Tb3+ Doped Transparent Germanate Glass Ceramics: Preparation and Enhanced Luminescence for X-ray Detection
2021-06-16CHENYuxuanHUANGLihuiZHAOJingtaoZHAOShilongXUShiqing
CHEN Yu-xuan, HUANG Li-hui, ZHAO Jing-tao,ZHAO Shi-long, XU Shi-qing
(1. College of Materials and Chemistry, China Jiliang University, Hangzhou 310018, China;2. Institute of Optoelectronic Materials and Devices, China Jiliang University,Hangzhou 310018, China;3. College of Optical and Electronic Technology, China Jiliang University, Hangzhou 310018, China;4. Laboratory of Glasses and Ceramics, Univ Rennes, CNRS, ISCR(Institut des Sciences Chimiques de Rennes) UMR CNRS 6226, University of Rennes 1, Rennes 35042, France)
Abstract: Tb3+ doped transparent germanate glass ceramics embedded with LaF3 nanocrystals were successfully prepared via melt-quenching and subsequent thermal treatment. The luminescent properties of the as-prepared glass and the glass ceramics were investigated in detail. The X-ray diffraction results prove that the crystalline phase precipitated in the glass matrix was pure LaF3 crystal and the crystal size was between 16 nm and 21 nm. Under the excitation of 377 nm ultraviolet light and X-ray, Tb3+ glass ceramics embedded with LaF3 nanocrystals show much intense green emission than Tb3+ doped germanate glass, and the green emission intensity increases with the increment of thermal treatment temperature and time. The maximum integrated X-ray excited luminescence intensity of the glass ceramics is about 40.3% of that of Bi4Ge3O12 crystal which is the commercial scintillating crystal. Our research shows that Tb3+ doped germanate glass ceramics embedded with LaF3 nanocrystals have potential application prospects in X-ray detection.
Key words: luminescence; Tb3+; germanate glass; glass ceramics; glass scintillator
1 Introduction
A scintillator is a luminescent material that emits ultraviolet/visible light under the excitation of high-energy particles or rays. Scintillator is widely used in high energy physics, nuclear medicine imaging, detection technology, public security, custom anti-smuggling, space physics, geological exploration and other fields. There are many kinds of scintillators, among which the crystal scintillators are most widely used[1-3]. In recent years, with the strengthening of human activities in the fields of nuclear physics, nuclear medicine imaging(such as X-CT, PET), industrial detection, nuclear radiation detection, petroleum logging, public safety and other X-ray detection fields, the demand for low-cost, large size scintillator is becoming more and more urgent. Although the crystal scintillator has high luminescent efficiency, its wide application in the corresponding fields is greatly limited by the long fabrication period, complicated preparation technology, limited size of the crystal, poor chemical stability and high cost. On the contrary, glass scintillator with composition of easy preparation, low cost, easy adjustment, good homogeneity and isotropy, easy processing, easy to be made into various shapes, easy system advantages of large size and realizing large-scale production has been brought to the attention of the material world and physicist, become a hotspot in the research of the scintillator materials[4-9]. However, the intrinsic amorphous structure of the glass results in the low luminescence efficiency ofRE3+and consequently limit the practical applications of glass scintillator[10-12]. Therefore, there is an urgent need to improve the luminescence efficiency.
As everyone knows that fluoride nanocrytals have low phonon energy, the controlled crystallization of fluoride crystals inRE3+activated oxyfluoride glass has been confirmed to be an effective method to improve their luminescent efficiency[13-14]. Therefore, many researchers reported the enhanced luminescence ofRE3+ions in glass ceramics(GCs) containing fluoride nanocrystals[15-16]. Therefore, the controllable crystallization of fluoride crystals inRE3+-doped scintillating glass is also an effective method to improve their luminescence efficiency[17]. Meanwhile,RE3+could give more intense emission in germanate glass than it in silicate glass since germanate glass has relatively low phonon energy. In the recent years, Tb3+activated germanate glass has been confirmed to be a promising scintillator material[18-21]. Consequently, Tb3+doped germanate glass ceramics may be a more promising scintillation material than Tb3+doped germanate glass.
In this paper, we prepared Tb3+doped transparent germanate glass ceramics embedded with LaF3nanocrystals and investigated their luminescent properties.
2 Experiments
Tb3+doped oxyfluoride germanate glass with composition of 50GeO2-12Al2O3-8Li2O-(20-x)LaF3-10LiF-xTbF3(x=2, 4, 6, 8, 10) was synthesized through conventional high temperature melt quenching method. 20 g of high-purity GeO2(99.999%), Al2O3(99.99%), Li2CO3(99.99%), LaF3(99.99%), LiF(99.99%), and TbF3(99.99%) were selected as raw materials. For each batch, starting materials were mixed thoroughly and uniformly in an agate mortar first, and then were put into an alumina crucible with a cover. Finally, the crucible was transferred to an electric furnace and melt at 1 480 ℃for 40 min. After melting, the melt was poured onto a stainless-steel plate and pressed by another stainless-steel plate to cool fast and obtain bulk glass. The as prepared bulk glass was annealed at 500 ℃ for 3 h to release the inner stress, and then cooled to room temperature. Three pieces of oxyfluoride germanate glass(the precursor glass, denoted PG) with Tb3+doping concentration of 8% were selected and heat treated at 620 ℃ for 3 h, 640 ℃ for 3 h and 640 ℃ for 6 h to form transparent glass ceramics, named as GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h, respectively. All samples were cut and polished elaborately with the regular size of 10.0 mm×10.0 mm×2.0 mm.
The thermal properties of 8% Tb3+doped germanate glass were characterized by differential thermal analysis(DTA) on powdered samples using a Netzsch DTA 404PC at a heating rate of 10 K/min. The X-ray diffraction(XRD) patterns were carried out on powdered samples using an X-ray diffraction (Bruker D2 PHASER) with Cu-Kα radiation and 2θdata were collected between 10° and 80° with a 0.1° step size. The transmittance spectra of the PG and GCs were recorded on a Shimadzu UV-3600 spectrophotometer in the range of 200-750 nm. The emission spectra and the excitation spectra were performed by a fluorescence spectrophotometer (Jobin-Yvon Fluorolog3) equipped with a Xe lamp.X-ray excited luminescence(XEL)spectra were recorded by a spectrometer(Ocean Optical QE65000) with an X-ray tube(Copper target, 80 kV, 1.5 mA). All of the structural and optical measurements were performed at room temperature.
3 Results and Discussion
Fig.1 shows the DTA curve of germanate precursor glass when Tb3+doping concentration is 8%. The glass transition temperature(TG) of this germinate glass is around 551 ℃. And two exothermic peaks can be easily observed at 657 ℃(Tx1) and 768 ℃(Tx2). According the XRD results, the first peak is due to the precipitation of LaF3nanocrystals and the second peak is due to the crystallization of the glass. The large difference up to 111 ℃ betweenTx1andTx2shows that it is easy to crystallize pure LaF3crystals in the glass matrix. On the basis of these results, 620 ℃ and 640 ℃ which are near the LaF3crystallization temperature were selected as the crystallization temperature.
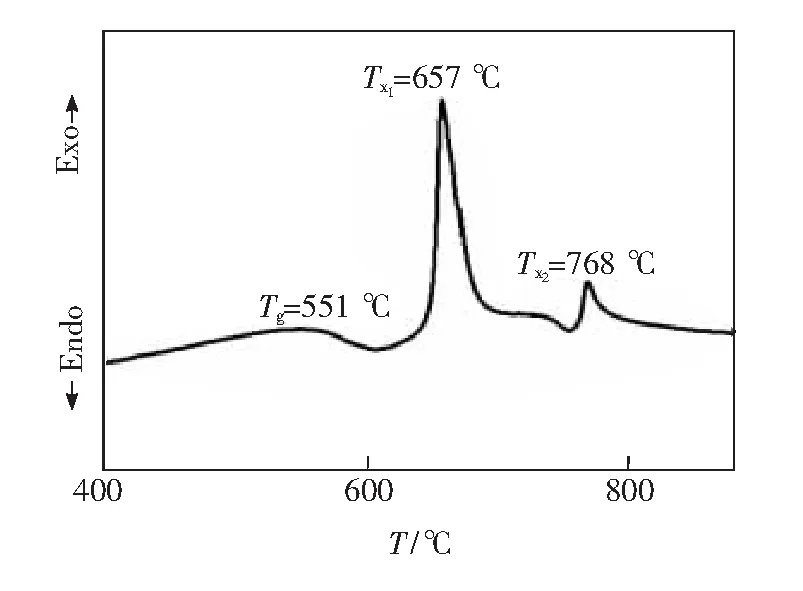
Fig.1 DTA curve of 8% Tb3+ doped germanate glass and GC640 ℃-6 h glass ceramics
The XRD patterns of 8% Tb3+doped PG and GCs are presented in Fig.2. The GCs present sharp diffraction peaks while the PG is completely amorphous with no crystalline diffraction peaks. For Tb3+doped GCs, the diffraction peaks can be clearly assigned to the hexagonal LaF3(JCPDS No.32-0483) and no other diffraction peaks were observed. Using the Scherrer equation[22], the size of LaF3nanocrystals in the glass ceramic can be estimated based on the XRD pattern. The sizes of LaF3nanocrystals were 16.7, 19.6, 21.6 nm for the samples GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h, respectively. The results imply that the crystal size in the GCs becomes bigger with increasing thermal treatment temperature and time.
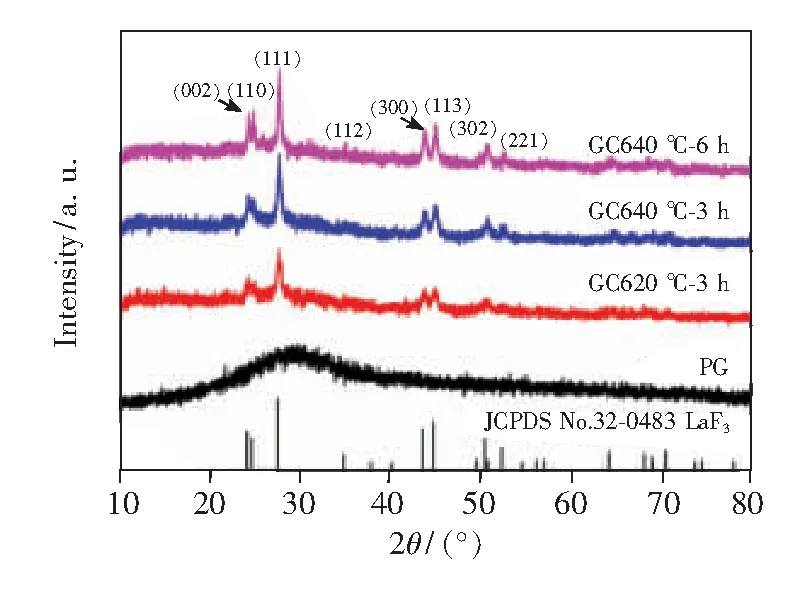
Fig.2 XRD patterns of PG glass, GC620 ℃-3 h, GC640 ℃-3 h and GC 640 ℃-6 h glass ceramics.
Fig.3 shows the transmittance spectra of PG glass, GC620 ℃-3 h, GC640 ℃-3 h, and GC640 ℃-6 h glass ceramics in the ranging from 200 nm to 750 nm. All samples remain agood transparency from 450 to 750 nm(as shown in the photographs of PG, GC620 ℃-3 h, GC640 ℃-3 h, and GC640 ℃-6 h in the inset of Fig.3). However, the transparency of GC620 ℃-3 h, GC640 ℃-3 h, and GC640 ℃-6 h have a slight decrease with increasing thermal treatment temperature and the thermal treatment time. Meanwhile, the characteristic optical transitions7F6→5H7,5D2,5D3and5D4of Tb3+ion induced absorption bands centered at 316, 351, 373, 485 nm appeared. It is also noted that an obvious red shift was observed from the UV cut-off wavelength of GCs, which could be originated to the reduction of non-bridging fluorine(NBF)[23].
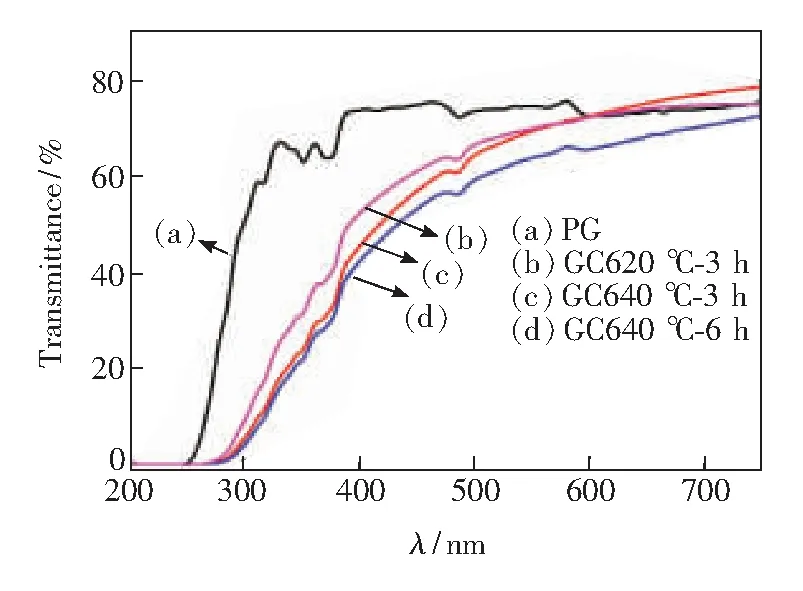
Fig.3 Transmittance spectra of PG glass, GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h glass ceramics, the thickness of all samples is 2 mm.
Greenish fluorescence is presented in Tb3+doped oxyfluoride germanate PG and GCs under the excitation of 377 nm light. Fig.4 is the emission spectra of oxyfluoride germanate glass upon 377 nm light at different Tb3+concentration. The spectra are constituted by four emission bands centered at 492, 546, 590, 624 nm, which are attributed to the transitions5D4→7F6,5D4→7F5,5D4→7F4and5D4→7F3of Tb3+, respectively. The emission intensity increases gradually with the increment of Tb3+concentration when it approaches 8%, and then reduces due to the concentration quenching. Thus the optimum concentration of Tb3+is 8% in these oxyfluoride germanate glass.
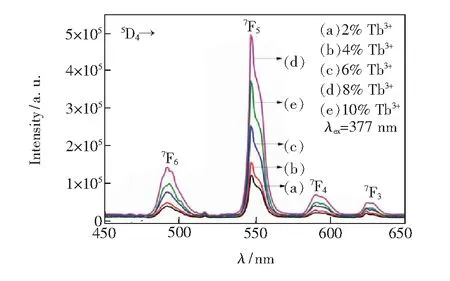
Fig.4 Emission spectra of Tb3+ doped germanate glass under 377 nm light excitation
Fig.5 presents the emission spectra of 8% Tb3+doped PG and GCs under the excitation of 377 nm light. The spectra of Tb3+doped GCs are similar to those of Tb3+doped oxyfluoride germanate glass, which also contain four emission bands peaked at 492, 546, 590, 624 nm. The intensities of green emissions of the glass ceramics increased significantly with the comparison of those of the PG, and also increased with increasing thermal treatment temperature and time. The peak intensity of 546 nm emission of the GCs which were prepared after thermal-treated at 620 ℃ for 3 h, 640 ℃ for 3 h and at 640 ℃ for 6 h increases by about 1.46, 2.05, 2.34 times that of the PG.
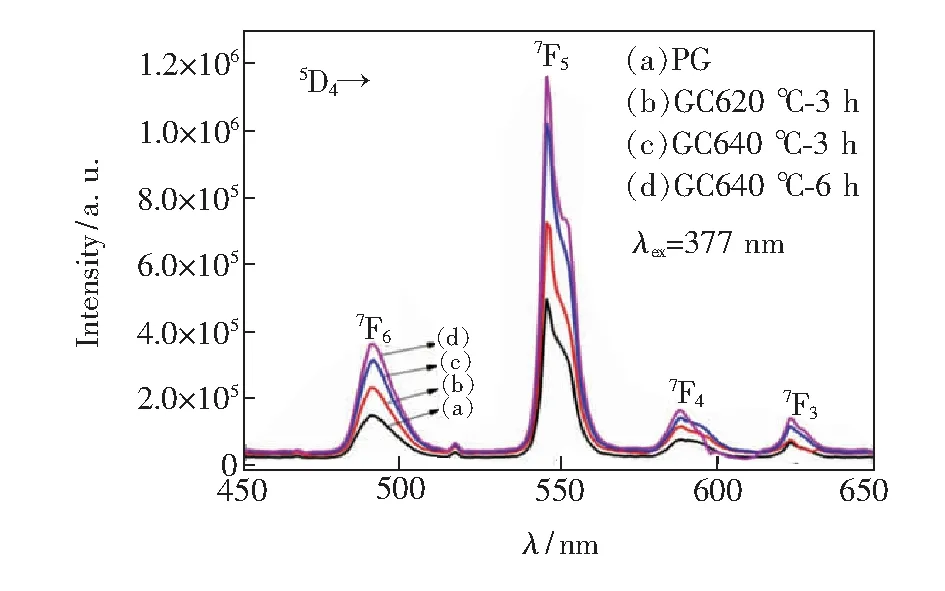
Fig.5 Emission spectra of the PG glass, GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h glass ceramics under the excitation of 377 nm light.
Fig.6 presents the excitation spectra of the PG and the GCs monitored at 546 nm emission from 290 nm to 510 nm. The excitation spectra show characteristic absorptions of Tb3+ion. Two groups are clearly observed from the overall excitation spectra. The strongest excitation band around 377 nm is attributed with the transition7F6→5D3of Tb3+, while the weak bands centered at 302, 317, 351, 369, 484 nm are originated from the transitions7F6→5H6,5H7,5D2,5D4of Tb3+, respectively. Moreover, the intensity of excitation band is enhanced with the increment of thermal treatment temperature and time, which is consisted with the emission spectra.
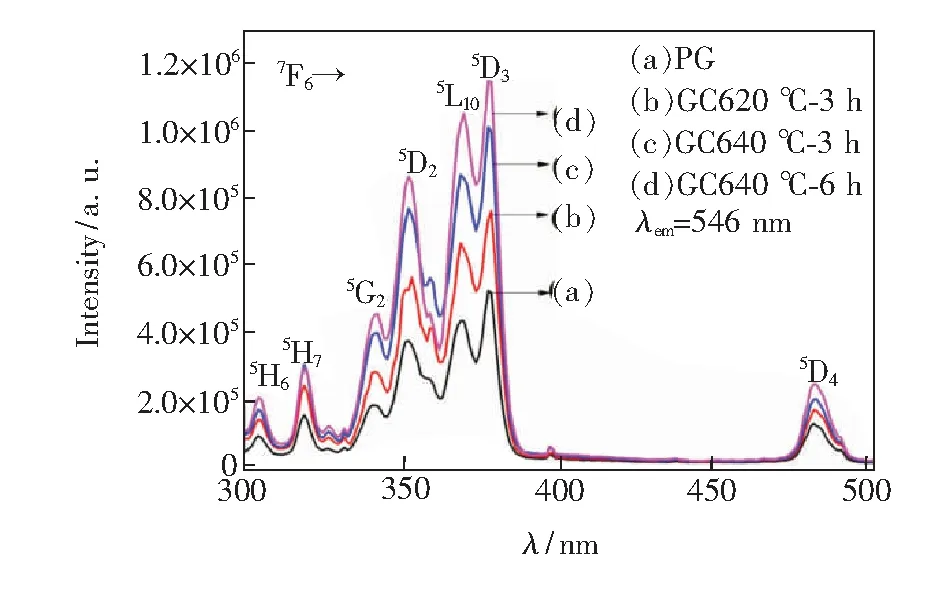
Fig.6 Excitation spectra of PG glass, GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h glass ceramics monitored at 546 nm emission.
The XEL spectra of Tb3+doped PG and GCs are presented in Fig.7, which were excited by X-ray(80 kV, 1.5 mA). The spectra are also consisted of four emissions peaked at 488, 542, 585, 620 nm, which are originated from5D4→7F6,7F5,7F4and7F3transitions of Tb3+ions, respectively. They are similar to the emission spectra under 377 nm light excitation, however, there is slight difference in the peak location due to the different luminescence mechanisms under two different excitations. In comparison with PG sample, XEL intensity of 542 nm emission increases by 6.36, 12.53, 15.76 times for GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h samples, respectively. The results present that the emission intensity increases significantly after thermal treatment, meanwhile it is enhanced with increasing thermal treatment temperature and time. The increased XEL intensity of GCs could be attributed to Tb3+preferentially incorporated into LaF3nanocrystals with low phonon energy. Moreover, as a comparison, the XEL spectra of BGO scintillating crystal with the same thickness under the same excitation were also showed in Fig.7. The integrated emission intensity ranging from 300 nm to 800 nm of PG, GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h samples is about 2.8%, 16%, 31.1%, 40.3% of that of the BGO crystal.The results approach to the oxyfluoride silicate scintillating glass ceramic containing Lu6O5F8nanocrystals[4]. The present results indicate that Tb3+doped transparent germanate glass ceramics embedded with LaF3nanocrystals have a potential application in X-ray detection.
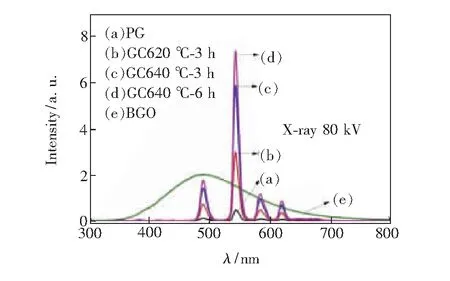
Fig.7 XEL spectra of PG glass, GC620 ℃-3 h, GC640 ℃-3 h and GC640 ℃-6 h glass ceramics and BGO scintillating crystal.
4 Conclusion
Tb3+doped transparent germanate GCs embedded with LaF3nanocrystals were successfully prepared by melt-quenching and subsequent thermal treatment. And their luminescent properties were investigated in detail. The XRD results demonstrate the crystalline precipitated in the glass matrix was LaF3and the corresponding crystal size is in the range of 16-21 nm. Under the excitation of 377 nm light and X-ray, Tb3+doped germanate GCs embedded with LaF3nanocrystals present more intense emission than Tb3+doped germanate glass, and the emission intensity is enhanced with the increment of thermal treatment temperature and time. The maximum integrated XEL intensity of the GCs is approximately 40.3% of that of the commercial BGO scintillating crystal. The results indicate that Tb3+doped transparent germanate GCs embedded with LaF3nanocrystals have potential application prospects in X-ray detection.
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20210117.
