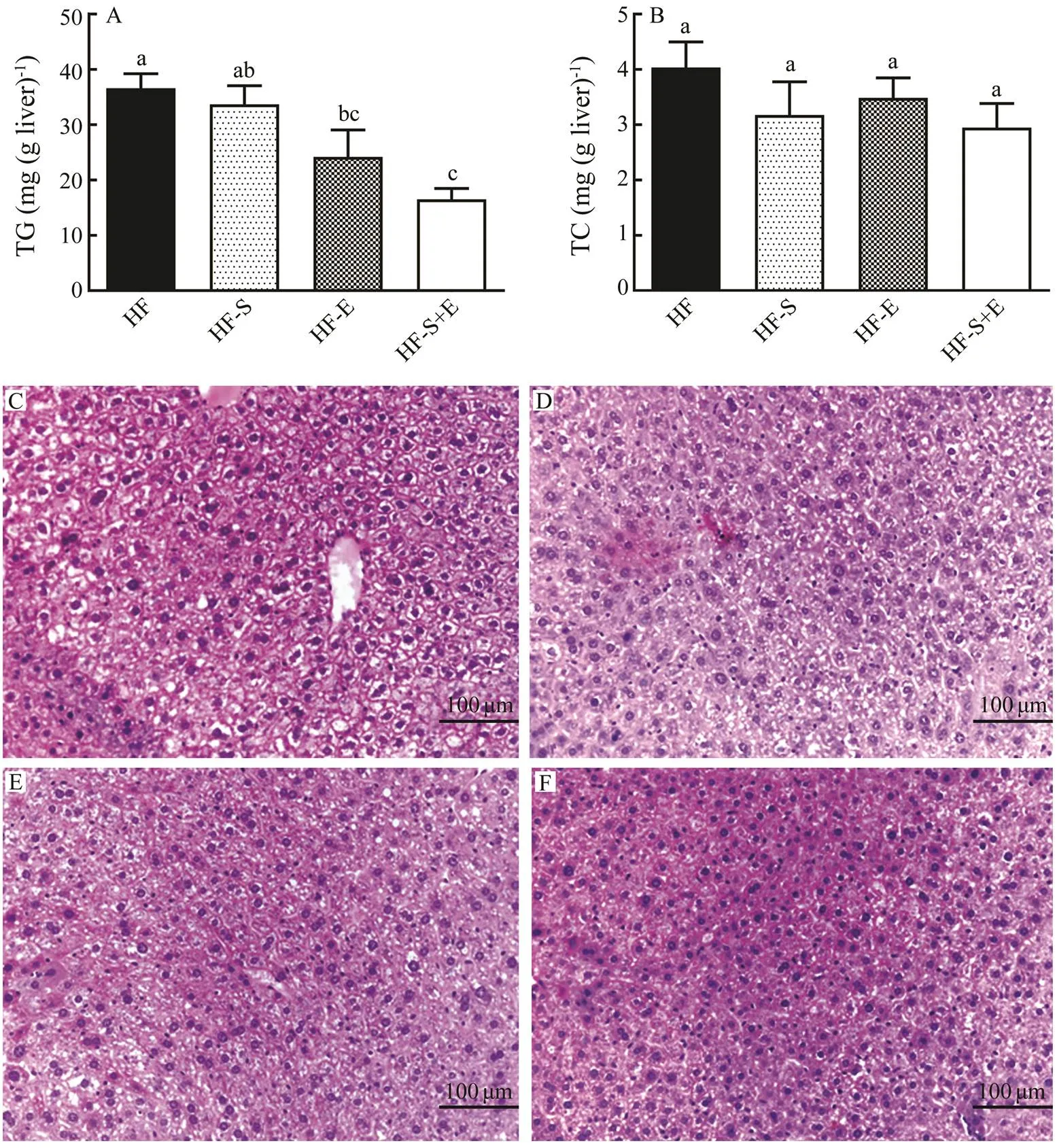
Fig.3 Hepatic contents of triacylglycerol (A) and cholesterol (B). H&E stains reflect morphological changes in the liver (C–F). Data reflect the mean±SEM. Different letters represent significant differences (P<0.05). TG, triacylglycerol; TC, total cholesterol; HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high- fat diet with saponins and exercise.
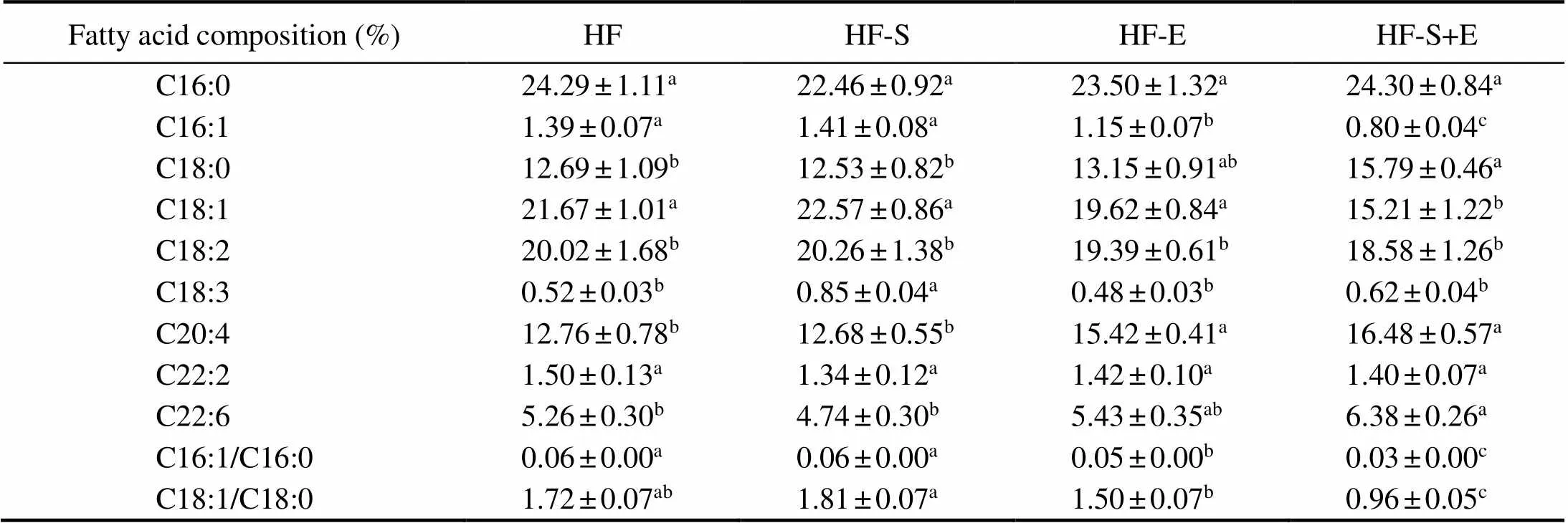
Table 3 Fatty acid composition of liver lipids
Notes: Values reflect the mean±SEM of eight mice. Different letters indicate significant differences at<0.05. HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.
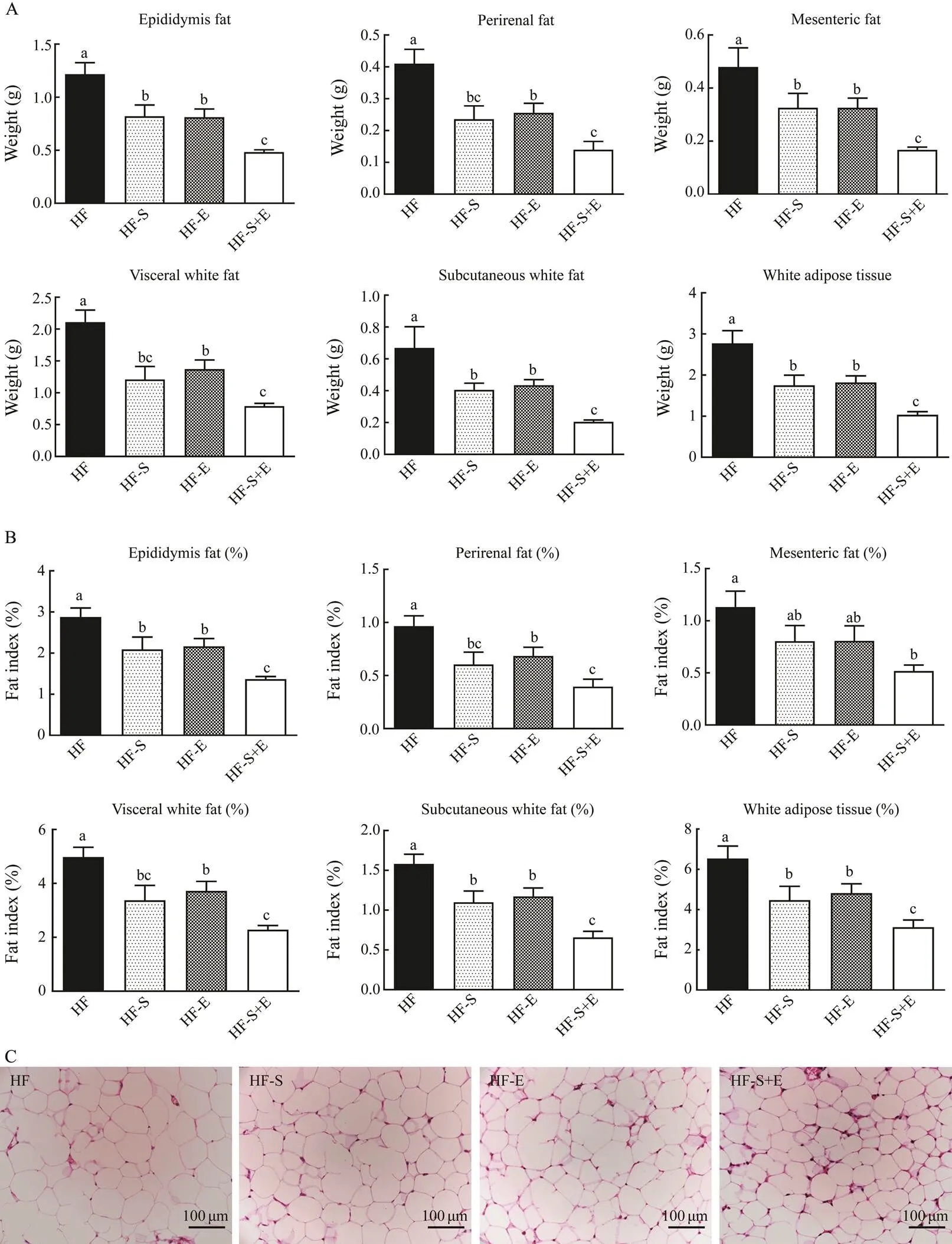
Fig.4 Weight (A) and body fat index (B) of KM mice.H&E stains reflect morphological changes in epididymal adipose tissue (C). Data reflect the mean±SEM. Different letters represent significant differences (P<0.05). HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.
3.5 Influence of Dietary SCS and Exercise on mRNA Expression Associated with β-Oxidation and Lipogenesis in Liver
The transcription level of genes associated with lipoge- nesis and β-oxidation was determined to assess the mecha- nism of SCS and exercise in reducing triacylglycerol le- vels. The regulation of lipogenesis in the liver is control- led by sterol regulatory element-binding protein (SREBP).is essential for TG synthesis because it regulates the expression of downstream target genes, such as fatty acid synthase (), acetyl-CoA carboxylase 1 (),and stearoyl-CoA desaturase-1 (). As shown in Fig.5A, the expression ofanddecreased in the treatment groups compared with that in the HF group (<0.05), but no downtrend in the mRNA expressions ofandwas observed. The expressions ofand+E group significantly decreased by 83% and 80%, respectively. SCD is the key enzyme responsible for converting saturated fatty acids into monounsaturated fatty acids, and changes inexpression were consistent with changes in the desaturation indices of the liver (Table 3). The mRNA expression levels ofand, which provide NADPH for thesynthesis of fatty acids, were not affected by dietary SCS or exercise.regulates mitochondrial and peroxisome oxidation to promote the oxidative decomposition of fatty acids, thereby regulating the expression of its tar- get genes in downstream pathways, including,,, and. As shown in Fig.5B, the three treat-ment groups showed increased mRNA expression ofand its target genegroup, but the differences observed were not significant. The expressions of,, anddid not change in the treatment groups relative to that in the HF group.
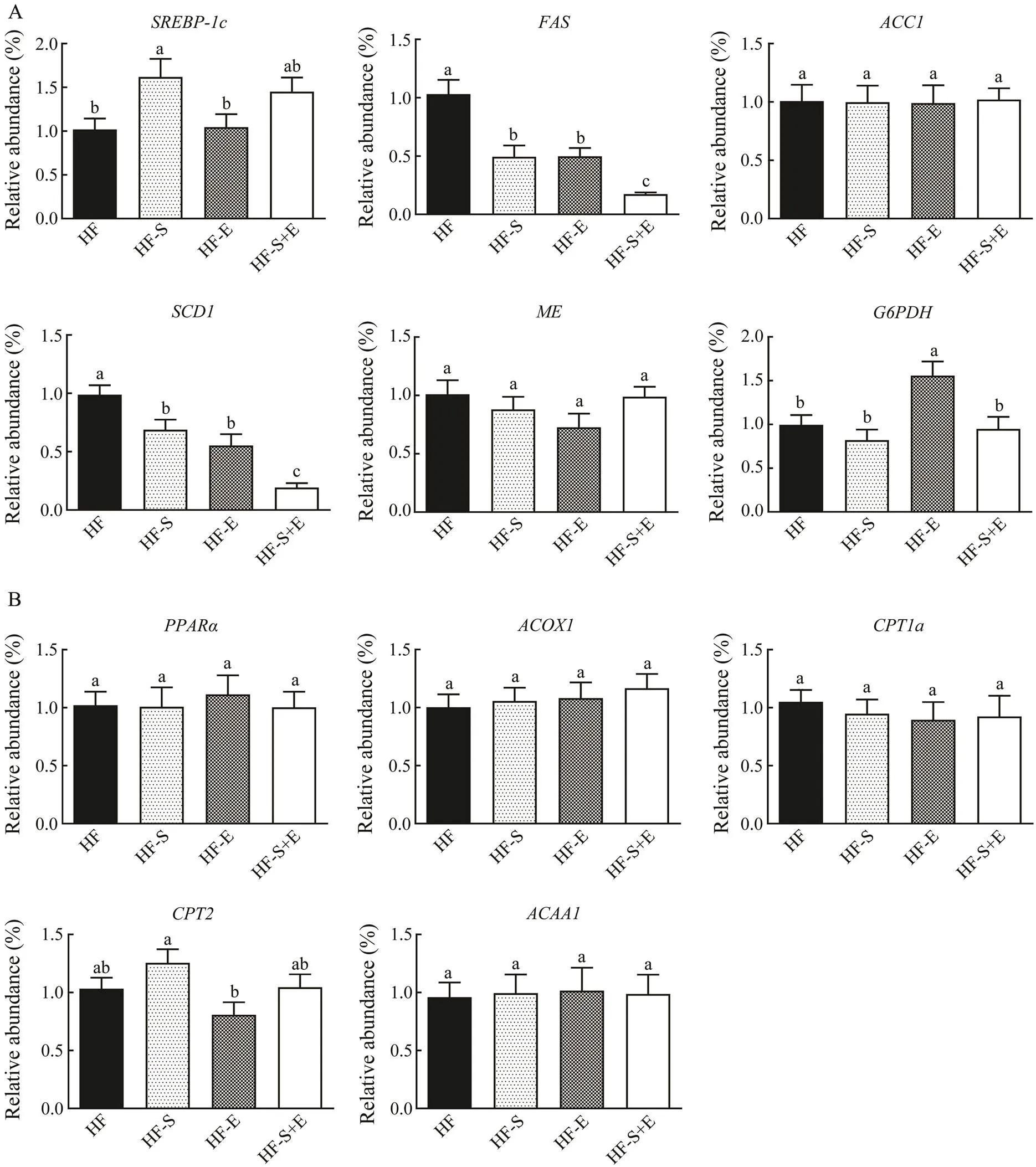
Fig.5 MicroRNA expression associated with lipogenesis (A) and β-oxidation (B) in the liver. Data reflect the mean±SEM (n=8).Different letters represent significant differences (P<0.05). SREBP-1c, sterol regulatory element binding transcrip- tion factor 1; FAS, fatty acid synthase; ACC1, 1-aminocyclopropane-1-carboxylate 1; SCD1, stearoyl-coenzyme A de- saturase 1; ME, malic enzyme; G6PDH, glucose-6-phosphate dehydrogenase; PPARα, peroxisome proliferator activated receptor alpha; ACOX1, acyl-coenzyme A oxidase 1; CPT1a, carnitine palmitoyl transferase 1a; CPT2, carnitine palmi- toyl transferase 2; ACAA1, acetyl-coenzyme A acyltransferase 1. HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.
3.6 Influence of Dietary SCS and Exercise on Expre- ssion of mRNA Associated with Lipogenesis and Lipolysis in Epididymal White Adipose Tissue
The mRNA level of genes associated with lipogenesis and lipolysis in epididymal white adipose tissue was stu- died to assess the mechanism of SCS and exercise in suppressing adipose accumulation. As shown in Fig.6A, the expression ofand its target genes,, and, all of which are related to lipogenesis, did not change in the treatment groups. By contrast, the expression ofin the HF-S and HF-E groups declined by 38% and 41%, respectively compared with that in the HF group. Interestingly, the expression ofdecreased by 74% in the HF-S+E group; this decline is greater than those in the HF-S and HF-E groups. The expression ofdid not change in the HF-E group but decreased in the HF-S+E group (<0.05). Moreover, the mRNA expression of(Fig.6B), which could enhance FFA solubility and transport to specific enzymes andcellular compartments, did not change among the three treatment groups. ATGL and HSL are the major enzymes involved in the activity of TG hydrolase in adipose tissue. In this study, the ex- pression ofin the HF-E and HF-S+E groups and that ofin the HF-E group significantly increased com- pared with the HF group. The expression of, which could cause an inflow of fatty acids into adipose tissue, was not modified in any of the groups. The expression ofdid not change in the HF-S and HF-E groups but increased significantly in the HF-S+E group (<0.05).
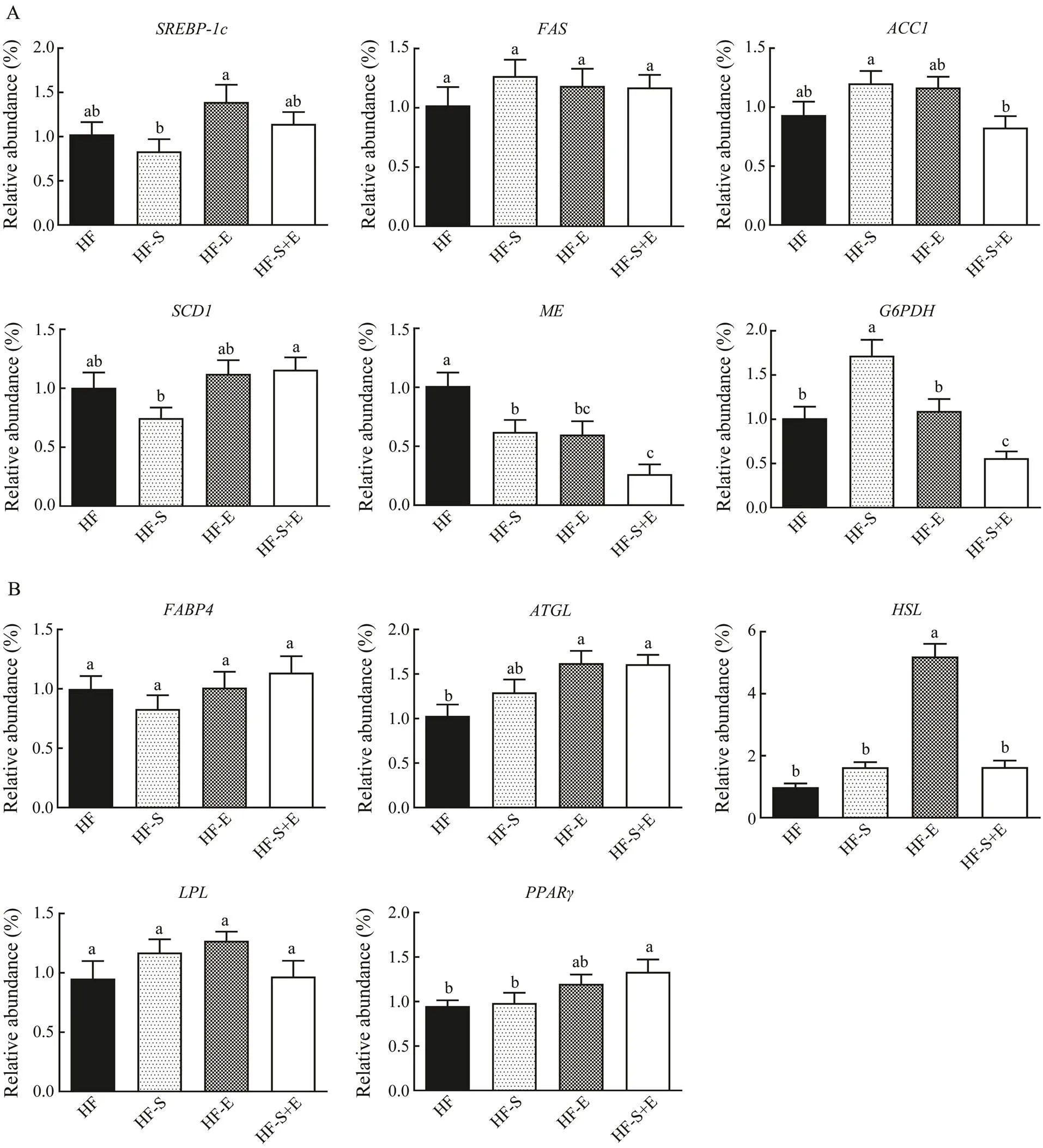
Fig.6 Expression levels of mRNA associated with lipogenesis (A) and lipolysis (B) in epididymal white adipose tissue. Data reflect the mean±SEM (n=8). Different letters represent significant differences (P<0.05). SREBP-1c, sterol regula- tory element binding transcription factor 1; FAS, fatty acid synthase; ACC1, 1-aminocyclopropane-1-carboxylate 1; SCD1, stearoyl-coenzyme A desaturase 1; ME, malic enzyme; G6PDH, glucose-6-phosphate dehydrogenase; FABP4, fatty acid binding protein 4; ATGL, adipose triglyceride lipase; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; PPARγ, peroxisome proliferator-activated receptor γ. HF, high-fat group; HF-S, high-fat diet with saponins; HF-E, high-fat diet with exercise; HF-S+E, high-fat diet with saponins and exercise.
4 Discussion
Obesity is closely related to an individual’s lifestyle and generally is considered to be resulted from a lack of phy- sical activity, excessive intake of dietary calories, and me- tabolic disorders. Proper aerobic exercise can reduce obesity by increasing the body’s total metabolic expenditure. Adequate exercise has clearly been proven to reduce cardiovascular risk and alleviate many cardiometabolic risk factors (Oktay., 2017; Swift., 2018). However, exercise is often ignored for different reasons.Thus, the use of dietary supplements, which requires considerably less effort than traditional behavioral changes, such as re- striction of diet, has become an increasingly popular means to lose weight(Pillitteri., 2008). Studies on the individual beneficial effects of exercise or various food ingre- dients, including dietary fiber, saponins, and mulberry an- thocyanins, are widely available; however, research focus-ed on the combined effects of exercise and food ingredients on the development of obesity is limited (Wu., 2013;Liu., 2017). The results of the present study re- vealed that the combination of dietary SCS and exercise significantly reduces adiposity. Furthermore, SCS supple- mentation with exercise improves peripheral markers, such as serum parameters and hepatic TG levels (Fig.7). Although several studies have demonstrated the independent effect of SCS on obesity, the present study further explores the anti-obesity effects of SCS under the condition of ex- ercise (Wang., 2014; Guo., 2016). Previous stu- dies demonstrated that the combination of dietary conjugated linoleic acids and exercise could decrease fat mass and increase lean mass in mice fed a high-fat diet(Bhattacharya., 2005), which supports our findings in this research. Another study demonstrated that polyunsaturatedfatty acids suppressed lipogenesis by inhibiting LXRα tran- sactivation to improve lipid metabolism in the livera mechanism similar to that of food-derived saponins (Takeuchi., 2010; Uemura., 2011).
Previous studies suggested that exercise may inhibit fat formation to suppress lipid accumulation in the liver (Car- lson and Winder, 1999; Lavoie and Gauthier, 2006). SCS canfurther suppressthe lipogenesis. Indeed, the combina- tion of exercise and SCS significantly reduced the mRNA expression ofand., 2011).activity is regulated by the amount of intracel-lular cholesterol, and LXR is activated by the oxidized cho- lesterol (oxysterol) as an endogenous ligand. LXRα has been reported to regulatetranscription, which can influencefatty acid synthesis-related genes, such asand(Chen., 2004). Exercise can increase the basal energy expenditure by increasing fat oxi- dation. In this study, the fatty acid β-oxidation gene ex- pression was mediated by SCS or exercise independently, differing from previous studies.The differences might be attributed to variations in the experimental conditions, such as mice strain (KM or C57/BL6) or intensity of exercise (Meng., 2018).

Fig.7 Effects of dietary supplementation with sea cucumber saponins together with exercise in suppressing adipose accumulation in high-fat diet-fed mice and the possible mechanism.
While an abundance of small adipocytes was observed in the dietary SCS+exercise treatment group, the HF con- trol group showed large adipocytes. Previous studies de- monstrated that the consistency of adipose tissue cells is the main determinant of metabolic activity and the enhanced lipolysis or blunted lipogenesis may contribute to differences in adipocyte size (Schweiger., 2006;Marques., 2010). In this study, we found that moderate-in- tensity exercise and diet could inhibit body fat accumulation due to increased lipolysis. Schweiger. (2006)proved that adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) were the major enzymes con-tributing to TG breakdown inassays and organ cul- tures of murine white adipose tissue.The combination of SCS and exercise can increase lipolysis mainly by increa- sing the expressions ofand(Karbowska and Kochan, 2012). The fat in adipose tissue is mobilized to release FFA and glycerol into the circulation system. Se- rum FFA and glycerol are mainly derived from the lipoly- sis in adipose tissue through the catalysis of HSL (Cho., 2010; Zhang., 2014). Endurance or resistance exercise can increase the serum free fatty acid levels in obese women (Davitt., 2013). Our results suggest that exercise promotes lipolysis. Ohyama. (2014) demon- strated that exercise could activate the phosphorylation of PKA to induce the activity of HSL, thereby enhancing li- polysis. Thus, the combination of SCS and exercise may promote lipolysis by activating the phosphorylation of PKA.The combination of exercise and dietary SCS also increased the expression ofto regulate lipid metabolism, which was consistent with a previous study (Brunani., 2008).
The skeletal muscle plays an important role in the body’s energy expenditure, and participatesglycolysis and lipid me- tabolic processes (Sylow., 2017). The fuel supply in- volves different enzymatic pathways responsible for obtaining energy from glucose and fatty acids through glycolysis and β-oxidation, respectively (Simon., 2002). Various stimuli, such as β-adrenergic agonists and exercise, trigger fatty acid generation (Dyck., 1998). In the pre- sent study, skeletal muscles may also be responsible for energy expenditure by increasing fat oxidation in the ex- ercise group, which requires further study.
5 Conclusions
The present study is the first to confirm that dietary SCS can significantly enhance the benefits of exercise in suppressing adipose accumulation. The combination of dietary SCS and exercise can significantly reduce body weight, adipose tissue accumulation, and serum and hepatic lipids, which are consistent with the liver and adipose tissue mor- phology. Inhibition of adipose accumulation may be achi- eved by suppressing lipid synthesis in the liver and promoting lipolysis in epididymal white adipose tissue. The synergistic effects of anti-obesity food ingredients and ex- ercise can effectively control body weight.
Acknowledgements
This work was supported by the National Key R&D Pro- gram of China (No. 2018YFD0901103) and the National Natural Science Foundation of China (Nos. 31901688 and 31571771).
Abbreviation List
, 1-aminocyclopropane-1-carboxylate 1;, acyl-coenzyme A oxidase 1;, adipose triglyceride li-pase;, acetyl-coenzyme A acyltransferase 1;, carnitine palmitoyl transferase 1a;, carnitine palmitoyl transferase 2; EA, echinoside A;, fatty acid synthase;, fatty acid binding protein 4; FFA, free fat- ty acid;, glucose-6-phosphate dehydrogenase; GC, gas chromatography; HA, holothurin A; HDL-C, density lipoprotein cholesterol; HF, high-fat diet group; HF-S, sea cucumber saponins group; HF-E, exercise group; HF-S+E, sea cucumber saponins and exercise group;, hormone-sensitive lipase;, lipoprotein lipase; LDL-C, low-density lipoprotein-cholesterol;, malic enzyme;, peroxisome proliferator activated receptor alpha;, peroxisome proliferator-activated receptor γ; SCS, sea cucumber saponins;, stearoyl-coenzyme A desaturase 1;, sterol regulatory element binding transcription factor 1; TC, cholesterol; TG, triacylglycerol.
Adegboye, A. R. A., and Linne, Y. M., 2013. Diet or exercise, or both, for weight reduction in women after childbirth.,7: CD005627.
Bhattacharya, A., Rahman, M. M., Sun, D., Lawrence, R., Mejia, W., McCarter, R., O’shea, M., and Fernandes, G., 2005. The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat-fed male balb/c mice.,135 (5): 1124-1130.
Brunani, A., Caumo, A., Graci, S., Castagna, G., Viberti, G., and Liuzzi, A., 2008. Rosiglitazone is more effective than metformin in improving fasting indexes of glucose metabolism in severely obese, non-diabetic patients.,10 (6): 460-467.
Carlson, C., and Winder, W., 1999. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise.,86 (2): 669-674.
Chen, G., Liang, G., Ou, J., Goldstein, J. L., and Brown, M. S., 2004. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver.,101 (31): 11245-11250.
Cho, A.S., Jeon, S.M., Kim, M.J., Yeo, J., Seo, K.I., Choi, M.S., and Lee, M.K., 2010. Chlorogenic acid exhibits anti-obe- sity property and improves lipid metabolism in high-fat diet-induced-obese mice.,48 (3): 937-943.
Davitt, P. M., Arent, S. M., Tuazon, M. A., Golem, D. L., and Henderson, G. C., 2013. Postprandial triglyceride and free fat- ty acid metabolism in obese women after either endurance or resistance exercise.,114 (12): 1743-1754.
Dyck, D. J., and Bonen, A., 1998. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxi- dation., 275 (5): E888-E896.
Eaton, S., 2002. Control of mitochondrial β-oxidation flux., 41 (3): 197-239.
Folch, J., Lees, M., and Stanley, G. S., 1957. A simple method for the isolation and purification of total lipides from animal tissues.,226 (1): 497-509.
Galani, C., and Schneider, H., 2007. Prevention and treatment of obesity with lifestyle interventions: Review and meta-analysis.,52 (6): 348-359.
Guo, L., Gao, Z., Zhang, L., Guo, F., Chen, Y., Li, Y., and Huang, C., 2016. Saponin-enriched sea cucumber extracts exhibit an antiobesity effect through inhibition of pancreatic lipase activity and upregulation of LXR-β signaling.,54 (8): 1312-1325.
Hu, X.Q., Wang, Y.M., Wang, J.F., Xue, Y., Li, Z.J., Nagao, K., Yanagita, T., and Xue, C.H., 2010. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in ratsPPARα and SREBP-1c signaling.,9 (1): 25.
Karbowska, J., and Kochan, Z., 2012. Fat-reducing effects of dehydroepiandrosterone involve upregulation of ATGL and HSL expression, and stimulation of lipolysis in adipose tissue.,77 (13): 1359-1365.
Kushner, R. F., 2014. Weight loss strategies for treatment of obesity.,56 (4): 465-472.
Lavoie, J. M., and Gauthier, M. S., 2006. Regulation of fat metabolism in the liver: Link to non-alcoholic hepatic steatosis and impact of physical exercise.,63 (12): 1393-1409.
Liu, R., Zheng, Y., Cai, Z., and Xu, B., 2017. Saponins and flavonoids from adzuki bean (L.) ameliorate high-fat diet-induced obesity in ICR mice.,8: 687.
Liu, X., Cui, J., Leng, K., Xue, C., Li, Z., Xue, Y., and Wang, Y., 2016. Docosahexaenoic acid-enriched phospholipids exhibit superior effects on obesity-related metabolic disorders to egg yolk phospholipids and soybean phospholipids in mice.,118 (11): 1712-1721.
Liu, X., Cui, J., Li, Z., Xu, J., Wang, J., Xue, C., and Wang, Y., 2014. Comparative study of DHA-enriched phospholipids and EPA-enriched phospholipids on metabolic disorders in diet- induced-obese C57BL/6J mice.,116 (3): 255-265.
Marques, C., Motta, V., Torres, T., Aguila, M., and Mandarim-de-Lacerda, C., 2010. Beneficial effects of exercise training (treadmill) on insulin resistance and nonalcoholic fatty liver disease in high-fat fed C57BL/6 mice., 43 (5): 467-475.
Meng, J., Hu, X., Zhang, T., Dong, P., Li, Z., Xue, C., Chang, Y., and Wang, Y., 2018. Saponin from sea cucumber exhibited more significant effects than ginsenoside on ameliorating high fat diet-induced obesity in C57BL/6 mice.,9 (4): 725-734.
Ohyama, K., Nogusa, Y., Suzuki, K., Shinoda, K., Kajimura, S., and Bannai, M., 2014. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice.,308 (4): E315-E323.
Oktay, A. A., Lavie, C. J., Kokkinos, P. F., Parto, P., Pandey, A., and Ventura, H. O., 2017. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease.,60 (1): 30-44.
Pillitteri, J. L., Shiffman, S., Rohay, J. M., Harkins, A. M., Bur-ton, S. L., and Wadden, T. A., 2008. Use of dietary supplements for weight loss in the United States: Results of a national survey.,16 (4): 790-796.
Schweiger, M., Schreiber, R., Haemmerle, G., Lass, A., Fledelius, C., Jacobsen, P., Tornqvist, H., Zechner, R., and Zimmer- mann, R., 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism.,281 (52): 40236-40241.
Sjögren, P., Sierra-Johnson, J., Gertow, K., Rosell, M., Vessby, B., De Faire, U., Hamsten, A., Hellenius, M.L., and Fisher, R., 2008. Fatty acid desaturases in human adipose tissue: Relationships between gene expression, desaturation indexes and insulin resistance.,51 (2): 328-335.
Sullivan, D. R., Kruijswijk, Z., West, C. E., Kohlmeier, M., and Katan, M. B., 1985. Determination of serum triglycerides by an accurate enzymatic method not affected by free glycero., 31 (7): 1227-1228.
Swift, D. L., McGee, J. E., Earnest, C. P., Carlisle, E., Nygard, M., and Johannsen, N. M., 2018. The effects of exercise and physical activity on weight loss and maintenance.,61 (2): 206-213.
Sylow, L., Kleinert, M., Richter, E. A., and Jensen, T. E.,2017. Exercise-stimulated glucose uptake-regulation and implications for glycaemic control., 13 (3): 133-148.
Takeuchi, Y., Yahagi, N., Izumida, Y., Nishi, M., Kubota, M., Teraoka, Y., Yamamoto, T., Matsuzaka, T., Nakagawa, Y., and Sekiya, M., 2010. Polyunsaturated fatty acids selectively sup- press sterol regulatory element-binding protein-1 through pro- teolytic processing and autoloop regulatory circuit.,285 (15): 11681-11691.
Uemura, T., Goto, T., Kang, M. S., Mizoguchi, N., Hirai, S., Lee, J. Y., Nakano, Y., Shono, J., Hoshino, S., and Taketani, K., 2011. Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic tri- glycerides in obese diabetic mice.,141 (1): 17-23.
Wang, T., Xue, C., Zhang, T., and Wang, Y., 2018. The improve- ments of functional ingredients from marine foods in lipid me-tabolism.,81: 74-89.
Wang, Y., Wang, J., Yanagita, R. C., Liu, C., Hu, X., Dong, P., Xue, C., and Xue, Y., 2014. Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction.,78 (1): 139-146.
Wu, T., Qi, X., Liu, Y., Guo, J., Zhu, R., Chen, W., Zheng, X., and Yu, T., 2013. Dietary supplementation with purified mulberry (Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice.,141 (1): 482-487.
Ye, S. Y., and Liu, J., 2016. Chinese adults’ physical exercise, static behaviors, trends and influencing factors.,28: 365-369.
Zhang, J., Zhao, Y., Xu, C., Hong, Y., Lu, H., Wu, J., and Chen, Y., 2014. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study.,4: 5832.
Zhao, Y. C., Xue, C. H., Zhang, T. T., and Wang, Y.M., 2018. Sa- ponins from sea cucumber and their biological activities., 66 (28): 7222-7237.
May 1, 2020;
August 3, 2020;
November 10, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
E-mail: zhangtiantian@ouc.edu.cn E-mail: wangyuming@ouc.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Case Study of a Short-Term Wave Energy Forecasting Scheme:North Indian Ocean
- Temporal and Spatial Characteristics of Wave Energy Resources in Sri Lankan Waters over the Past 30 Years
- Vibration Deformation Monitoring of Offshore Wind Turbines Based on GBIR
- Dependence of Estimating Whitecap Coverage on Currents and Swells
- The Variation of Microbial (Methanotroph) Communities in Marine Sediments Due to Aerobic Oxidation of Hydrocarbons
- 3-Aminopropyltriethoxysilane Complexation with Iron Ion Modified Anode in Marine Sediment Microbial Fuel Cells with Enhanced Electrochemical Performance
