Antifertility effects of 60-day oral gavage of ethanol extract of Spondias mombin leaves in guinea pigs:A biochemical,reproductive and histological study
2021-04-01OlalekanBukunmiOgunroMusaToyinYakubu
Olalekan Bukunmi Ogunro,Musa Toyin Yakubu
1Phytomedicine,Toxicology,Reproductive and Developmental Biochemistry Research Laboratory,Department of Biochemistry,University of Ilorin,Ilorin,Nigeria
2Department of Biological Sciences,KolaDaisi University,Ibadan,Oyo State,Nigeria
ABSTRACT
KEYWORDS:Anacardiaceae; Spondias mombin; Guinea pig;Testicular dysfunction; Anti-spermatogenic; Antifertility
1.Introduction
Spondias(S.)mombin(Linn)(family,Anacardiaceae),known as hog plum(English),Ichikara(Igbos of the Eastern Nigeria),Tsardarmasar(Hausas of Northern Nigeria)and Akika etikan or Iyeye(Yorubas of the South western Nigeria)is widely distributed in Ivory Coast,Brazil,Mexico and Western Nigeria[1].S.mombin is a deciduous,often deeply grooved tree with blunt and spinelike projections.The stem bark is thick,greyish-brown and deeply fissured.The trunk with branches that are 2-10 m above the ground level form a spreading crown that resembles an open or densely closed canopy.The pinnate leaves which are alternately arranged have 5-10 pairs of elliptic leaflets.
Various parts of S.mombin had been acclaimed in folk medicine to be used in the management of diarrhoea,dysentery,stomach aches,inflammation,hemorrhoids,gonorrhoea,urethritis,cystitis,dystocia,post-partum haemorrhage as well as expectorant,emetic,purgative,diuretic,febrifuge and abortifacient[1,2].The leaves have also been specifically touted as traditional contraceptive for controlling or regulating fertility and conception.
Studies reported earlier on S.mombin in the open scientific literature included the chemical constituents of the plant leaves[3],analgesic and inflammatory activities of the methanol leaf extract[4,5],hepatotoxic,nephrotoxic and haematotoxic effects of the ethanol leaf and aqueous stem bark extracts[6,7]and haematinic potential of the ethanol leaf extract[8].The effects of aqueous extracts of S.mombin leaves on arsenite-induced sperm cells distortions,testicular and epididymal parameters have been documented[9,10].Also,the anti-gonadotropic effects of aqueous leaf extracts of S.mombin on male rats and the effects of ethanol leaf extract of S.mombin on pituitary-gonadal axis of female Wistar rats have been separately documented[11,12].In addition,Pakoussi et al[13]have documented the effects of hydroethanolic extract of S.mombin on labour time,uterus,sex steroids,organ weight and coagulation time whilst Raji et al[14]reported the alterations of some serum chemistry parameters,white blood cells,red blood cells and functional status of accessory sex organs of male rats after the administration of aqueous extract of S.mombin bark.Finally,the effects of ethanolic extract of S.mombin leaves on haematological parameters,liver and kidney function indices and histomorphology of the testes,epididymis and prostate gland have also been reported[15,16].
Although different studies have separately concluded on the antifertility effects of S.mombin extracts and fractions at several doses[1,9-16],to the best of our knowledge,there is still dearth of scientific information on the effects of ethanol extract of S.mombin leaves on the function indices and histoarchitectural changes of the testes of male guinea pigs; reproductive,maternal and foetal outcomes after pairing the female guinea pigs with the ethanol extract of S.mombin leaves-treated males.Accordingly,the present study reported the impact of 60 days of administration of ethanol extract of S.mombin leaves at the doses of 100,250 and 500 mg/kg body weight on the sperm profile,male reproductive hormones,biochemical parameters of testicular function and testicular histoarchitecture as well as maternal and fetal outcomes of guinea pigs.
2.Materials and methods
2.1.Plant material and authentication
The plant was obtained from Isaba Ekiti in Ikole Local Government Area of Ekiti State,Nigeria,in August 2015.It was authenticated at the Herbarium Unit of the Department of Plant Biology,University of Ilorin,Ilorin,Nigeria.A voucher specimen(UILH/001/1147)was deposited for future references.
2.2.Drugs,chemicals and assay kits
Alkaline phosphatase(ALP),acid phosphatase(ACP),glutamate dehydrogenase,gamma glutamyl transferase,17-β-hydroxy steroid dehydrogenase,3-β-hydroxy steroid dehydrogenase,glucose-6-phosphate dehydrogenase,total protein,and total cholesterol assay kits were products of Randox Laboratory,Co-Atrim,United Kingdom.Estradiol,follicle stimulating hormone and luteinizing hormone assay kits were manufactured by Diagnostics Laboratories,Freiburg,Germany.Assay kits for 3-hydroxy-3-methylglutaryl CoA reductase,sorbitol dehydrogenase,malic enzyme,glutamate dehydrogenase and lactate dehydrogenase were products of Sigma Aldrich Ltd,St.Louis,Missouri,USA.All other chemicals used in this study were of analytical grade obtained from Sigma Aldrich Ltd.,Buchs,Canada.The pH cooperative paper strips were product of British Drug House Chemical Limited,Poole,England.
2.3.Experimental animals
Forty-eight sexually matured male guinea pigs(Cavia porcellus)[(1000.40 ± 8.12)g]and twenty-four female guinea pigs [(810.00 ±4.22)g]used in this study were obtained from the Nigerian Institute for Trypanosomiasis Research,Kaduna,Nigeria.The guinea pigs were housed in clean,aluminium cages that were placed in wellventilated room [light/dark cycle:12 hours light and 12 hours dark cycle; temperature:(23±2)℃ and relative humidity:40%-45%].The guinea pigs were allowed free access to animal pellets(Premier Feed Mills Company Limited,Ibadan)and clean tap water.Fourteen days of acclimatisation was observed before the commencement of the study.The cages were also cleaned on daily basis.
2.4.Preparation of ethanol extract of S.mombin leaves
Fresh leaves of S.mombin(13 700 g)were collected,washed under running tap and oven-dried at 40 ℃ for 72 h to a constant weight(4 740 g)using Uniscope SM9053 Laboratory Oven,Surgifriend Medicals,England.The leaves were pulverized with an electric blender [Crown Star Blender CS- 242B,Trident(H.K.)Ltd,China].The powder(1 000 g)was extracted in 3 litres of 98% ethanol for 96 h at 25 ℃ with constant shaking.The resulting solution was filtered using Whatman No.1 filter paper and afterwards concentrated under reduced pressure in a rotary vacuum evaporator(Model:RE-52A,Shanghai Ya Rong Biochemistry Instrument Factory,China)to give a yield of 81.93 g which corresponded to percentage yield of 0.60%.Calculated amount of the freeze-dried sample was reconstituted in physiological saline(vehicle)to give the equivalent doses of 100,250 and 500 mg/kg body weight that were used in this study.The doses of 100,250 and 500 mg/kg body weight previously used by Pakoussi et al[13]were adopted in the present study.
2.5.Secondary metabolite screening
The ethanol extract of S.mombin leaves was screened for the presence of secondary metabolites such as saponins,alkaloids,flavonoids,tannins,steroids,phenolics,cardiac glycosides,anthraquinones,cardenolides and dienolides,chalcones,phlobatannins and terpenes by adopting the procedure described by Egbuna et al[17].Furthermore,the presence of saponins,alkaloids,flavonoids,tannins,phlobatanins,steroids,phenolics,cardiac glycosides,cardenolides and dienolides were quantified by adopting the procedures described by Egbuna et al[17].
2.6.Animal grouping and administration of ethanol extract of S.mombin leaves
Twenty-four sexually matured male guinea pigs were randomly assigned into four groups(A,B,C and D)of 6 animals each and treated once daily for 60 days(53 days of 1 complete cycle of spermatogenesis and maximum of 7 days of final transit through the epididymis).Group A received 1 mL of physiological saline and served as the control.Group B received 1 mL of ethanol extract of S.mombin leaves corresponding to 100 mg/kg body weight.Group C received 1 mL of ethanol extract of S.mombin leaves corresponding to 250 mg/kg body weight.Group D received 1 mL of ethanol extract of S.mombin leaves corresponding to 500 mg/kg body weight.All administrations were done through oral gavage between 8:00-9:00 a.m.to the various groups of animals using oropharyngeal cannula.The experimental animals were allowed free access to rat pellets and tap water after their daily doses of ethanol extract of S.mombin leaves and physiological saline.
2.7.Preparation of serum and testicular supernatants
The weight of the guinea pigs was determined at exactly twentyfour hours after the last administration.Immediately after weighing,the animals were anaesthetized with intraperitoneal injection of 60 mg/kg body weight of 6% sodium pentobarbital.The jugular veins were then cut with a sterile dissecting blade.Exactly 5 mL of blood was collected from the jugular veins into plain sample tubes and left to clot at 25 ℃ for 10 min.The tubes were then centrifuged at 685 ×g for 15 min using Hermle Bench Top Centrifuge(Model Hermle,Z300,Hamburg,Germany)after which the resulting sera were aspirated into sample bottles using Pasteur pipettes.The sera were used for the hormonal assay within 12 h of collection.
The testes were excised,freed from fat,blotted with blotting paper and weighed for the computation of testes-body weight ratio.Thereafter,the testes were cut very thinly with sterile blade and homogenised separately in ice cold 0.25 M sucrose solution(1:5 w/v).The homogenates were centrifuged at 1 398 ×g for 15 min to obtain the testicular supernatants.The resulting supernatants were then collected into sample bottles,kept frozen for 24 h at 4 ℃ and afterwards diluted appropriately with 0.25 M sucrose solution before being used for the analyses.
2.8.Collection of semen and analysis of semen parameters
The procedure reported by Quadri and Yakubu[18]was adopted for the collection of semen from the epididymis of the guinea pigs.Briefly,the caudal epididymis was lacerated with sterile scissors.The semen was standardised to minimise and/or avoid variation in secretion by aspirating 10 µL from the caudal epididymis of each guinea pig with the aid of polyester capillary tube and releasing such into Petri dishes containing 1.0 mL of 0.1 M phosphate buffer(pH 7.4).The dishes were gently swirled for homogeneity and dispersal of the sperm cells into the solution was done for 10 min at 37 ℃.The semen pH and volume,sperm count,viability,motility,density and morphology were determined accordingly as previously described by Quadri and Yakubu[18].
In specific terms,the pH was determined on 10 µL of the semen collected from the caudal epididymis using a pH cooperative paper(strip)that ranged from 5-9.The change in colour was then matched with those of the standard colour that was supplied with the kit.
The connective tissue capsule around the caudal epididymis was bugged out and the epididymal duct was uncoiled,exposed and incised.The whole semen that transuded into the cavity block was quickly sucked into a graduated cylinder and the volume was recorded as the semen volume.
For the determination of sperm count,the caudal epididymis from the right testes was lacerated to release the semen,and a 1:20 dilution from each well-mixed sample was prepared by diluting 50 µL of liquefied semen with 950 µL of the diluent(prepared by adding 50 g of sodium carbonate,10 mL of 35%(v/v)of formalin and 0.25 g of trypan blue to distilled water and making up the solution to a final volume of 1 000 mL)to form a suspension from which the sperm count was determined using the Neubauer Improved Haemocytometer(Marienfeld Laboratory Glassware,Laudakonigshofen,Germany)under the Olympus Bright Field Binocular Microscope(Model CX22 LED,Japan)at ×40 magnification.
For the determination of sperm morphology,the 10 µL of the semen obtained from the caudal epididymis was diluted with Tris buffer solution to make 1.0 mL solution.Neutral buffered formalin(10%,1:20)was further used to dilute the aliquot of the resulting mixture.This was then followed by the addition of two drops of warm Eosin/Nigrosin stain to the semen on a pre-warmed slide.A uniform smear was then made,air-dried after which the stained slide was then immediately examined for any morphological changes under the microscope at ×400 magnification.Five fields of the microscope were randomly selected and the types and number of abnormal spermatozoa were evaluated from the total number of spermatozoa in the five fields.Classification of the sperm cells was based on the presence of one or more of abnormal features including tail defects,neck defects(neck and middle piece defects)and head defects.The number of abnormal spermatozoa was expressed as a percentage of the total number of spermatozoa.
The sperm viability(live/dead ratio)was determined by adding a drop of warm Eosin/Nigrosin stain to a drop of semen on a prewarmed slide(to minimize the risk of cold shock)after which a uniform smear was then made and air-dried.The stained slide was immediately examined under the Olympus Microscope at×40 magnification.The live sperm cells were unstained,while the dead sperm cells absorbed the stain.The stained and unstained spermatozoa were counted and the percentage calculated.
The number of motile spermatozoa was determined by adding two drops of warm 2.9%(w/v)sodium citrate to exactly 10 µL of semen that was placed on a pre-warmed(27 ℃)microscope slide.The slide was then covered with a pre-warmed cover slip(22 mm ×22 mm)and examined under the microscope at ×40 magnification.Ten fields of the microscope were randomly selected,and the motility of 10 sperm cells was assessed in each field.Thereafter,the motility of 100 sperm cells was assessed randomly.Sperm cells were designated as motile,sluggish or immotile.The percentage of motile sperm cells was determined by the progressive and non-progressive movement of the sperm cells under the microscope.Percentage motile sperm cells were defined as the number of motile sperm cells divided by the total number of counted sperm cells.
For the determination of sperm density,a sample of sperm suspension was heated in a water bath for exactly 30 seconds to kill all the sperm and was later counted with a haemocytometer.
The viscosity of the semen was evaluated traditionally by determining the length in centimetres of a thread that viscous semen formed when released through a plastic pipette.In addition,viscosity status of the semen was determined by estimating the time taken by a fresh semen sample to fully load a chamber with tapered ends.Briefly,Eppendorf micropipette(10 µL)was used to load semen into the filling area of a single chamber in a Leja 20 µm Disposable Standard Count,2 Chamber Slide(Leja,Nieuw-Vennep,the Netherlands; Art.number SC20-01-02-B).The tip of the pipette was placed at the filling area of the chamber at an angle,without touching the entrance.The stopwatch was simultaneously started immediately after releasing the piston,and the time taken in seconds for the liquid to reach the air outlet of the chamber was recorded.
2.9.Determination of testicular and serum parameters
The procedures described by Scriver et al[19],Makela et al[20],Lee et al[21]and Afshari et al[22]were adopted for the determination of serum concentrations of FSH,LH,estradiol,and serum and testicular testosterone,respectively.The ratio of serum testosterone:estradiol was also computed for all the different groups of animals.
Other testicular function indices investigated in this study were as described for alkaline phosphatase[23],acid phosphatase[24],glutamate dehydrogenase[25],γ-glutamyl transferase[26],3-hydroxy-3-methylglutaryl CoA reductase[27],sorbitol dehydrogenase[28],malic enzyme[29],3-β-hydroxysteroid dehydrogenase and 17-β-hydroxysteroid dehydrogenase[30],lactate dehydrogenase[31],glucose-6-phosphate dehydrogenase[32],cholesterol[33],glycogen[34],sialic acid[35],total protein[36],ascorbic acid[37],catalase[38],and superoxide dismutase[39].
2.10.Histopathological examination of the testes
The testes of the guinea pigs were fixed in 10%(v/v)formaldehyde,dehydrated through ascending grades of ethanol(70%,90% and 95%v/v),cleaned in xylene and embedded in paraffin wax(melting point 56 ℃).Tissue sections were stained with haematoxylin & eosin(H & E).The resulting slides were then read with a light microscope(OLYMPUS,Model:CX21,New York Microscope Company Inc.,New York).Cross section of the testes was captured at ×400 with Canon! Image Folio package software(Model:Powershot A2500,Japan).
2.11.Fertility study
Another set of twenty-four,sexually matured,male guinea pigs,were randomly assigned into four groups(A,B,C and D)of 6 animals each.The animals in groups A,B,C and D were orally administered the physiological saline,100,250 and 500 mg/kg body weight respectively,once daily for 60 days.Thereafter,female guinea pigs were brought into artificial oestrous by administering estradiol benzoate at 10 µg/100 g body weight and progesterone at 0.5 mg/100 g body weight,subcutaneously,48 h and 4-6 h,respectively before pairing with the male guinea pigs.One receptive female guinea pig was introduced into the cage containing another male guinea pig(1:1).The pairing lasted for 33 days during which two oestrous cycles must have elapsed.Thereafter,the female guinea pigs were allowed to carry the pregnancy for another 32 days making a total of 65 days.During this period,feed and water were supplied ad libitum.On day 66,the female guinea pigs were sacrificed as previously described in Section 2.7(Preparation of serum and testicular supernatants)and fetal parameters that included total number of pups,circumference of the pups,length of pups(from the snout to the tail)and weight of the pups were recorded[17].Furthermore,maternal outcome like number of fetal resorption and fertility index were also computed using the following expression:
Fertility index(%)=(Number of pregnant guinea pigs×100)/Number of paired guinea pigs
2.12.Data analysis
Data were expressed as the mean of six determinations±standard deviation(mean±SD).The normally distributed data were analysed using both the one-way analysis of variance and Duncan's new multiple range test at P<0.05(confidence level = 95%).All the analyses were done with SPSS version 20.0 software(SPSS Inc,Chicago,IL,USA).The data on total number of pups and number of fetal resorption which were non-normally distributed were expressed as median(interquartile range).
柔性垂直防渗技术充分利用了原有场区地下相对不透水层地质,在垂直防渗技术应用过程中,涉及到开槽挖土作业,需掌握场地详细地层条件与物理力学参数,查明场地相对不透水层地层的深度、厚度及特性,依据相对不透水层得出柔性垂直防渗设计深度,依据地层条件与物理力学参数指导柔性垂直防渗技术的实施。
2.13.Ethics statement
The study was conducted after obtaining a letter of approval(UERC/ASN/2015/206)dated 15th October,2015 from the University of Ilorin Ethical Review Committee.
3.Results
3.1.Secondary metabolite constituents of ethanol extract of S.mombin leaves
The ethanol extract of S.mombin leaves contained saponins,alkaloids,flavonoids,tannins,steroids,phenolics,phlobatannins,cardiac glycosides,cardenolides and dienolides whilst anthraquinones,chalcones and terpenes were not detected(Table 1).Quantitatively,saponins(4.80 mg/mL)were the most abundant whilst cardenolides and dienolides(0.08 mg/mL)were the least abundant of all the secondary metabolites(Table 1).
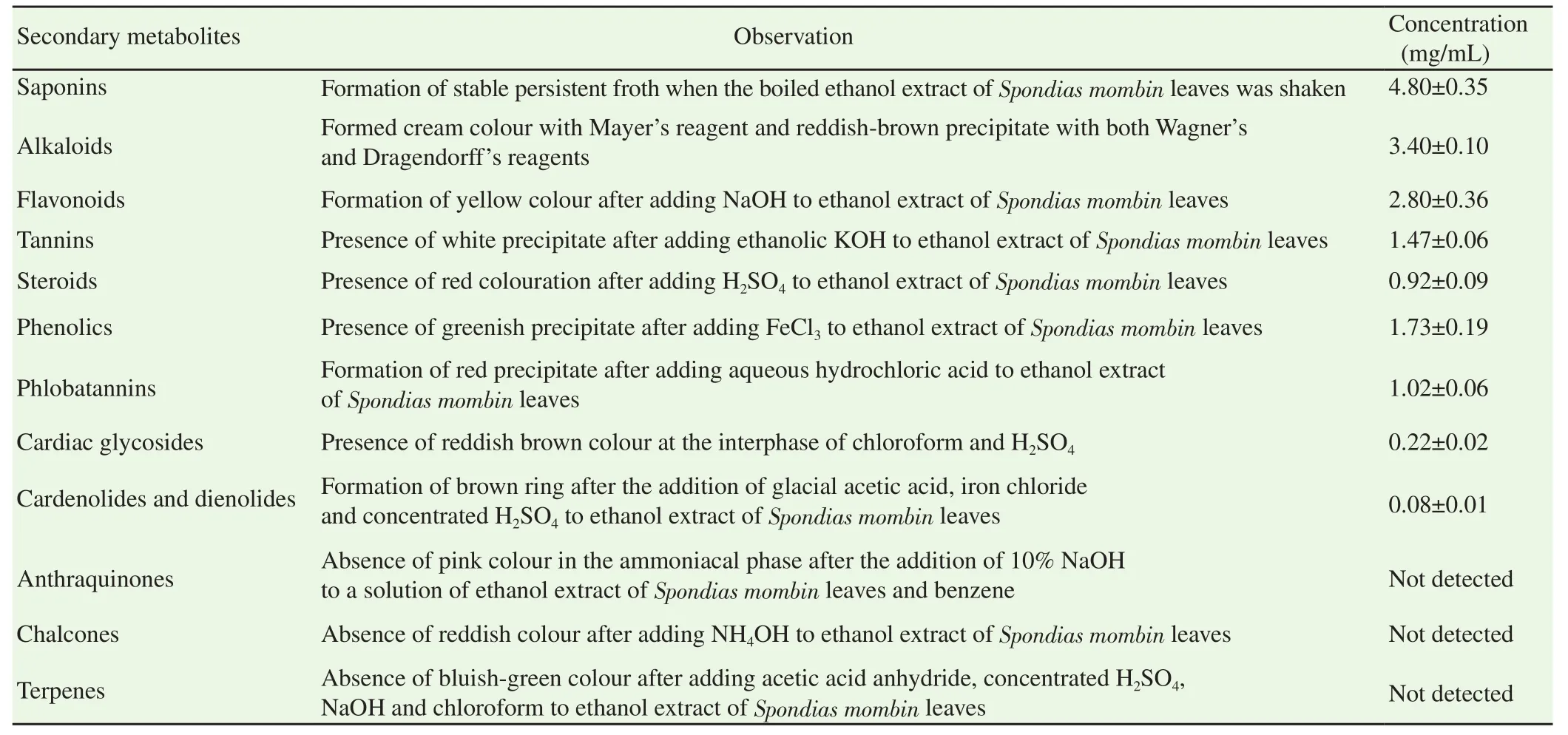
Table 1.Secondary metabolite constituents of ethanol extract of Spondias mombin leaves.
3.2.Testicular function indices of male guinea pigs after 60 days of oral administration of ethanol extract of S.mombin leaves
Administration of ethanol extract of S.mombin leaves significantly and dose dependently reduced the activities of alkaline phosphatase,glutamate dehydrogenase,3-hydroxy-3-methylglutaryl Co enzyme A reductase,malic enzyme,17-β-hydroxysteroid dehydrogenase,lactate dehydrogenase,catalase and superoxide dismutase as well as the levels of testosterone,glycogen,total protein and ascorbic acid in the testes of the male guinea pigs when compared with those of physiological saline-treated control guinea pigs(P<0.05)(Table 2).Furthermore,the highest dose(500 mg/kg body weight of ethanol extract of S.mombin leaves)significantly decreased the levels of alkaline phosphatase,glutamate dehydrogenase,3-hydroxy-3-methylglutaryl Coenzyme A reductase,malic enzyme,17-βhydroxysteroid dehydrogenase,lactate dehydrogenase,catalase,superoxide dismutase,testosterone,glycogen,total protein and ascorbic acid by 62.54%,49.40%,66.67%,61.34%,58.72%,43.45%,24.61%,31.07%,67.43%,71.05%,37.26% and 19.26% respectively when compared with those of physiological salinetreated control guinea pigs(P<0.05)(Table 2).Also,the activities of sorbitol dehydrogenase,3-β-hydroxysteroid dehydrogenase,glucose-6-phosphate dehydrogenase and sialic acid were significantly decreased by the ethanol extract of S.mombin leaves(P<0.05)in a manner that were not dose-dependent when compared with those of physiological saline-treated control guinea pigs(Table 2).However,the 500 mg/kg body weight of ethanol extract of S.mombin leaves significantly reduced the levels of sorbitol dehydrogenase,3-βhydroxysteroid dehydrogenase,glucose-6-phosphate dehydrogenase and sialic acid by 21.08%,18.31%,3.92% and 51.61% respectively when compared with those of physiological saline-treated control guinea pigs(P<0.05).In contrast,all the doses of ethanol extract of S.mombin leaves significantly and dose dependently increased the activities of acid phosphatase,gamma glutamyl transferase and the levels of total cholesterol when compared with those of physiological saline-treated control guinea pigs,with the highest dose(500 mg/kg body weight of ethanol extract of S.mombin leaves)(P<0.05)increasing the levels of acid phosphatase,gamma glutamyl transferase and total cholesterol by 1.69,1.17 and 2.58 folds of the physiological saline -treated control male guinea pigs(Table 2).
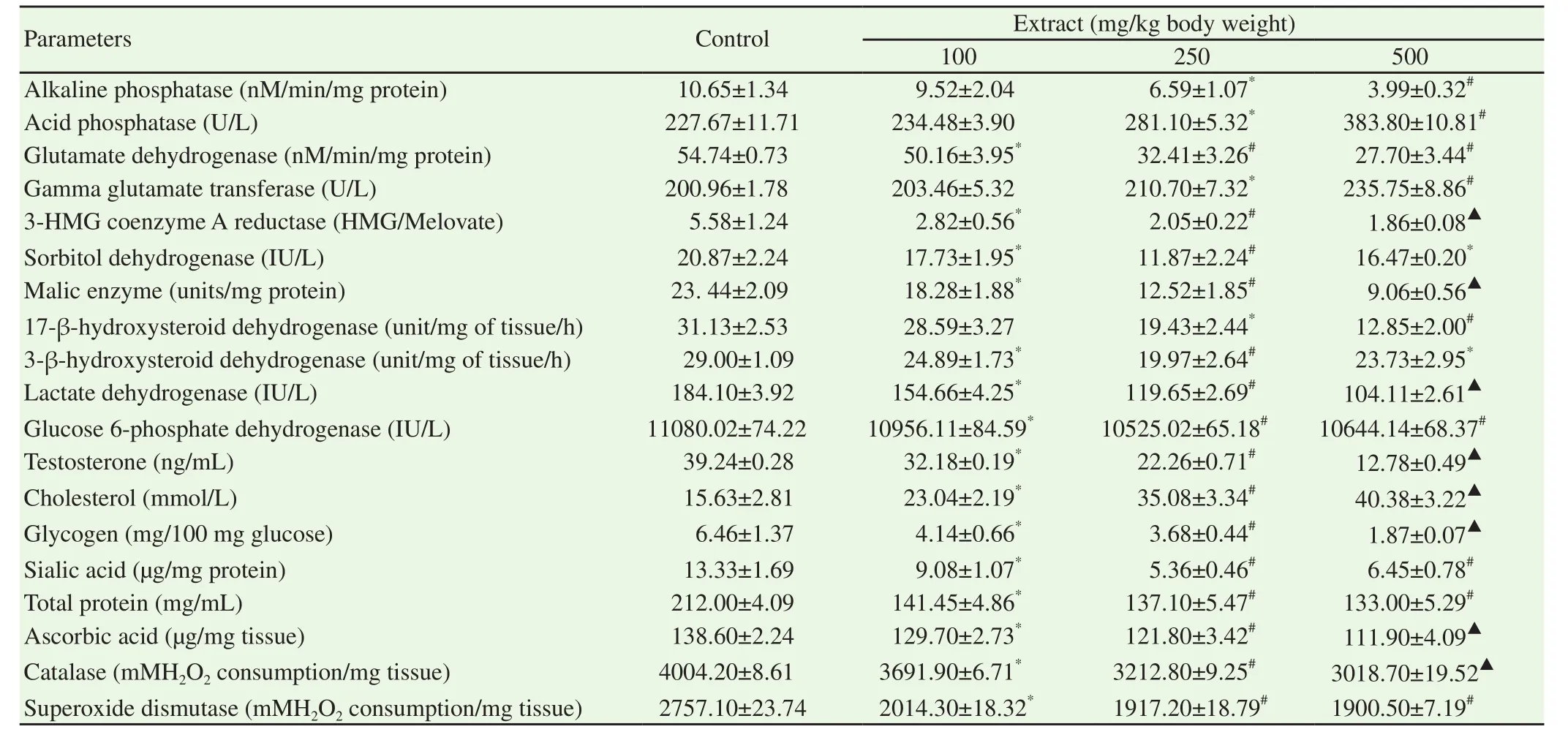
Table 2.Testicular function indices of male guinea pigs after 60 days of oral administration of ethanol extract of Spondias mombin leaves.
3.3.Serum hormones of male guinea pigs after oral administration of ethanol extract of S.mombin leaves for 60 days
Administration of ethanol extract of S.mombin leaves significantly reduced the concentrations of serum luteinizing hormone,testosterone and estradiol(P<0.05)whereas the ethanol extract of S.mombin leaves significantly increased the levels of follicle stimulating hormone in the serum of the male guinea pigs when compared with those of physiological saline-treated control guinea pigs(P<0.05)(Table 3).The highest dose of ethanol extract of S.mombin leaves(500 mg/kg body weight)reduced the concentrations of serum luteinising hormone,testosterone and estradiol of the male guinea pigs by 48.27%,66.70% and 46.48% respectively when compared with those of physiological saline-treated control guinea pigs whereas the same dose increased the serum content of follicle stimulating hormone of the male guinea pigs by 2.67 folds of the physiological saline-treated control value(Table 3).Also,the ethanol extract of S.mombin leaves did not produce a general pattern of effect on the computed serum testosterone to estradiol ratio of the animals.In specific terms,the testosterone:estradiol produced by the 100 mg/kg body weight of ethanol extract of S.mombin leaves was at par with that of the physiological saline-treated control male guinea pigs whilst that produced by the 250 mg/kg body weight of ethanol extract of S.mombin leaves was higher than that of the physiological saline-treated control guinea pig(Table 3).Furthermore,the administration of 500 mg/kg body weight of ethanol extract of S.mombin leaves reduced the computed testosterone:estradiol by 36.54% when compared with that of physiological saline-treated control guinea pigs(Table 3).
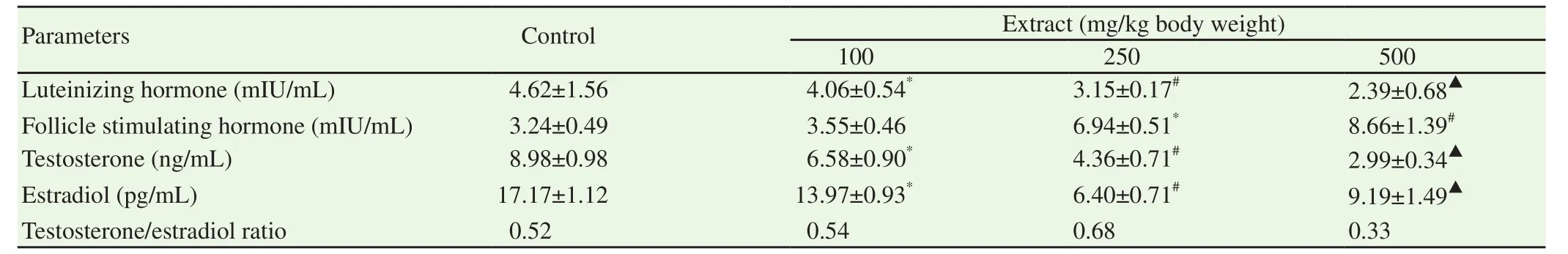
Table 3.Serum hormones of male guinea pig after oral administration of ethanol extract of Spondias mombin leaves for 60 days.
3.4.Sperm characteristics of male guinea pigs after 60 days of oral administration of ethanol extract of S.mombin leaves
Administration of the ethanol extract of S.mombin leaves at the doses of 100,250 and 500 mg/kg body weight significantly decreased the sperm viability,sperm count,sperm density,sperm motility and semen viscosity when compared with those of physiological saline-treated control guinea pigs(P<0.05)(Table 4).The impact of the ethanol extract of S.mombin leaves on these sperm parameters was most pronounced with the 500 mg/kg body weight,reducing the sperm motility,sperm count,sperm density,sperm viability and sperm viscosity by 63.30%,70.46%,73.21%,49.23% and 74.73%,respectively when compared with those of physiological saline-treated control guinea pigs(Table 4).The dose dependent reduction(P<0.05)in the normal sperm morphology after exposure to ethanol extract of S.mombin leaves was accompanied by increase(P<0.05)in the number of sperm cells with head,neck and tail defects when compared with those of physiological salinetreated control guinea pigs.The 500 mg/kg body weight of ethanol extract of S.mombin leaves significantly decreased the normal sperm cell morphology by 42.74% whilst the same dose increased the head defect,neck defect and tail defect by 2.0,3.4 and 1.8-folds respectively,of their control.In contrast,all the doses of the ethanol extract of S.mombin leaves did not significantly alter the pH and the volume of the semen of the male guinea pigs when compared with those of physiological saline-treated control guinea pigs(P>0.05)(Table 4).
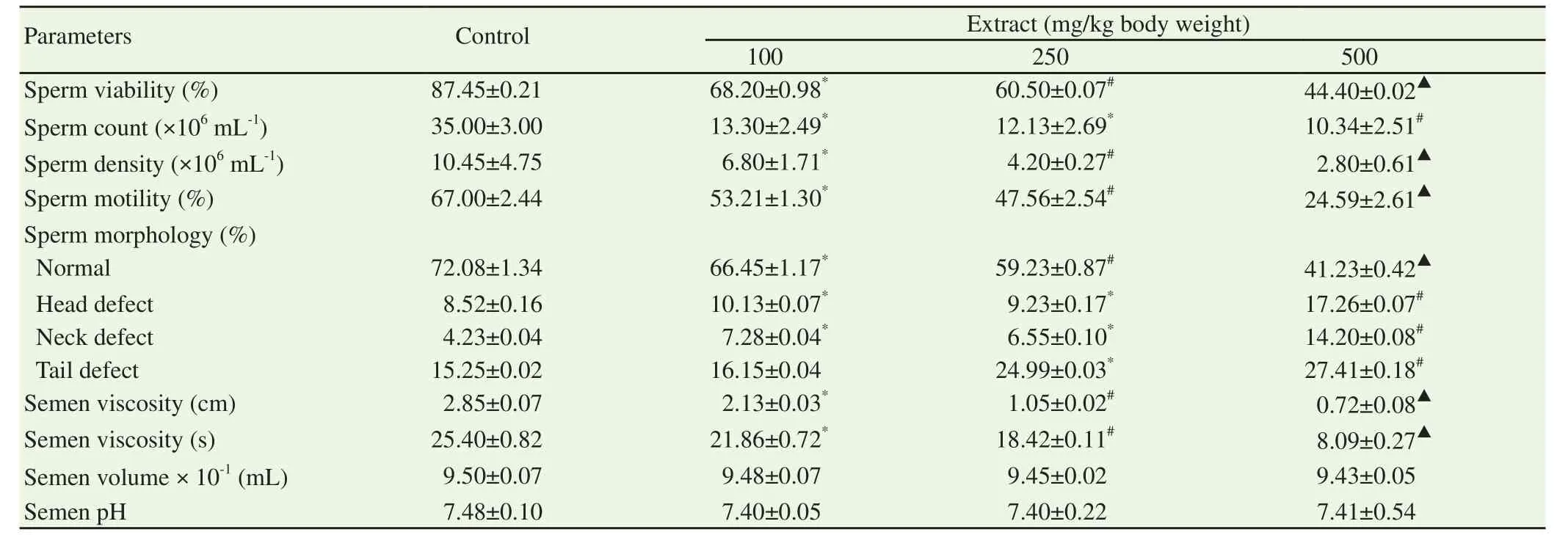
Table 4.Sperm characteristics of male guinea pigs after 60 days of oral administration of ethanol extract of Spondias mombin leaves.
3.5.Testicular histoarchitectural changes
Compared with the physiological saline-treated control male guinea pigs where the germinal epithelium lining of the seminiferous tubules of the testes of the animals were within normal histology with different stages of spermatogenesis(Figure 1A),the testicular histoarchitecture of the male guinea pigs treated with the 100 mg/kg body weight of ethanol extract of S.mombin leaves were essentially the same with those of the physiological saline treated control guinea pigs(Figure 1B).In contrast,the 250 mg/kg body weight of ethanol extract of S.mombin leaves induced mild necrotic changes in the seminiferous tubules with reduction in the number of spermatocytes(Figure 1C)whereas the 500 mg/kg body weight of ethanol extract of S.mombin leaves produced degenerative and necrotic changes in the seminiferous tubules with vacuoles in the germinal epithelium and scanty spermatocytes(Figure 1D).
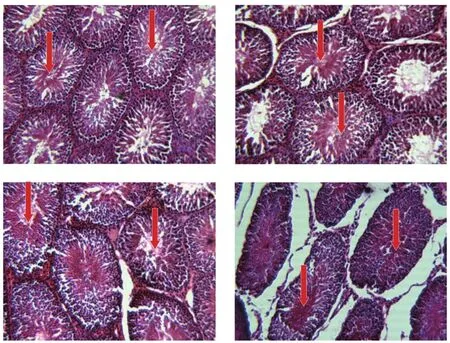
Figure 1.Cross section of the testis of male guinea pigs after 60 days of oral gavage with ethanol extract of Spondias mombin leaves(×400; H & E).A:The control group treated with physiological saline.The arrows depict that the germinal epithelium lining of the seminiferous tubules of the testes of male guinea pigs are within normal histology with different stages of spermatogenesis.B:Male guinea pigs treated with 100 mg/kg body weight of ethanol extract of Spondias mombin leaves.The testicular histoarchitecture is similar to that of physiological saline-treated control guinea pigs.The arrows show that the germinal epithelium lining of the seminiferous tubules of the testes of male guinea pigs is within normal histology with different stages of spermatogenesis.C:Male guinea pigs treated with 250 mg/kg body weight of ethanol extract of Spondias mombin leaves.The arrows indicate mild necrotic changes in the seminiferous tubules with reduction in the number of spermatocytes.D:Male guinea pigs treated with 500 mg/kg body weight of ethanol extract of Spondias mombin leaves.The arrows depict degenerative and necrotic changes in the seminiferous tubules with vacuoles in the germinal epithelium and scanty spermatocytes.
3.6.Fertility indices of female guinea pigs after 60 days of oral administration of ethanol extract of S.mombin leaves
The 100 and 250 mg/kg body weight of ethanol extract of S.mombin leaves-treated male guinea pigs produced pregnancy in 3(50%)and 1(17%),respectively out of 6 female guinea pigs paired,as against the 6(100%)out of the 6 female guinea pigs that were paired with the physiological saline-treated male guinea pigs.No episode of pregnancy was recorded in the female guinea pigs that were paired with the 500 mg/kg body weight of ethanol extract of S.mombin leaves-treated male guinea pigs(Table 5).Also,the total number of pups found in the female guinea pigs that were paired with the 100,250 and 500 mg/kg body weight of ethanol extract of S.mombin leaves-treated male guinea pigs were 3,1 and 0 respectively as against 19 in the physiological saline-treated animals(Table 5).The female guinea pigs that were paired with the male guinea pigs that received 100 mg/kg body weight of ethanol extract of S.mombin leaves produced the highest number of fetal resorption when compared with those of 250 and 500 mg/kg body weight of ethanol extract of S.mombin leaves and that of physiological saline-treated control guinea pigs.The circumference of the pups,length of the pups and weight of the pups were all reduced in the female guinea pigs that were paired with the 100 and 250 mg/kg body weight of ethanol extract of S.mombin leaves-treated male guinea pigs(Table 5).In contrast,there was no pup in the female guinea pigs that were paired with the male guinea pigs that received the 500 mg/kg body weight of ethanol extract of S.mombin leaves(Table 5).
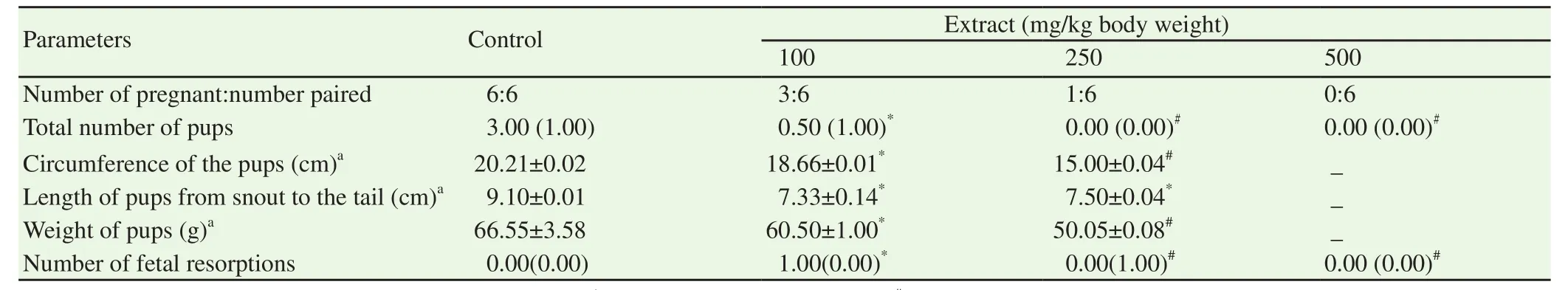
Table 5.Maternal and fetal outcomes of female guinea pigs after pairing with ethanol extract of Spondias mombin leaves-treated male guinea pigs.
4.Discussion
Chemical compounds and natural products are capable of inducing profound and irreversible injury to mammalian testes.Medicinal plants and their products induce testicular damage that manifest in the disruption of normal secretory and synthetic function and alterations in the histoarchitecture of the testes.The accumulating evidence on the decline in male fertility has necessitated the need to screen for the impact of ethanol extract of S.mombin leaves on the biochemical indices of testicular function including testicular hormones,testicular enzymes,non-enzymatic parameters,serum reproductive hormones,semen and sperm quality,maternal and fetal outcome as done in the present study.
The normal structure and functioning of the testes depends exclusively on bioavailability of testosterone and other androgens.Testosterone,when available in the required amount stimulates the growth,synthetic and secretory activities of the testes.Therefore,a decrease in the concentration of testicular testosterone obtained in this study after the administration of ethanol extract of S.mombin leaves will impair the number and functioning of the cells of the testes most especially the Leydig cells as corroborated by the reduction in the activity of glucose-6-phosphate dehydrogenase.Furthermore,the drop in the activity of glucose-6-phosphate dehydrogenase will diminish the production of reducing equivalent that is essential for the conversion of cholesterol to testosterone.Also,since testicular proteins are androgen dependent,the drop in the levels of total protein content of the testes of guinea pigs by the ethanol extract of S.mombin leaves in the present study,further corroborates the depletion of testosterone by the ethanol extract of S.mombin leaves and this may adversely affect spermatogenesis and more specifically,sperm maturation.Such depletion of testicular testosterone may also confer anti-androgenic activity on ethanol extract of S.mombin leaves.
Testicular phosphatases(ACP and ALP)are also known to be involved in the secretion of gonadotropins and in the intercellular and intracellular transportation of metabolites during steroidogenesis[40].The dose dependent decrease in the testicular ALP activity after the administration of ethanol extract of S.mombin leaves,most especially at the 500 mg/kg body weight might adversely affect the transportation of required metabolites within and outside the cells of the testes during steroidogenesis.Furthermore,the increase in the activity of ACP in the present study could be linked to indiscriminate hydrolysis of phosphate esters which should have served as potential source of energy to the cells.Lactate dehydrogenase is associated with the maturation of germinal epithelial layer of seminiferous tubules and post meiotic spermatogenic cells.Therefore,the reduction in the activity of lactate dehydrogenase in the testes of the male guinea pigs in the present study suggests hindrance and/ or impairment in the ability of the animals to transform spermatocyte to spermatozoa.Also,the drop in the sperm count of the male guinea pigs in this study could be linked to the degenerative and necrotic changes in the seminiferous tubules by the 250 and 500 mg/kg body weight of ethanol extract of S.mombin leaves.Also,glutamate dehydrogenase which is densely expressed in the Sertoli cells is known to provide the spermatids with lactate and other nutrients.Therefore,a reduction in the activity of glutamate dehydrogenase might have deprived the spermatids the much needed lactate and other nutrients.In addition,the reduction in the activity of the enzyme might be linked with the drop in the sperm motility of the male guinea pigs in this study.All the doses of ethanol extract of S.mombin leaves also impaired the normal functioning of the Sertoli cells as corroborated by the increase in gamma glutamyl transferase in the testes of the guinea pigs.Due to their roles in the energy metabolism of sperm cells,the reduction in the activities of sorbitol dehydrogenase and malic enzyme as well as glycogen in the testes of the male guinea pigs could be linked to the drop in sperm motility,since provision of adequate energy to propel the sperm cells would have been hindered.Also,the reduction in the testicular sialic acid content,a lubricant that facilitates easy movement of the sperm cells by reducing friction among the spermatozoa,supports the reduction in the motility of sperm cells by ethanol extract of S.mombin leaves in the present study.All these consistently emphasis drop in sperm quality and hence,the fertilising capacity of the sperm cells of the male guinea pigs.
The maintenance of normal activity of steroidogenic enzymes is required for proper testicular steroidogenesis and spermatogenesis.3-hydroxyl-3-methylglutaryl coenzyme A reductase is the ratelimiting step in the sterol biosynthesis/mevalonoate pathway whilst 3-β-hydroxyl steroid dehydrogenase and 17-β-hydroxyl steroid dehydrogenase are key androgenic enzymes that are essential in steroidogenesis and steroid degradation[41].Therefore,the dose dependent reduction in the activities of these enzymes indicate that ethanol extract of S.mombin leaves have interfered with the biosynthesis of steroid hormone which impacted negatively on spermatogenesis and by extension,the fertility of the male guinea pig.The reduction in the activity of these enzymes by the constituents of ethanol extract of S.mombin leaves is reflected by the dose-dependent increase in the concentrations of testicular cholesterol.The implication of this is that cholesterol will not be available for the synthesis of testosterone,hence,the depleted levels of testosterone in the present study.All these adverse effects on the testicular function indices by ethanol extract of S.mombin leaves in the male guinea pigs consistently emphasis testicular toxicity and dysfunction,which culminated in the reduced fertility of the male guniea pigs in this study.
The presence of different antioxidant defense systems in guinea pigs and mammals has been well documented.Superoxide dismutase generally dismutates the superoxide anion radicals into hydrogen peroxide which is then degraded by catalase and glutathione peroxidase.As observed in this study,ethanol extract of S.mombin leaves significantly overwhelmed the defense capacity of the testicular enzymatic(superoxide dismutase and catalase)and non-enzymatic(ascorbic acid)antioxidants.This suggests that the ethanol extract of S.mombin leaves caused impairment to the testes of the male guinea pigs through induction of oxidative stress,notwithstanding the non-investigation of indicator of lipid peroxidation marker,malondialdehyde,in this study.The testosterone-induced depletion of the testicular antioxidant defense system in the male guinea pigs may be one of the mechanism by which ethanol extract of S.mombin leaves elicited its poor sperm quality and reduction in fertility.This is the first report that has comprehensively addressed the impact of ethanol extract of S.mombin leaves on the function indices of the testes of experimental animals,guinea pigs in this instance.
Testosterone is the most important androgen essential for spermatogenesis that is regulated by lutenising hormone.Luteinising hormone acts on the Leydig cells of the testes and enhance the synthesis and secretion of testosterone.The testosterone in conjunction with follicle stimulating hormone acts on the spermatogonia to stimulate sperm production.The pituitary-testicular axis is therefore central in the regulatory ability of the testes that culminates in the production of spermatozoa.Therefore,the reduction in the concentration of serum testosterone after the 60 days of administration of ethanol extract of S.mombin leaves suggests suppression of testicular steroidogenesis and spermatogenesis as earlier opined.Furthermore,the reduction in the concentration of luteinising hormone and increase in the concentration of follicle stimulating hormone may not be unconnected with the feedback inhibition mechanism of testosterone.Estradiol is very vital in modulating libido,erectile function and spermatogenesis.Expectedly,the reduction in the concentration of estradiol in the present study has consequentially affected spermatogenesis of the male guinea pigs,arising from the degenerative and necrotic changes in the seminiferous tubules earlier reported in this study.The least value of testosterone:estradiol by the 500 mg/kg body weight of ethanol extract of S.mombin leaves suggests that the testosterone deficiency might partly or entirely be due to the accompanying decline in estradiol and further corroborates the negative impact on sexual arousal and spermatogenesis[42].It is also possible that the ethanol extract of S.mombin leaves might have acted as an endocrine disruptor in this study.The patterns of reduction in the levels of serum luteinising hormone and estradiol of the male guinea pigs in this study are similar to the dose-dependent decreases in the serum hormones reported by Asuquo et al[12]after 14 daily administration of 250,350 and 500 mg/kg body weight of ethanol extract of S.mombin leaves to female Wistar rats.The highest dose(500 mg/kg body weight)in the present finding reduced the levels of serum luteinising hormone and estradiol in the male guinea pigs by 48.27% and 46.48%,respectively as against the 93.75% and 56.13%,respectively reported by Asuquo et al[12]in the female Wistar rats.The follicle stimulating hormone of the male guinea pigs dose-dependently increased in the present study whereas Asuquo et al[12]reported a dose dependent decrease in the female rats serum hormone.Although Asuquo et al[13]used extraction solvent(aqueous),experimental animals(male rats),doses(400 and 800 mg/kg)and duration of experiment(28 days)that are different from those of the present study,the findings on dose-dependent reduction in the levels of serum follicle stimulating hormone,luteinising hormone and testosterone are similar to those reported herein.
The decrease in sperm count,motility,density,viscosity,viability and the increase in the morphological abnormalities in the male guinea pigs after 60 days of administration of ethanol extract of S.mombin leaves are indications of impairment of spermatogenesis in the animals as a result of direct effect on the testicular tissue.In specific terms,disruption at the Sertoli-germ cell tight junctions will lead to failure of spermatogenesis.Profound testicular damage by the ethanol extract of S.mombin leaves in this study is evident from the degenerative and necrotic changes of the seminiferous tubules as revealed by the testicular histology and may account for the progressive sloughing of immature germ cells which resulted in abnormalities in early sperm development and thus the increase sperm morphological abnormalities in this study.The decrease in sperm count and quality is correlated with decrease in testosterone levels and testicular oxidative damage as evident from depleted levels of superoxide dismutase,catalase and ascorbic acid.Semen pH is determined by the acid secretion of the prostate gland and the alkaline secretion of the seminal vesicle.Therefore,the absence of an effect on the semen pH by all the doses of ethanol extract of S.mombin leaves might imply that the plant extract did not adversely affect the balance between the acid secretion of the prostate gland and alkaline secretion of the seminal vesicle.Furthermore,the volume of the semen is largely made up of secretion from the seminal vesicles which make up about 60% and most of the remainder coming up from the prostate gland with only a very small volume from the secretion of the bulbourethral(Cowper's)gland.Thus,the absence of significant difference between the physiological saline-treated guinea pigs and the ethanol extract of S.mombin leaves-treated animals suggest that the ethanol extract of S.mombin leaves did not affect the accessory glands(seminal vesicle,prostate gland and Cowper's glands).All these indicate that ethanol extract of S.mombin leaves did not affect the accessory glands,at least with respect to these semen parameters,but exhibited localised toxicity on the testes of the male guinea pigs.Although the findings reported here with respect to dose-dependent decrease in sperm progressive motility,sperm count,viability and dose-dependent increase in percentage abnormal spermatozoa are in line with those of Raji et al[15],the current study administered 100,250 and 500 mg/kg body weight of ethanol extract of S.mombin leaves,once daily for 60 days to male guinea pigs whereas Raji et al[15]administered 8.4,16.8 and 33.6 mg/kg body weight per day of aqueous extract of S.mombin leaves stem bark for 4 weeks to male Wistar rats.
The dose-dependent reduction in the number of pregnant female guinea pigs,percentage of pregnant guinea pigs and fertility success could be linked to the dose-dependent reduction in the quality of sperm cells produced by the male guinea pigs.It is worthy of note that pregnancy did not occur in the female guinea pigs that were paired with the highest dose(500 mg/kg body weight)treated male guinea pigs.All these confers anti-conception/contraceptive property on the ethanol extract of S.mombin leaves as previously reported by Uchendu and Isek[1]after the administration of 800 mg/kg of aqueous ethanol leaf extract of S.mombin to rats.Since the number of resorption evaluates the correlation between the number of implanted blastocytes and those of the undeveloped blastocytes,the higher number of fetal resorption confers pregnancy failure property on the ethanol extract of S.mombin leaves.The fetotoxic effects of ethanol extract of S.mombin leaves in this study were evident from the reduction in the total number of pups,circumference,length and weight of the pups.The reduction in the number and weight of litters(pups)in the present study contrast those of Pakoussi et al[14]who reported absence of an effect on the number and weight of litter after challenging the pregnant rats from day 19 of pregnancy with 100,250 and 500 mg/kg body weight of hydroethanol extract of S.mombin leaves.
Studies have shown that medicinal plants exhibit antifertility effects in male animals by various mechanisms such as immobilization of spermatozoa(by saponins,phenolics,and triterpenes),sperm aggregation and spermicidal activity(by saponins,phenolics and alklaoids),reduced sperm density,antispermatogenic activity and alteration in sperm structure(by phenolics),anti-androgenic activity and decreasing testosterone levels(alkaloids and glycosides),cessation of spermatogenesis and degeneration of the seminiferous tubules(flavonoids).Therefore,the antifertility effects of ethanol extract of S.mombin leaves in the present study was exhibited through mechanisms such as anti-androgenic activity,antispermatogenic activity/suppression of spermatogenic activity,antisteroidogenic activity,anti-gonadotropic and contraceptive effects.The presence of saponins,alkaloids,flavonoids,tannins,steroids,phenolics,and cardiac glycosides contained in the ethanol extract of S.mombin leaves which might have acted singly or in combination may be responsible for these mechanisms of antifertility effects of S.mombin leaves.
The specific bioactive principle of antifertility effects of S.mombin leaves may have to await further studies.
In conclusion,S.mombin leaves have induced infertility in the male guinea pigs via endocrine dysregulation,anti-androgenic activity,anti-spermatogenic activity,testicular dysfunction,antioxidant activity and degeneration of the seminiferous tubule.All of these were made possible due to the presence of saponins,alkaloids,flavonoids,tannins,steroids,phenolics,and cardiac glycosides.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
The authors acknowledge the technical assistance of Mr.Dele Aiyepeku of the Department of Biochemistry,University of Ilorin,Ilorin.Nigeria.
Authors' contributions
The conception and design of the study were done by Olalekan Bukunmi Ogunro and Musa Toyin Yakubu.The literature search was done by Olalekan Bukunmi Ogunro while Olalekan Bukunmi Ogunro and Musa Toyin Yakubu conducted the experiment.The data acquisition and analysis as well as drafting of the manuscript were done by Olalekan Bukunmi Ogunro.The editing and reviewing of the draft manuscript as well as revising the manuscript for intellectual content were done by Musa Toyin Yakubu.Both Olalekan Bukunmi Ogunro and Musa Toyin Yakubu approved the completed article.
猜你喜欢
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Association between estradiol levels and clinical outcomes of IVF cycles with single blastocyst embryo transfer
- Combined effects of Gymnema sylvestre and Pergularia daemia on letrozole-induced polycystic ovarian syndrome in rats
- Aphrodisiac potential of Polyalthia bullata(Tongkat Ali)in fowl
- Semen characteristics of the three genetic types of boars reared in Benin
- Evaluation of pig oocyte in vitro maturation and fertilization using three gonadotropin-based hormonal compounds
