Removal of Sulfamethazine by Corn Biochars from Aqueous Solution: Sorption Mechanisms and Efficiency
2021-06-25ZHANGMoZHAOYangguoWANGJinpengBAIJieandLiKuiran
ZHANG Mo, ZHAO Yangguo, WANG Jinpeng, BAI Jie, and Li Kuiran
Removal of Sulfamethazine by Corn Biochars from Aqueous Solution: Sorption Mechanisms and Efficiency
ZHANG Mo1), 4), ZHAO Yangguo1), 2), WANG Jinpeng1), BAI Jie1), 2), and Li Kuiran3), *
1)College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China 2) Key Laboratory of Marine Environment and Ecology(Ocean University of China), Ministry of Education,Qingdao 266100, China 3)College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China 4)The People’s Government of Laiwu District, Jinan 271100, China
Sorption mechanisms of sulfamethazine (SMT) in different pH solutions are complicated. It has not been sufficiently investigated to enhance removal of SMT from alkalescent aqueous solution. In this study, sorption isotherms and kinetics of SMT by corn biochars pyrolyzed at 300℃ and 600℃ (300C, 600C) under diverse pH conditions were compared. In order to improve the sorption efficiency of SMT from alkalescent aqueous solution, the biochar 300C was modified by acid or base. Different mathematic models were used to describe sorption driving force and sorption process. Results showed that the biochar 300C possessed more amorphous organic carbon and polar functional groups, which led to a higher sorption capacity than biochar 600C. The sorption rate of biochar 300C was greater than 600C under diverse pH as the physisorption of 300C outweighed 600C. The SMT presented stronger hydrophobicity at pH 5 and higher electrostatic repulsion at pH 1 or 8, which resulted in a higher combining capacity of SMT with both biochars at pH 5 than other pH values. In addition, the acid modified biochar had better removal effect on SMT than alkali at pH solution around 8. The research provides a theoretical basis for the removal of SMT from alkalescent aqueous solution.
sulfamethazine; sorption; mechanisms; process; modification
1 Introduction
Among various treatment methods, sorption has been one of the most simple and effective methods to remove pollutants from the environment (Reguyal and Sarmah, 2018). Compared with activated carbon, biochar presents many advantages such as lower production cost and wider sources (Kumar., 2020). Biochar refers to a solid carbonaceous material producedpyrolysis of biomass such as agricultural crop residues, animal manure and sewage sludge in reactors under conditions of low oxygen and moderate temperature. (Zhang., 2015; Sepehri and Sarrafzadeh, 2018). Due to its soil fertility and carbon sequestration ability, biochar has attracted much attention in agricultural science as a means of sequestering carbon (Heitkotter and Marschner, 2015). In addition, due to aromatic hydrophobic surface and the rearrangement of microcrystalline structure, biochar presents strong potential in water remediation and ability to remove various contaminants such as heavy metals, oxygen-containing anions and organic compounds (Li., 2017). Con-sequently, the biochars become more popular in environmental engineering research field.
Antibiotics are often detected in surface water, groundwater and other water environments (Zhang., 2015). The accumulation of antibiotics in the environment may lead to enrichment of bacterial resistance, which makes it more difficult to control the diseases and easily imbalance the microbial ecosystems (Lorenzo., 2018; Xiong., 2019). Some of these antibiotics are in substantial amounts and found to have potential toxic effects on human and wildlife (Reguyal and Sarmah, 2018). In China, most of aquaculture farms are directly connected to the external aquatic environments, which lead to discharge of residual antibiotics into the surface water through the aquaculture wastewater (Rico., 2013). Ocean is becoming a major reservoir of antibiotic residues (Chen., 2015). Hence, it is urgent to remove antibiotics from mariculture wastewater before discharge. The removal of antibiotics by sorption is a simple but important method to control their release (Zheng., 2013).
Sulfonamides have been widely used in aquaculture to control the disease (Bilkova., 2019). Since they are poorly metabolized, much of sulfonamides residues still exist in aquaculture wastewater (Liu., 2018). Research (Teixido., 2011) found the sulfonamides showed an unconventional sorption behavior due to complex ionic form at various pH levels, which led to hardly remove sulfonamides from natural water and mariculture wastewater at about pH 8. However, present researches mainly care about the sorption of the same charged sulfonamides by different sources biochars under consant pH environment. Few researches focus on the sorption me- chanisms of sulfonamides with various electric charges by biochars at different pyrolysis temperatures (Ahmed., 2017; Jia., 2017). It should be noted that none of the studies above report the method about improving removal of sulfonamides from alkalescent aqueous solution. Hence, to improve sorption efficiency, it is necessary to reveal the sorption mechanisms and behavior of sulfonamides by the biochars under different pH solutions. Besides the research about modified biochar removing sulfonamides in alkalescent aqueous solution should be investigated.
In this study, sulfamethazine (SMT) was selected as the target pollutant, and corn straw biochars with different pyrolysis temperatures (300℃ and 600℃) were used as the sorbent. To remove SMT from alkalescent aqueous solution, sorption mechanisms and sorption process of SMT by corn biochars at various pH solutions were studied by using sorption isotherms and kinetics. To improve sorption capacity, the biochars were modified and the sorption performance was investigated. The results will provide a theoretical basis and technical support for antibiotics removal from alkalescent aqueous solution such as mariculture wastewater.
2 Materials and Methods
2.1 Chemicals
Sulfamethazine (purity >95%) standard compound was purchased from Supelco Chemical Co. (USA). Physicochemical properties of sulfamethazine include: molecular weight=278.34,pK,1=2.65±0.2 andpK,2=7.4±0.2 (Lertpaitoonpan., 2009). The logKow of SMT+, SMT0and SMT−are −1.3, 0.27 and −2.09, respectively (Carda- Broch and Berthod, 2004). Acetonitrile (HPLC grade) was purchased from Tedia Company, Inc. (Fairfield, USA). A stock solution of 100mgL−1of sulfame- thazine dissoluted with 0.1molL−1HCl solution was prepared.
2.2 Biochar Preparation and Characterization
Corn straws were used as the feedstock to produce biochars and collected from farmland in Laiwu, Shandong Province, China. After being washed and dried at 80℃ for at least 12h, the corn straws were ground and transferred to the tube furnace. The heating speed of tube furnace was 10℃min−1under nitrogen protection and heat preservation time was 2h. The corn straws were pyrolyzed at 300℃ and 600℃ respectively. Then the residues were ground and sieved with a 60 mesh sieve, and recorded as 300C and 600C. The elemental composition of the biochars was determined by the CHN elemental analyzer (Vario MICRO, Germany). The surface functional group were examined using FTIR (Miracle-10, Shimadzu, Japan). The spectra were obtained by measuring the absorbance from 400 to 4000cm−1using a combined 40 scans. Zeta potential values of the biochar particles were detected by using zeta potential analyzer (90Plus, Brookhaven, USA).
2.3 Sorption of Sulfamethazine
2.3.1 Effect of pH changes on SMT sorption by biochars
Biochar (50 mg 300C or 600C) was prewetted in 5mL Milli-Q water shaking at 120rmin−1for 24 h at 25℃. Then 2 mL SMT stock solution (100mgL−1) was added to the tubes. Afterwards, the solution pH was amended from 1 to 9 using 1molL−1HCl or 1molL−1NaOH solution. Milli Q water were added to keep all the samples in a total volume of 10mL. The tubes were shaken at 120r min−1for 48h at 25℃ in the dark. All experiments were conducted in triplicates. After shaking, 1.0mL suspension was filtrated by using 0.45μm microfiltration membrane.
The concentration of SMT in the filtrate was analyzed using HPLC (Agilent, USA) as following conditions. The UV detector at 265nm was selected. A reverse phase Zorbax Bonus RP C18 column (5.0μm, 2.1mm×1.50mm, Agilent Technologies, USA) was used for the separation. Mobile phase A was composed of acetonitrile and mobile phase B was composed of Milli-Q water and acetic acid (0.1%). The elution contained 40% of A and 60% of B at a flow rate of 0.8mLmin−1.
2.3.2 Sorption isotherms
Sorption isotherms experiments were conducted in triplicates using batch mode described in Section 2.3.1 except that the final SMT concentrations in tubes were 2, 5, 10, 20 and 40mgL−1and the pH values of the solution were amended at 1, 4 or 8. The time intervals were 0.5, 1, 2, 4, 8, 16, 24, 48 and 72h. And the filtrates were used to HPLC analysis.
2.3.3 Sorption kinetics
Similarly, the sorption kinetics experiments were conducted in triplicates using batch mode as the above except that, the final SMT concentrations in tube was 20mgL−1and the pH values of the solution were amended at 1, 4 or 8. The three solution samples were obtained on time 0.5, 1, 2, 4, 8, 16, 24, 48, 72h, respectively, and the filtrates were used to HPLC analysis.
2.3.4 Modification of corn biochar
To improve the sorption capacity of the biochar for the SMT, the biochar 300C was modified by acids (H2SO4or HNO3) or alkalis (NaOH or KOH). About 10g of 300C was soaked in 200 mL water containing the above acids or alkalis. The acids or alkalis were kept at 0, 0.2, 1.0, 20, 100 and 200mmol per gram biochar 300C. Solutions were stirred at room temperature (25℃±2℃) for 24h. The precipitated biochars were washed with distilled water until the washing solution reached neutral pH. The biochars were then dried overnight at 80℃. The biochars modified by acids were labeled as 300CAC (0.2, 1.0, 20, 100, 200), while alkali modified biochar as 300CAL (0.2, 1.0, 20, 100, 200). Single point sorption (20mgL−1) of SMT was conducted using modified 300C. 1molL−1NaOH was used to adjust the solution pH at 8. After shaking, 1.0mL suspension was filtrated and used for detecting the residual SMT by the HPLC.
2.4 Data Analysis
Different mathematic models were used to describe sorption driving force and sorption process. Langmuir, Freundlich and Adsorption-partition models were employed to fit the sorption isotherms of SMT to further understand the sorption mechanisms. Pseudo-first-order (PFO), Pseudo-second-order (PSO), Intra-particle diffusion model (IDM) and Boyd model were used to fit the sorption kinetic experimental data to infer the sorption rate limiting step and the relevant sorption mechanisms. The detailed information for each model was provided in the supplemental files. The parameters were estimated by nonlinear regression analysis using a Sigma plot 10.0 software and OriginPro 8.0.
3 Results and Discussion
3.1 Characterization of Biochars
The elemental composition and atomic ratio of corn straw biochars were shown in Table 1. As the improvement of pyrolysis temperature, the H, N and O contents of biochars decreased while the C content increased. The value of H/C decreased but the degree of carbonization increased. Compared to 300C, the values O/C and surface polarity index (O+N)/C of 600C decreased indicating hydrophilicity and surface polar functional groups reduced. The results of zeta potential values of 300C and 600C were shown in Fig.1. The surfaces of two corn biochars were positive at pH 1 while at pH 5 or 8, the surfaces were negative.
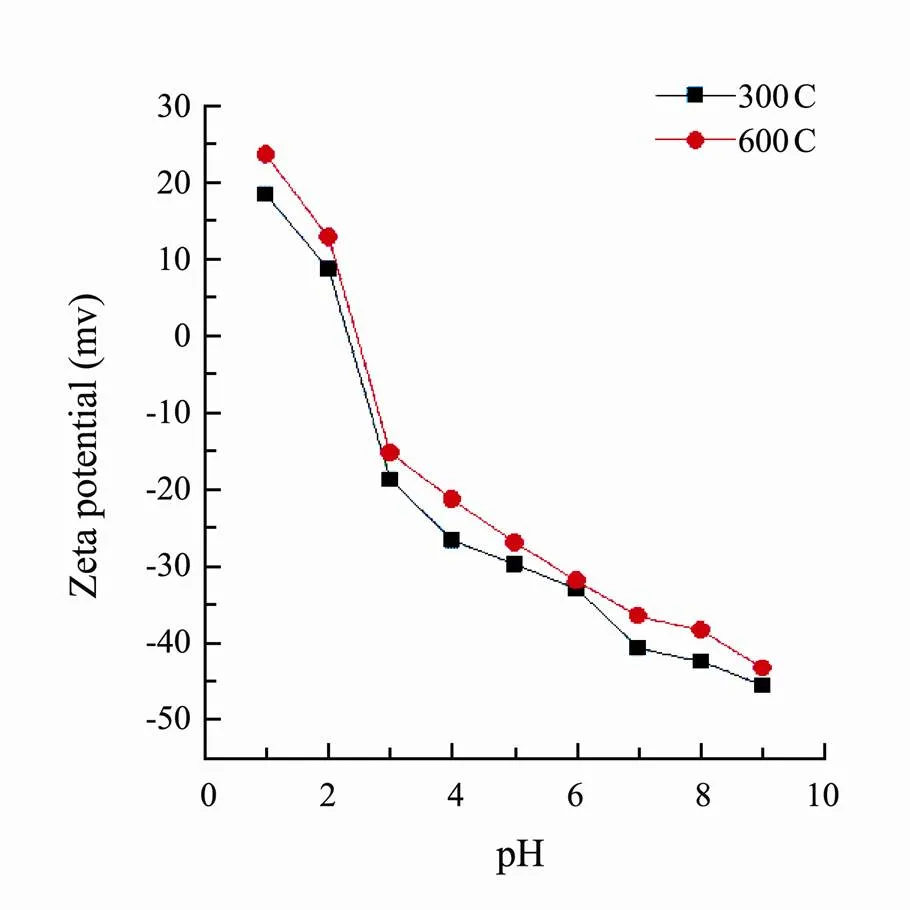
Fig.1 Zeta potential-pH curves of corn straw biochars pyrolyzed at 300℃ (300C) and 600℃ (600C).

Table 1 Physico-chemical properties of corn straw biochars
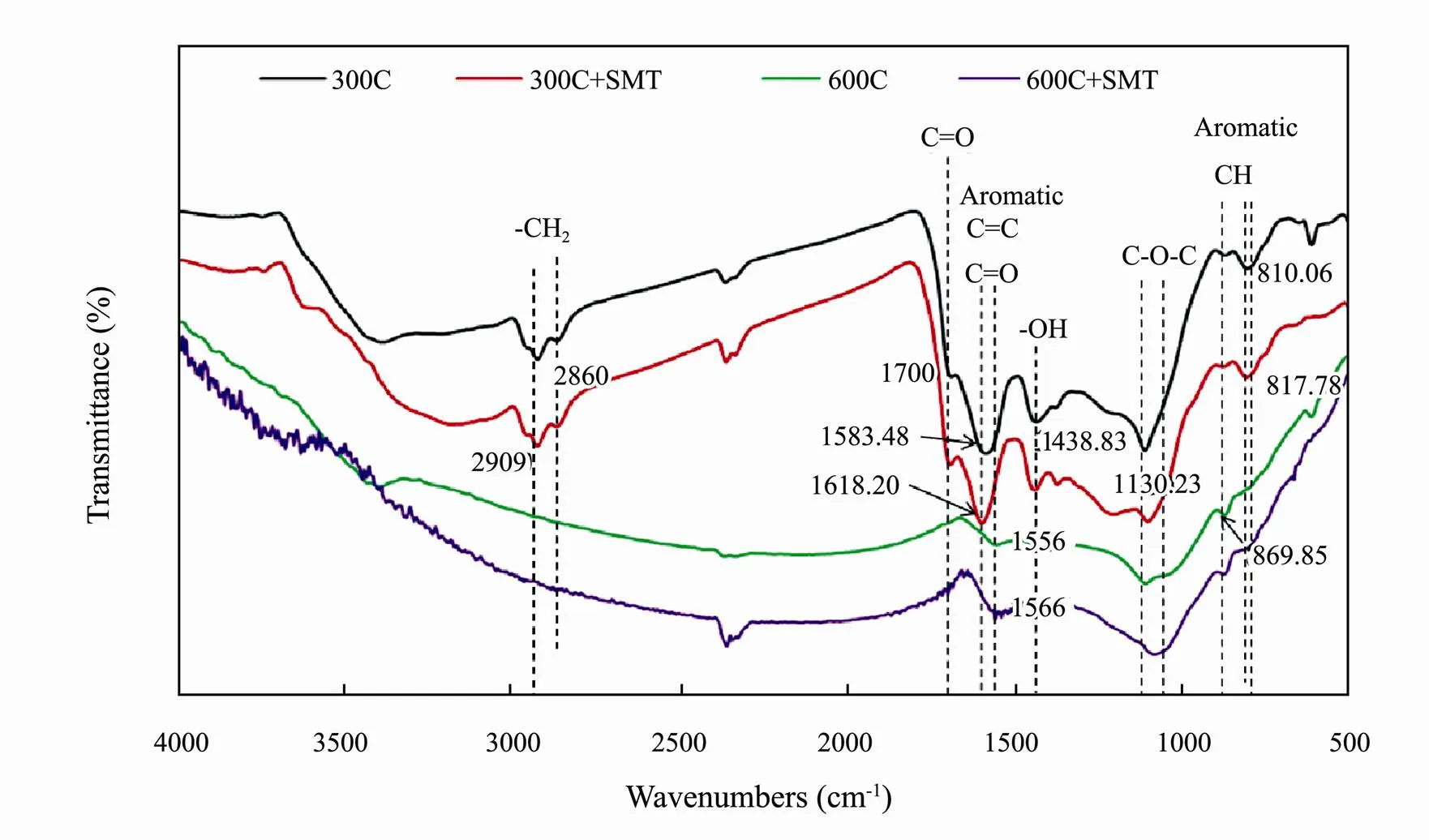
Fig.2 FTIR spectra of corn straw biochars pyrolyzed at 300℃ and 600℃ before (300C, 600C) and after (300C+SMT, 600C+SMT) sorption of sulfamethazine (SMT).
The FTIR spectra of biochars pyrolyzed at 300℃ and 600℃ were shown in Fig.2. Compared with biochar 300C, the sorption peaks of biochar 600C significantly decreased and some even disappeared. This was mainly due to the loss of –OH (3400cm−1) and aliphatics C-O-C C=O (1130.23, 1700cm−1) on the surface of biochar caused by pyrolysis. As the pyrolysis temperature increased, the aliphatic alkyl group and aromatic C=O (810, 1600cm−1) which protected the aromatic core were destroyed. Finally, the aromatic C-O and phenol –OH (1438.83 cm−1) linked to the aromatic core were destroyed. For the biochar 300C and 600C, the peak (1583.48cm−1) associating with the C=C bond and the peak (869.85, 810.06cm−1) associating with the C-H bond on the aromatic ring shifted to higher wavenumbers after reaction with SMT (Fig.2), indicating a possible sorption mechanism involving π-π EDA interaction occurring in the sorption process (Jia., 2018).
3.2 Effect of pH
Fig.3 showed the sorption of SMT by corn straw biochars (300C and 600C) was pH dependent, which was consistent well with previous research (Ahmed., 2017), where they found that pH affected the sorption of SMT by bamboo biochar. In present research, sorption capacity varied significantly with the change of pH values as a result of simultaneous and numerous sorption mechanisms.
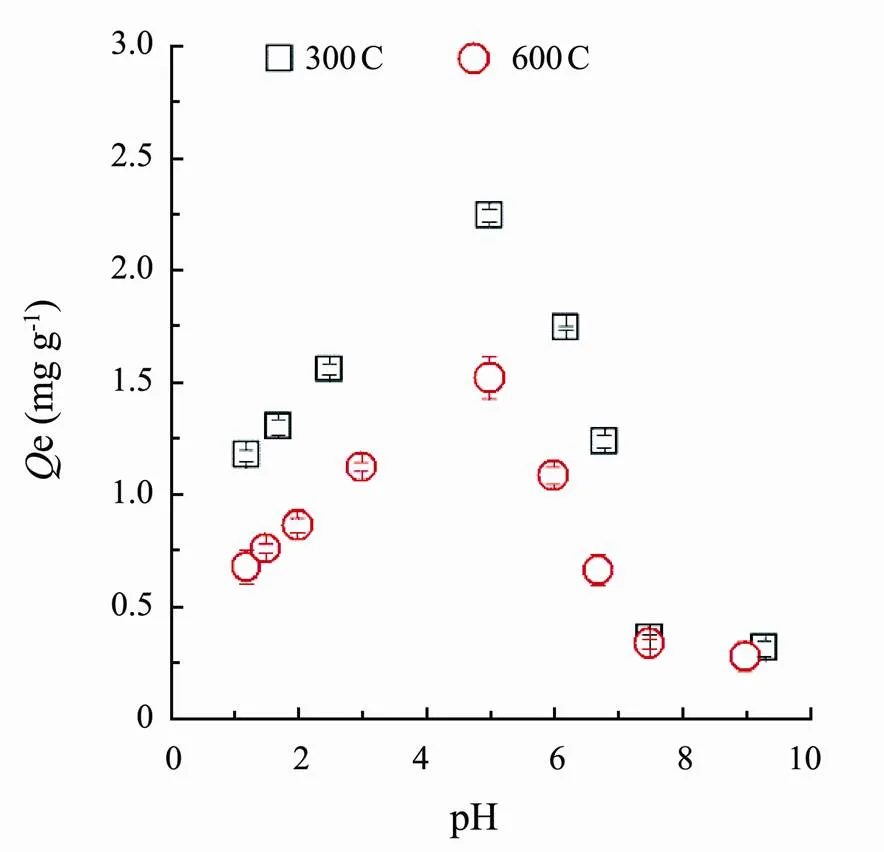
Fig.3 Effect of pH changes on sorption amounts of SMT by corn straw biochars (300C and 600C).
The biochar 300C or 600C reached a maximum sorption capacity at pH around 5.Then adsorption capacity gradually decreased with pH coming up or down. This was mainly due to the complicated species of SMT at different pH condition, and biochars possessing diverse charges at various pH prompted the biochars presenting unusual sorption behaviors. SMT has two dissociation constants containingpK1=2.65 andpK2=7.4 (Lert- paitoonpan., 2009), and SMT species were shown in Fig.4. Apparently, when the solution pH was lower than 2.65, SMT mainly existed as cation (SMT+). The SMT would mainly be presented as neutral molecule (SMT0) between the pH 4 and 6. When the solution pH approached to 7.4, the anionic SMT (SMT−) appeared. In order to explain the possible sorption mechanisms and the sorption processes of SMT by two biochars, the sorption isotherms and kinetics of SMT at pH around 1, 5 and 8, where the SMT emerged as SMT+, SMT0and SMT−, were further investigated.

Fig.4 Transition of SMT species with the continuous change of the environmental pH.
3.3 Sorption Isotherms of SMT by the Biochars
As was shown in Fig.5, the equilibrium sorption amounts of SMT by corn biochars 300C and 600C appeared as following decreasing order: 300C (pH=5), 600C (pH=5), 300C (pH=1), 600C (pH=1), 300C (pH=8) and 600C (pH=8).Under different pH conditions, the sorption capacity of biochar 300C was obvious higher than biochar 600C. At low initial SMT concentrations, the equilibrium sorption capacity of the two biochars almost presented no difference. As the solution pH increased, the sorption capacity of the biochar 300C was significantly higher than that of 600C. Both 300C and 600C had maximum sorption of SMT at pH around 5.
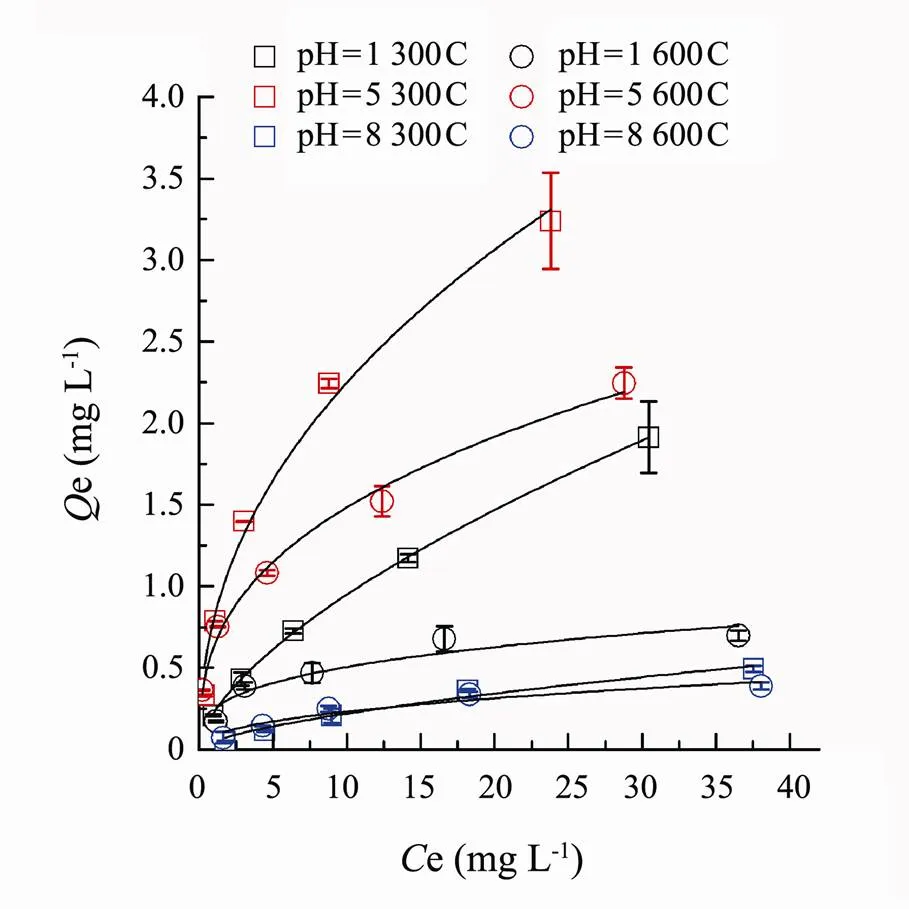
Fig.5 Sorption isotherms of SMT by the corn stalk biochars 300C and 600C at environmental pH 1, 5 and 8. Dots are experimental data, and lines are the Freundlich model fitted curves.
To explore the sorption mechanisms, Langmuir and Freundlich isotherm models were used to fit the sorption data. Langmuir isotherm model was usually used to describe monolayer sorption on homogeneous surface (Ahmed., 2015). In this research, the2value of Langmuir was 0.855–0.996. Except for the sorption of SMT by biochar 600C at pH 5, the other experimental data were well fitted to the Langmuir. Jia. (2018) revealed that the standard Gibbs free energy (Δ0) of physisorption ranged from −20 to 0kJmol−1and Δ0of chemisorption ranged from −400 to −80kJmol−1. In the present research, Δ0calculated from Langmuir equation ranged from −22.893 to −27.788kJmol−1(Table 2), indicating that the sorption of SMT by biochars 300C and 600C at pH 1, 5 and 8 were the combined results of physisorption and chemisorption.

Table 2 Parameters from the fitting of the Langmuir and Freundlich equation for SMT by the biochars 300C and 600C at environmental pH 1, 5 and 8
The Freundlich isotherm model could describe the reversible sorption of heterogeneous system (Ho., 2005). The2values of two biochars 300C and 600C under various pH were between 0.863 and 0.999.Kvalues could reflect the sorption capacities of biochars (Foo and Hameed, 2013), and the decreasing order ofKvalues were: 300C (pH=5), 600C (pH=5), 600C (pH=1), 300C (pH=1), 300C (pH=8) and 600C (pH=8), which was consistent with the actual equilibrium sorption quantity. Both biochars 300C and 600C,Kvalue at pH 5 was far larger than pH 1 and 8. There might be two reasons to ex- plain the sorption difference. Firstly, the electrostatic repulsion reduced the combination between SMT and biochars since the same electrical charge presented on the surface of SMT and biochars 300C and 600C. At pH 1, SMT mainly existed as cation (SMT+) and the biochars possessed positive charge, while at pH 8, SMT−species dominated in solution and the biochars possessed negative charge. Secondly, at pH 5, SMT mainly existed as neutral molecule (SMT0). The logKow of SMT+, SMT0and SMT−are −1.3, 0.27 and −2.09 (Carda-Broch and Berthod, 2004). Thus the hydrophobicity of SMT0was stronger than SMT+(pH 1) and SMT−(pH 8). The hydro- phobicity of SMT0improved the sorption amount at pH 5.
Table 2 showed that thevalues of all sorption isotherms are 0.311–0.629, which concluded that the sorption of SMT by the two biochars was non-linear.values were less than 1, which indicated that the partition from Van der Waals force and surface adsorption from chemical force simultaneously influenced the sorption process (Li., 2010). It also showed that the sorption sites on biochars were gradually saturated with the increase of the initial concentration of SMT. Pignatello and Xing (1996) had proved that, at lowvalue, the adsorbents possessed more heterogeneous glassy, and had higher sorption site energy distribution. In present study, thevalue of the biochar 600C (0.311–0.435) was lower than the biochar 300C (0.446–0.639) at diverse pH (Table 2), which revealed that biochar 600C became more aromatized and needed more energy to sorb SMT. This result was consistent with the conclusion of FTIR spectra results (Fig.2), which showed biochar 600C had more aromatic structure. For the biochar 300C, the increasing order ofvalue was: pH=5, pH=1 and pH=8, indicating that the effect of surface heterogeneity at pH 5 outweighed other pH situations. For the biochar 600C, there was no obvious difference forvalue, reflecting that the surface heterogeneity had similar effects at various pH.
Adsorption-partition model was used to assess the quantitative contributions of surface adsorption and partition (Fig.6). Partition and adsorption contributed to sorption of the biochars 300C and 600C at different pH implying that Van der Waals force and π-π electron interaction were the driving forces of sorption. Jia. (2018) reported that the main driving force of partition was Van der Waals force. The main driving force of adsorption contained π-π EDA interaction, electrostatic forces and H-bonding. In present research, π-π EDA interaction was one of the sorption driving force. According to FTIR spectra results (Fig.2), the peak associating with the C=C bond and the C-H bond on the aromatic ring shifted to the higher wavenumbers after sorption of SMT by 300C and 600C, proving that π-π EDA effect occurred in the sorption process (Jia., 2018). At pH 1, 5 and 8, the partition contribution of biochar 300C all outweighed the 600C. Wang and Xing (2007) concluded that the partition dominated by amorphous organic carbon of biochar while surface adsorption dominated by dense aromatic carbon. According to Fig.2 and Table 1, the biochar 300C contained more amorphous organic carbon than 600C. Thus, based on the principle of the dissolution in the same material structure, the partition contribution of 300C outweighing the 600C was the results that 300C possessed more amorphous organic carbon and contained more polar functional groups on the surface.
Overall, as for the sorption isotherms, the sorption of SMT by biochars 300C and 600C at pH 1, 5 and 8 were the combined results of physisorption caused by Van der Waals force and chemisorption caused by π-π electron interaction. Partition was the main sorption mechanism for the biochar 300C while adsorption was the main sorption mechanism for the biochar 600C, due to the diverse carbon structure. At pH 5, the sorption amounts outweighed other pH situations since the biochars and SMT possessed same charges at other pH values.

Fig.6 Contributions of partition and adsorption to total sorption of SMT by corn straw biochars 300C and 600C under the 1, 5 and 8.
3.4 Sorption Kinetics of SMT by the Biochars
Pseudo-first-order model (PFO) and pseudo-second-order model (PSO) were used to fit the kinetics data and the results were shown in Fig.7. The kinetic parameters were listed in Table 3. Results showed that, the sorption kinetics of SMT were fitted to PSO equation, but failed to fit the PFO equation. PFO equation fit well the initial stage of SMT sorption by two kinds of biochars while it deviated from the sorption process later. PSO equation could predict well the sorption related to sorption sites. In present research, the PSO equation fit the kinetic data with a slightly higher2value between 0.879–0.933 (Table 3), which showed that the sorption capacities of SMT by the two biochars were not only related to the chemical bonding force. Thus the sorption process in our research was a combined result of physisorption and chemisorption, which was consistent with the conclusion of sorption isotherms.
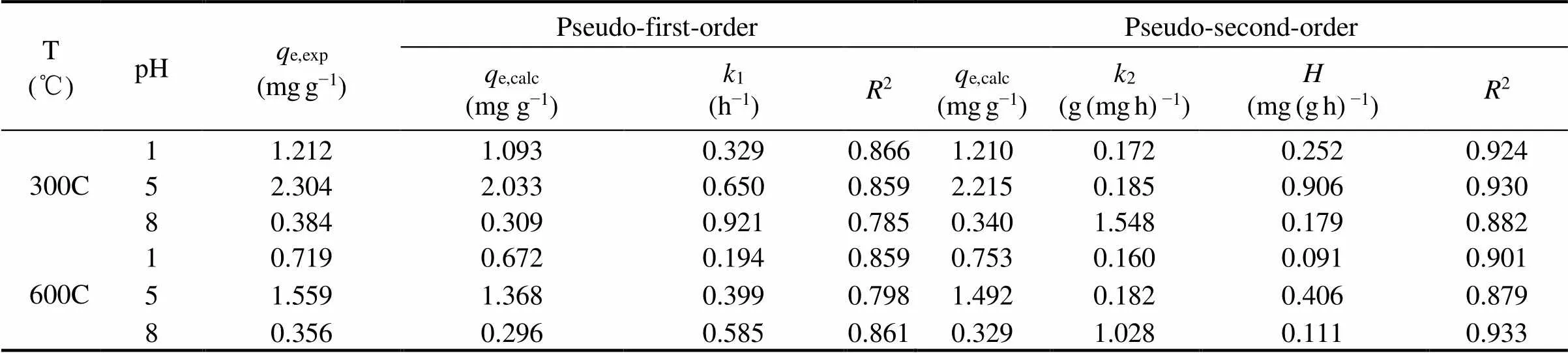
Table 3 Kinetic curve parameters of sorption SMT by the biochars 300C and 600C under the pH 1, 5 and 8

Fig.7 SMT sorption kinetics by corn straw biochars 300C and 600C under the various environmental pH 1, 5 and 8. Dots are experimental data, and lines are the pseudo-first- order model and pseudo-second-order model model fitted curves.
To further investigate the sorption processes of SMT by the biochars, the intraparticle diffusion model was applied to analyze the kinetic data and the results were shown in Fig.8 and Table 4. The results showed that all the sorption of SMT by the biochars were multilinear, thus intraparticle diffusion was not the only limiting factor in the sorption process. In this research, the sorption process of SMT by each biochar included three stages at every pH level, which included instantaneous sorption, gradual sorption and final equilibrium sorption stage respectively.
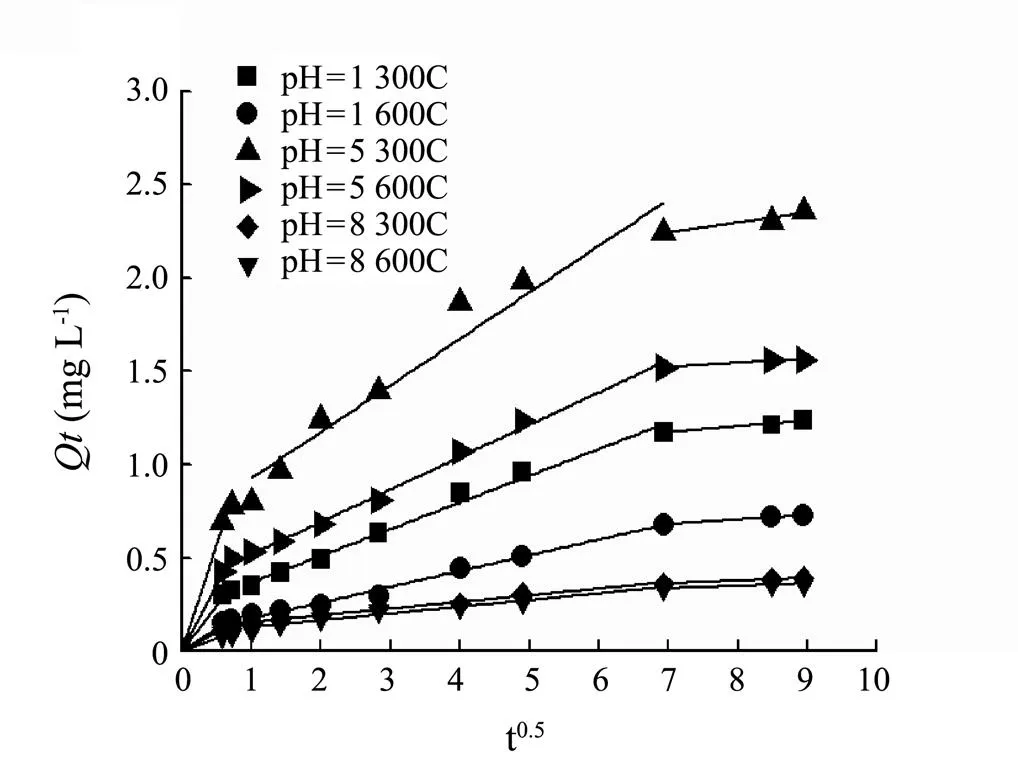
Fig.8 Intraparticle diffusion during the process of sorption SMT by the biochars 300C and 600C under the pH 1, 5 and 8.

Table 4 Intraparticle diffusion during the process of sorption SMT by the biochars 300C and 600C under the pH 1, 5 and 8
Kvalue represented diffusion rate of every sorption stage (Foo and Hameed, 2013). At the same pH, for the both biochars 300C and 600C, the decreasing order of diffusion rateskvalue were:k1,k2andk3. This was mainly due to the diverse driving forces in every sorption stage. Ioannou and Simitzis (2009) reported that the first stage (called instantaneous sorption stage) depicted external mass transfer during the retention of adsorbate occurring at short contact times. This step usually depends on variables such as solute concentration, temperature, and adsorbent particle size (Wu., 2009). The second and third stages were gradual sorption process attributing to the less accessible sorption sites. Hence, it took a longer time than instantaneous sorption stage. The instantaneous sorption rate constantk1of 300C was greater than 600C under any pH, mainly owing to physisorption caused by Van der waal forces of 300C outweighing 600C. Physisorption had the lower energy consumption and higher speed than chemisorption. The interceptCwas proportional to the thickness of the boundary layer. The larger intercept reflected the stronger boundary layer effect (Foo and Hameed, 2013). At any pH, the decreasing order ofCvalues of biochars were:1,2,3, indicating the effect of boundary layer increased with sorption process time. Thekvalue (pH 5) was higher than others (pH 1, 8). It was mainly related to the sorption capacity at various pHs, and consistent with the conclusion of sorption isotherms.
The sorption process was the combined result of the film diffusion, outer surface diffusion, intraparticle diffusion, sorption on the sorbent (Jia., 2018). Boyd model was further used to distinguish between the pore and film diffusion step. From the plots of Boyd models (Fig.9), the points were scattered around the plots and did not pass through the origin. Thus, the sorption process of SMT by two biochars at various pH were mainly governed by film diffusion.

Fig.9 Plots of the SMT sorption by corn biochars 300C and 600C fitted with the Boyd model.
Overall, fitting sorption kinetics analyses using different models, demonstrated that physisorption and chemisorption coexisted simultaneously. The sorption process was affected by both film diffusion and intraparticle diffusion, and film diffusion was the rate-limiting factor. The carbon structure also affected diffusion rate.
3.5 Sorption of SMT by Modified Corn Biochars
The pH value of aquaculture wastewater which is recognized as alkalescent wastewater was around 8. Yet the sorption amount of SMT by biochar was obviously low at pH 8 though present research. In order to remove SMT in actual aquaculture wastewater, it was necessary to modify biochar to remove SMT from alkalescent solution. In present research, 300C was used for modified precursor due to its higher sorption capability compared with 600C. Activating agents contained H2SO4, HNO3, NaOH and KOH. The sorption amounts of SMT by modified biochar 300C were different with acid and alkali modification. From Fig.10, it was observed that when impregnation ratios of strong acids/bases and biochar were 0, 0.2, 1.0 and 20mmolg−1, and the sorption amounts were almost same for both acid or alkali modification. When impregnation ratio was 100 and 200mmolg−1biochar, the sorption amounts of acid modified biochar 300C were higher than alkali modified 300C. In addition, for acid modified 300C, the sorption amounts increased with the improvement of impregnation ratio.
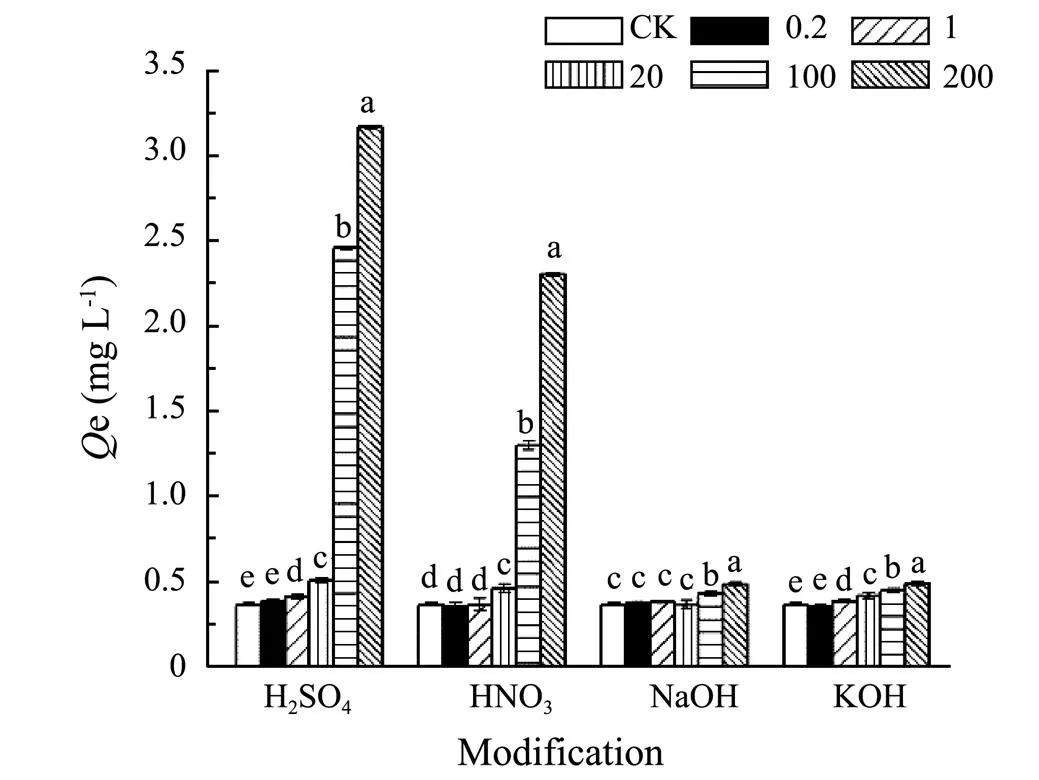
Fig.10 Sorption of SMT by corn biochar 300C modified by different concentration of H2SO4, HNO3, NaOH and KOH (0.2, 1, 20, 100, 200mmolg−1). Statistically significant was at a level of P<0.05 (Duncan).
It implied that the SMT sorption capacity by acid modified biochar 300C was better than alkali modified 300C at pH around 8. SMT species was mainly SMT−and the surface of the biochar owned negative charge at pH 8 (Fig.1 and Fig.4). Acid modification could decrease the negative charge while alkali modification could increase the negative charge on the surface of biochar. In addition, the sorption capacity of sulfuric acid modified 300C was higher compared to nitric acid modified 300C, which might be that the sulfuric acid offer more positive charge than nitric acid under the same impregnation ratio to weaken electrostatic repulsion. Hence, the effects of acid and alkali modification also further verified the electrostatic repulsion inhibitory effects between SMT and biochar at pH 8. Reducing the negative charge on the biochar surface could improve the sorption amounts of SMT by biochar under actual alkaline environment.
4 Conclusions
Sorption behavior of SMT by biochars 300C and 600C was related to pH solution. Under various pHs, sorption isotherms and sorption kinetics of SMT by corn straw biochars 300C and 600C were different. At various pH values, the SMT species and surface charge properties both affected sorption mechanisms. The sorption of SMT by the biochars 300C and 600C at different pH was the combined result of physisorption and chemisorption. The Van der Waals force and π-π EDA interactions were the main sorption driving force. At pH 5, the strong hydrophobicity of SMT increased sorption amounts, while pH at 1 or 8, electrostatic repulsion could impact the sorption. Sorption process was affected by both film diffusion and intraparticle diffusion, and film diffusion was the rate- limiting factor. Compared with alkali modification, acid modification could improve the sorption capacity of the biochar 300C at pH around 8. Acid modified biochar 300C could be used as adsorbent to remove SMT from alkalescent aqueous solution. The research provides a theoretical basis for the removal of SMT using biochar from mariculture wastewater.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities of China (No. 201964 004) and the National Natural Science Foundation of China (No. 41977315).
Ahmed, M. B., Zhou, J. L., Ngo, H. H., and Guo, W. S., 2015. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges., 532: 112-126.
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Guo, W. S., Johir, M. A., and Sornalingam, K., 2017. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water., 311: 348-358.
Bilkova, Z., Mala, J., and Hrich, K., 2019. Fate and behaviour of veterinary sulphonamides under denitrifying conditions., 695: 133824.
Carda-Broch, S., and Berthod, A., 2004. Countercurrent chromatography for the measurement of the hydrophobicity of sulfonamide amphoteric compounds., 59 (1-2): 79-87.
Chen, H., Liu, S., Xu, X. R., Liu, S. S., Zhou, G. J., Sun, K. Y., Zhao, J. L., and Ying, G. G., 2015. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure., 90 (1-2): 181-187.
Foo, K. Y., and Hameed, B. H., 2013. Utilization of oil palm biodiesel solid residue as renewable sources for preparation of granular activated carbon by microwave induced KOH activation., 130: 696-702.
Heitkotter, J., and Marschner, B., 2015. Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production., 245: 56-64.
Ho, Y. S., Chiu, W. T., and Wang, C. C., 2005. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust., 96 (11): 1285-1291.
Ioannou, Z., and Simitzis, J., 2009. Adsorption kinetics of phenol and 3-nitrophenol from aqueous solutions on conventional and novel carbons., 171 (1-3): 954-964.
Jia, M. Y., Wang, F., Bian, Y. R., Stedtfeld, R. D., Liu, G. X., Yu, J. P., and Jiang, X., 2018. Sorption of sulfamethazine to biochars as affected by dissolved organic matters of different origin., 248: 36-43.
Kumar, R., Strezov, V., and Weldekidan, H., 2020. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels., 123: 109 763.
Lertpaitoonpan, W., Ong, S. K., and Moorman, T. B., 2009. Effect of organic carbon and pH on soil sorption of sulfamethazine., 76 (4): 558-564.
Li, H., Mahyoub, S. A. A., Liao, W. J. E., Xia, S. Q., Zhao, H. C., Guo, M. Y., and Ma, P. S., 2017. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue., 223: 20-26.
Li, Y., Chen, B., and Zhu, L., 2010. Enhanced sorption of polycyclic aromatic hydrocarbons from aqueous solution by mo- dified pine bark., 101 (19): 7307-7313.
Liu, X., Lu, S., and Guo, W., 2018. Antibiotics in the aquatic environments: A review of lakes, China., 627: 1195-1208.
Lorenzo, P., Adriana, A., and Jessica, S., 2018. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem., 206: 70-82.
Pignatello, J. J., and Xing, B. S., 1996. Mechanisms of slow sorption of organic chemicals to natural particles., 30 (1): 1-11.
Reguyal, F., and Sarmah, A. K., 2018. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17 alpha-ethinylestradiol., 628-629: 722-730.
Rico, A., Phu, T. M., Satapornvanit, K., Min, J., Shahabuddin, A. M., Henriksson, P. J. G., Murray, F. J., Little, D. C., Dalsgaard, A., and van den Brink, P. J., 2013. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia., 412: 231-243.
Sepehri, A., and Sarrafzadeh, M. H., 2018. Effect of nitrifiers community on fouling mitigation and nitrification efficiency in a membrane bioreactor., 128: 10-18.
Teixido, M., Pignatello, J. J., Beltran, J. L., Granados, M., and Peccia, J., 2011. Speciation of the ionizable antibiotic sulfamethazine on black carbon (Biochar)., 45 (23): 10020-10027.
Wang, X. L., and Xing, B. S., 2007. Sorption of organic contaminants by biopolymer-derived chars., 41 (24): 8342-8348.
Wu, F. C., Tseng, R. L., and Juang, R. S., 2009. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics., 153 (1-3): 1-8.
Xiong, J. Q., Govindwar, S., and Kurade, M. B., 2019. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga,., 218: 551-558.
Zhang, Q. Q., Ying, G. G., Pan, C. G., Liu, Y. S., and Zhao, J. L., 2015. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance., 49 (11): 6772-6782.
Zheng, H., Wang, Z. Y., Zhao, J., Herbert, S., and Xing, B. S., 2013. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures., 181: 60-67.
January 12, 2020;
November 15, 2020;
December 2, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. Tel: 0086-532-82031853 E-mail: likr@ouc.edu.cn
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Case Study of a Short-Term Wave Energy Forecasting Scheme:North Indian Ocean
- Temporal and Spatial Characteristics of Wave Energy Resources in Sri Lankan Waters over the Past 30 Years
- Vibration Deformation Monitoring of Offshore Wind Turbines Based on GBIR
- Dependence of Estimating Whitecap Coverage on Currents and Swells
- The Variation of Microbial (Methanotroph) Communities in Marine Sediments Due to Aerobic Oxidation of Hydrocarbons
- 3-Aminopropyltriethoxysilane Complexation with Iron Ion Modified Anode in Marine Sediment Microbial Fuel Cells with Enhanced Electrochemical Performance
