Isotope shift of the 2s 2S1/2 →2p 2P1/2,3/2 transitions of Li-like Ca ions*
2021-05-06DenghongZhang张登红FangjunZhang张芳军XiaobinDing丁晓彬andChenzhongDong董晨钟
Denghong Zhang(张登红), Fangjun Zhang(张芳军), Xiaobin Ding(丁晓彬), and Chenzhong Dong(董晨钟)
Key Laboratory of Atomic and Molecular Physics and Functional Materials of Gansu Province,College of Physics and Electronic Engineering,Northwest Normal University,Lanzhou 730070,China
Keywords: isotope shift,multi-configuration Dirac–Hartree–Fock(MCDHF),mass shift,field shift
1. Introduction
Isotopes have different mass and charge distributions,which lead to the difference on the physical observable quantities. The energy or wavelength shifts of the specific transition line in the spectrum from different isotopes which were known as isotope shift(IS),mainly come from two contributions: the mass shift(MS)and the field shift(FS).The MS is due to the finite mass of the nucleus, whereas the FS is caused by the difference in the nuclear charge distribution of different isotopes. The MS plays a dominated role in the isotope shifts of light elements and then decreases rapidly with the increase of mass number. By contrast, the FS is dominant for heavy elements.[1]By studying IS,the difference between the meansquare nuclear charge radii and nuclear deformation could be obtained.[2,3]Meanwhile, it has recently been demonstrated that the measurements of nonlinear isotope shift can be used to search for the new boson.[4,5]Experimentally,the IS can be measured by muonic atoms method,[6]electron scattering,[7]x-ray[8]as well as laser spectroscopy.[9]Among these methods, the laser spectroscopy makes it possible to measure stable and radioactive isotopes.[10]From the IS observation and sophisticated theoretical calculation, the nucleus information could be deduced,which provides a new possibility to understand the nuclear effects on atoms. However, due to the high sensitivity of IS parameters to electron correlation,especially for neutral atoms and ions, the determination of the parameters is a great challenge for the atomic theory. The effect of the FS on the isotope shifts of light atoms is small compared with the total IS, separating the tiny FS from the light elements is a complex task that can only be performed on very simple and stable atoms or ions. Therefore, the experiment and theory must have high precision and accuracy in the determination of the radii difference of the light multi-electron isotope.[11]In the past two decades,Artemyev et al.calculated the relativistic nuclear recoil corrections of low-lying states for H-like ions by using the B-spline method for the Dirac equation.[12,13]The numerical results were presented for the IS of the lowest-lying states of helium atoms by Pachucki et al.,[14]and the relativistic nuclear recoil corrections of He-like ions were calculated by Shabaev and Zubova et al.[15,16]As far as Li-like ions are concerned, most of researches have been done on lithium atoms,[1,17]and some isotope shifts of lowlying levels along the lithium isoelectronic sequence were also calculated. For example,Li et al. and Zubova et al. calculated and discussed the relativistic mass-and field-shift parameters based on the MCDHF and large-scale configuration interaction Dirac–Fock–Sturm(CI-DFS)methods,respectively.[18,19]Kozhedub et al. recently calculated the relativistic mass shift for the 2p1/2→2s and 2p3/2→2s transitions along the Lilike isoelectronic sequence.[20]Zubova et al. calculated the isotope shifts in Be-like thorium and uranium ions by using the CI-DFS method. Xiang Zhang et al. calculated the field shift and mass shift parameters in Be-like ions by using the MCDHF and RCI methods.[21,22]The isotope shifts of the 2p3/2→2p1/2transition in B-like ions were evaluated by using a large-scale CI-DFS method by Zubova et al.[23]Recently, Silwal et al. have extended their study to the complex electronic systems such as Na-like,Mg-like,and Al-like ions.[24,25]
Calcium is an essential element for metabolism and the fifth most abundant elements in the Earth’s crust. Calcium and its ions produce strong spectral lines in the atmospheres of the sun and other stars. Their shape are strongly influenced by IS.[26]For decades, hyperfine structure and IS in various transitions of calcium isotopes have been studied theoretically[27,28]and experimentally.[29,30]This element is also widely used in biomedicine, planetary science, archeology, and other fields.[31]Ca has six stable isotopes which are40Ca,42Ca,43Ca,44Ca,46Ca, and48Ca, the abundances are 96.9%, 0.647%, 0.135%, 2.09%, 0.004%, and 0.187%, respectively.
In this work, the contributions of the MS, FS, and IS of Li-like Ca ions are calculated by using MCDHF methods.The electronic correlation effects on the MS, FS, and IS are included by systematically increasing the active space to n ≤8 to take the electron correlation effects into account efficiently.A separated relativistic configuration interaction calculation is performed to include the Breit-interaction and quantum electrodynamics(QED)contributions(such as,self-energy corrections and vacuum polarization) which are important for the highly charged ions. Finally, the IS on the transition wavelength are calculated.
2. Theory and computational methodology
The relativistic MCDHF method is one of the most widely used methods for study on the complex atomic structure and properties research.[32–38]The GRASP family codes are based on the MCDHF method which developed in the past 40 years.[39–44]The present work is performed by newly developed GRASP2K[43]and RIS4 package.[45]The detailed theory was expounded in the monograph of I.P.Grant.[46]Only a brief description on the method is given below.
In the MCDHF method, the atomic state wave function(ASFs)Ψ(γPJMJ)can be expressed in terms of configuration state functions (CSFs) Φ(γνPJMJ) with same parity P, total angular momentum J,and its z component MJ,i.e.,

where N is the number of CSFs,cνis the expansion coefficient for the state ν,γ and γνrepresent all additional quantum numbers in addition to the parity P,total angular momentum J,and its z component MJto defines the state uniquely.The CSFs are built from the antisymmetric products of one-electron Dirac orbital. These one-electron Dirac orbital and the expansion coefficients cνin CSFs are obtained by using the relativistic self-consistent field procedure. The MCDHF calculations are followed by the RCI,the Breit interaction and QED correction are included as perturbations in a separated RCI calculations by using GRASP2K package.

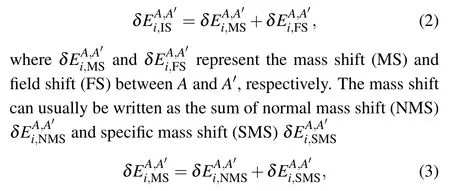
NMS and SMS corresponding to the one-body and two-body nuclear-recoil terms in the relativistic Hamiltonian, respectively. The MS contribution can be further written as
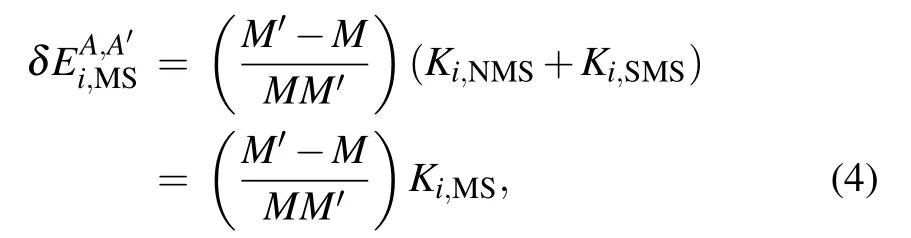
where M and M′are the nuclear masses of the isotopes A and A′, respectively. Ki,NMSand Ki,SMSrepresent the NMS parameters and SMS parameters obtained in the(αZ)4m2/M approximation from the following expectation values:

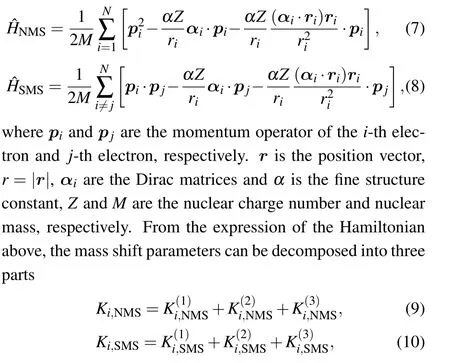
where the K(1)term is generally referred to as the nonrelativistic contribution and the sum of the last two terms,written as K(2)+(3), are the lowest-order relativistic correction in the Breit approximation.Considering a transition k connecting the upper level u and lower level l,the line frequency isotope mass shift can be expressed as
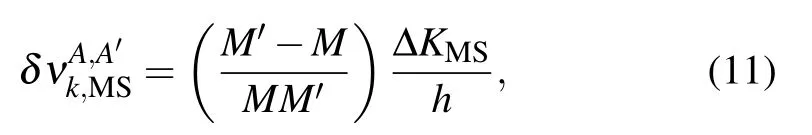
where ∆KMS=(Ku,MS−Kl,MS) is the line mass shift parameters between the upper (u) and lower (l) levels. Field shift parameter Fifor the state i can be given by[47]

where the |Ψ(0)|2is the total electron probability density at the origin. So the line frequency field shift for the transition k can be approximated as


3. Electron correlation model and calculation strategy
The isotope shift is sensitive to the electron correlation effects. In order to take the electron correlation effects into account efficiently, an electron correlation model is constructed by using active space method in this work. The reference configurations of Li-like Ca ions are 1s22s and 1s22p for the ground and the first excited states, respectively. The Dirac–Fock (DF) wave functions are firstly calculated for the40Ca isotope. The active space method is used to generate configuration space systematically. The active space is extended up to n ≤8 with single and double excitation from the reference configuration in the present calculation. The electron correlation model is labeled as nn′ll′. For example, n4l3 represents that the correlation configuration space consists of all the single and double excitation from{1s,2s,2p}orbital within n=1 to 4 and l =0 to 3, respectively (n represents the principal quantum number and l stands for the orbital angular momentum quantum number(lmax=6)). The active space is enlarged layer by layer until the physical quantities under investigation converged. For each iteration, only the newly added layer is optimized.
4. Results and discussion
The total energy and excitation energy of 2s2S1/2and 2p2P1/2,3/2transitions of Li-like Ca ions from the different electron correlation models are given in Table 1. It can be found that the total energy and the excitation energy relative to the ground state tends to be converged with the increase of the active space,respectively. Comparing our results with the data from NIST database,[48]the relative difference between two results are about 0.04% and 0.03% for the 2p2P1/2and 2p2P3/2, respectively. This indicates that the most important electron correlation effects are included in the present calculation.

Table 1. Total energy of 2s 2S1/2, 2p 2P1/2,3/2 and the excitation energy relative to 2s 2S1/2 in Li-like Ca ions.
The calculated wavelengths and transition probabilities for the 2s2S1/2→2p2P1/2,3/2transitions are given in Table 2 with other available data. In this table,λ is the transition wavelength(in ˚A),ABand ACare the transition probability in the Babushkin and Coulomb gauges, which is corresponding to the length and velocity gauge in non-relativistic quantum mechanics,respectively. It can be found that the wavelengths calculated in present work are in good agreement with the values of previous work.[49–51]The consistency of the transition probability from two different gauge is fairly good,which indicate that the wave function used in the present work is good in some extent. It can also be found that the transition probabilities in this work are in good agreement with many-body perturbation theory (MBPT) by Johnson et al. and relativistic coupled-cluster method(RCC)by Das et al. Although the methods used are different, the electronic correlation effect,Breit interaction and QED correction are almost same. This lead to a good agreement. The agreement of the transition wavelength and probability also indicates the validation of the electron correlation model used in this work.

Table 2. Calculated wavelengths(in ˚A)and transition probabilities(s−1)for the 2s 2S1/2 →2p 2P1/2 and 2s 2S1/2 →2p 2P3/2 transitions in Li-like Ca ions. Numbers in square brackets designate powers of 10.
The NMS, SMS parameters (in GHz u) and FS parameters (in GHz/fm2) of the 2s2S1/2→2p2P1/2,3/2transitions of Li-like Ca ions from different correlation models are given in Table 3. The result shows a good convergency with the increase of active space. For the 2s2S1/2→2p2P1/2transition, the MS and FS parameters of n6l5 differ by 0.0087%and 0.0349% from that of n7l6, while at n7l6 only differ by 0.0090%and 0.0159%from that of n8l6. For the 2s2S1/2→2p2P3/2transition MS and FS parameters of n6l5 differ by 0.0107% and 0.0380% from that of n7l6, while at n7l6 only differ by 0.0215%and 0.0159%from that of n8l6. The electron correlation contribution for these parameters increases significantly from DF calculation to n3l2,and then converges gradually.This means the electron correlation effects have significant contribution to the MS and FS parameters. Additionally,we also compare our results with other available theoretical calculations.[20]There are about 0.8%difference between the present work and the results calculated by Kozhedub et al.[20]This difference are mainly come from the different treatment on the electron correlation.
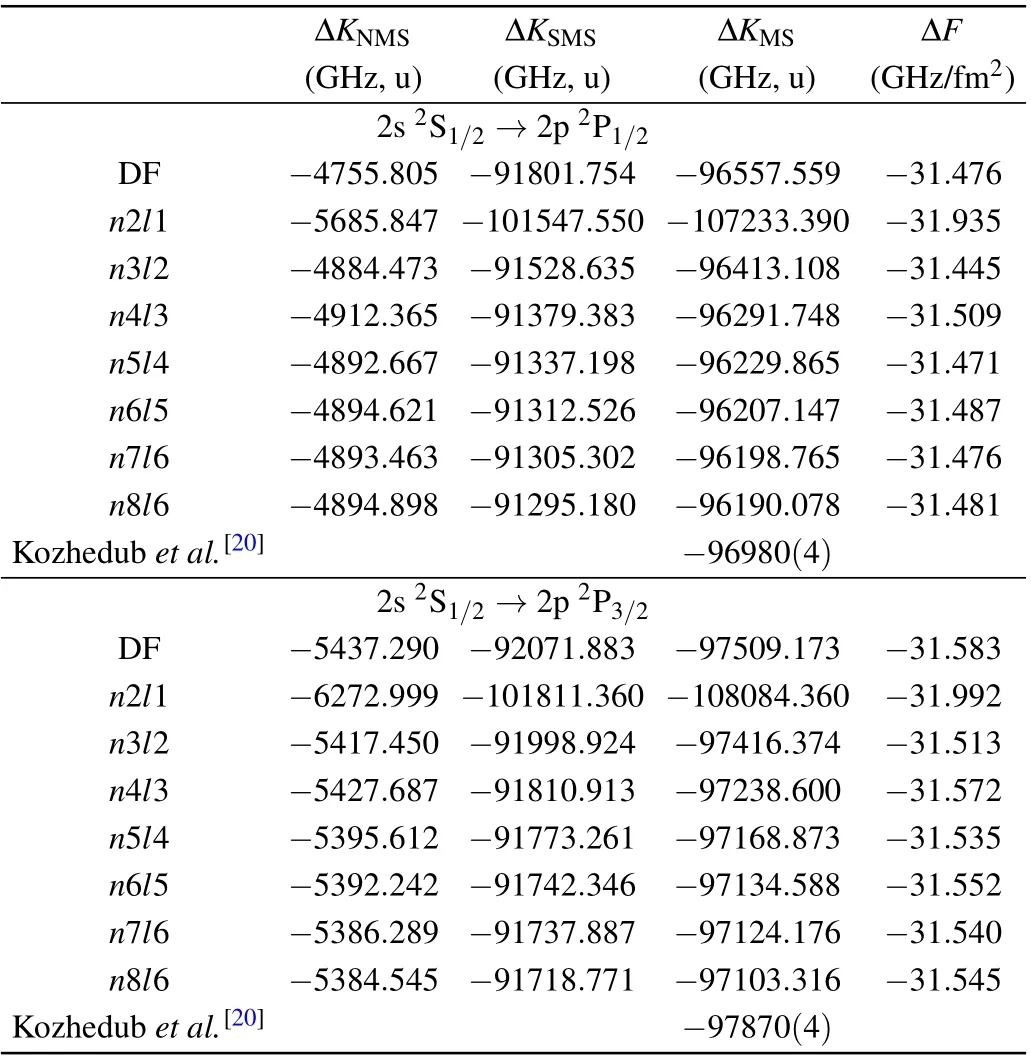
Table 3. NMS, SMS parameters and FS parameters of the 2s 2S1/2 →2p2P1/2,3/2 transitions of Li-like Ca ions under different correlation models.
The shifts of the wavelength relative to the wavelength ofA,40Ca due to MS, FS and IS (in units of fm) of the 2s2S1/2→2p2P1/2and 2s2S1/2→2p2P3/2transitions in Li-like Ca ions for the isotopes (A=40, 42, 43, 44, 46, 48) are given in Table 4. It can be found that the contribution of the MS is obviously larger than that of FS.The MS contribution,which mainly comes from the SMS,grows rapidly with the increase of mass number,while the FS contribution remains almost unchanged. It can be concluded that the IS on calcium almost completely comes from MS, while the contribution of FS to IS is negligible small. Li et al.[18]found the MS is dominant in IS for 6 ≤Z ≤34 elements,which agrees with the present calculations. They also suggest that the situation to might be inversed for heavy elements with Z ≥35. For these isotopes,the largest contribution is IS of Ca48,40and the most prominent shifts is about 0.01 ˚A which is possible to be observed in the experimental observation. For the convenience to the experimentalists,the IS on the transition wavelength in the units of meV are given in Fig.1.

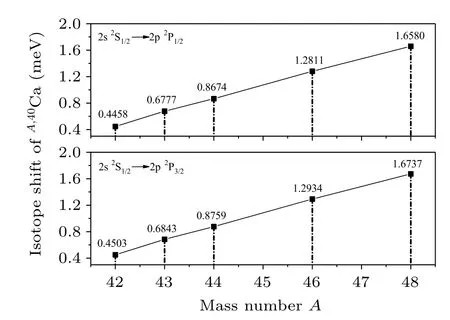
Fig.1. The values of the IS for the 2s 2S1/2 →2p 2P1/2 and 2s 2S1/2→2p 2P3/2 transitions in Li-like Ca ions for the isotope pair A,40Ca.
5. Conclusion

Acknowledgments
The authors would like to thank Ji-Guang Li(Institute of Applied Physics and Computational Mathematics) and Weiqiang Wen(Institute of Modern Physics,Chinese Academy of Sciences)for their good suggestions and helpful discussions.
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Speeding up generation of photon Fock state in a superconducting circuit via counterdiabatic driving∗
- Micro-scale photon source in a hybrid cQED system∗
- Quantum plasmon enhanced nonlinear wave mixing in graphene nanoflakes∗
- Restricted Boltzmann machine: Recent advances and mean-field theory*
- Nodal superconducting gap in LiFeP revealed by NMR:Contrast with LiFeAs*
- Origin of itinerant ferromagnetism in two-dimensional Fe3GeTe2∗
