Microalgal Cultivation in Secondary Effluents: Enhancement of Algal Biomass, Nutrient Removal, and Lipid Productivity
2020-11-30ZHANGBoMENGFanpingCUIHongwuDOUXiangDUShuhaoandPENGXiaoling
ZHANG Bo, MENG Fanping, 2), *, CUI Hongwu, DOU Xiang, DU Shuhao, and PENG Xiaoling
Microalgal Cultivation in Secondary Effluents: Enhancement of Algal Biomass, Nutrient Removal, and Lipid Productivity
ZHANG Bo1), MENG Fanping1), 2), *, CUI Hongwu3), DOU Xiang1), DU Shuhao1), and PENG Xiaoling1)
1)Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, China 2) College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100,China 3)Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
The growth performance, nutrient removal, lipid accumulation and morphological changes ofOUC1 andwhich were cultured in secondary effluents from two wastewater treatment plants: Tuandao Wastewater Treatment Plant (ETD) and Licun River Wastewater Treatment Plant (ELR) were investigated. The results showed that bothOUC1 andhave superior growth performances in both undiluted effluents, while the better of them was that in ETD effluent, with cell densities ofOUC1 andincreased by 159% and 66% over that of BG11 (control), respectively. Regarding nutrient removal,could completely remove inorganic phosphorus, and decrease ammonia nitrogen in ETD effluent by 81%. In addition, bothOUC1 andcultivated in ETD exhibited extraordinary potential for biofuel production, increasing lipid productivities by 133% and 89% of that cultivated in ELR, respectively. As to ultrastructural changes, the differences in the lipoidal globules and glycogen granules ofandOUC1 among the ETD and ELR treatments were mostly related to phosphorus limitations. The findings from this research reveal the probability using the secondary effluents as cultivation media to enhance algal biomass, nutrient removal and lipid productivity.
secondary effluent; nutrient removal; lipid;OUC1;
1 Introduction
Accelerated urbanization generates increasing quantities of secondary effluents discharged from municipal wastewater treatment plants (WWTPs), which contain residual nutrients and organic compounds (Ge and Champagne, 2016; Gojkovic., 2019; Yu., 2019). Residual nutrients in secondary effluent, in particular nitrogen and phosphorus, may induce eutrophication in rivers and coastal areas and endanger the stability of the ecosystem (Deegan., 2012; Yu., 2019). In this sense, urgent demand for more efficient approaches to diminish the consequences of nutrients in effluent discharge from WWTPs has attracted extensive attention from researchers (Yao., 2015, Mirzaei., 2019).
Simultaneously, concerns about the energy crisis, coupled with the burgeoning demand for energy and an expanding consciousness of the global effect of associated gaseous and particulate emissions, have made the advancement of sustainable and environmentally friendly energy sources indispensable (Tripathi., 2019). Along with the development of biofuels, the use of oil-rich microalgae as a replaceable biofuel feedstock is acquiring booming interest due to its promising advantages, including extraordinarily adaptive capacity, uncomplicated cell structures, high lipid content and a lipid composition suitable for biodiesel production (Markou and Georgakakis, 2011; Cai., 2013; Sakthivel., 2018). Nevertheless, production of biodiesel by microalgae at an industrialised scale has still not been shown to be economically and technically feasible on account of the high cost of cultivation, extraction, and harvesting techniques as well as the low yield (Tang., 2020). To solve this problem, adopting secondary effluents to produce microalgae-based biofuel has exhibited potential in contemporaneously resolving water eutrophication issues and the energy crisis for sustainable development (Chen., 2015).
Microalgae have been cultivated in diverse categories of effluent media to accomplish simultaneous biofuel production and nutrient elimination (Ge and Champagne, 2016; Cheng., 2018; Marella., 2019). Li. (2010) found thatsp. LX1 achieved the highest biomass (0.11gL−1, dry mass) and lipid content (31%-33%, dry mass) when cultivated in secondary effluents after 15 days and had an extremely high inorganic nutrient assimilation efficiency (98%) in 10days. Al Momani and Örmeci (2016) compared the dissimilar treatment efficiencies ofandfor the removal of ammonia (NH4+-N), nitrate (NO3−-N), total inorganic nitrogen (TIN), and total dissolved phosphorus (TDP) in the secondary effluents after 28 days or 25 days of cultivation. Overall, microalgae cultivation in the presence of secondary effluents can promote microalgal growth and nutrient removal efficiency, and the results ordinarily rely on the microalgal species, cultivation time, and type of secondary effluent. Accordingly, it would be worthwhile to investigate the corresponding tolerance and growth characteristics of microalgae in a secondary effluent containing different levels of nutrients.
The present work aimed to explore the feasibility of growth and lipid production of microalgae cultivated in secondary effluents from two municipal WWTPs under different dilution ratios; the removal efficiency of nutrients from secondary effluents by microalgae was also considered. In order to fulfil this purpose, cell densities, lipid contents and productivities, biomass production, nutrient concentration, and cell ultrastructure were determined. Two species of microalgae were used here:andOUC1. The former is a unicellular green alga, which has been reported to be used for wastewater treatment due to its high tolerance to a wide range of nitrogen and phosphorus loads and higher lipid yields (Arias., 2019). The latter is a unicellular blue-green alga, which has a tolerance to higher temperature (surviving at 20℃ to 50℃) and acidic conditions (Meng, 2018), as well as being able to grow rapidly.
2 Material and Methods
2.1 Effluent Sampling
The secondary effluent samples used in this study were taken from the Tuandao Wastewater Treatment Plant (ETD) and Licun River Wastewater Treatment Plant (ELR) in Qingdao (Shandong Province, China) (Table 1). For each plant, the effluent samples were collected at four different times (1:00, 7:00, 13:00, and 19:00) and mixed in equal volumes followed by precipitation (12h) and filtration (0.45μm filter membrane). The mixed effluents were sterilized at 121℃ for 20min, kept at 4℃. Before use, the effluents were diluted with distilled water to achi- eve various ratios of effluent volume to total volume (v/v = 1/5, 2/5, 3/5, 4/5, and 5/5) and used for algal culture.
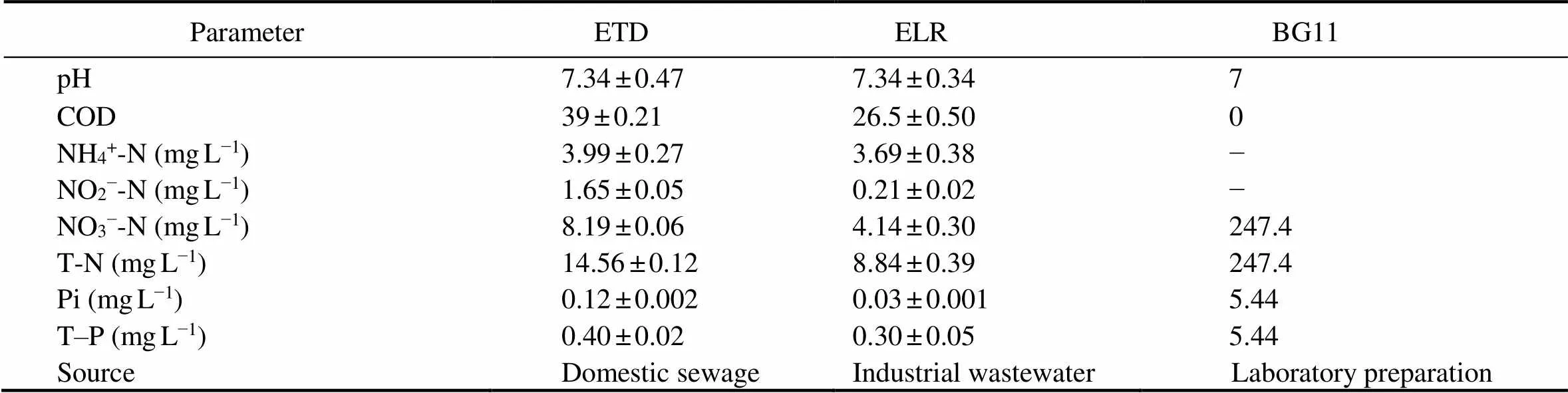
Table 1 Characteristics of the ETD, ELR, and BG11 medium
Notes: Data are shown as mean ± standard deviation (SD) (= 3). ETD, effluent from the Tuandao Wastewater Treatment Plant; ELR, effluent from the Licun River Wastewater Treatment Plant; NH4+-N, ammonia nitrogen; NO2−-N, nitrite nitrogen; NO3−-N, nitrate nitrogen; T-N, total nitrogen; Pi, orthophosphate; T-P, total phosphorus
2.2 Algal Strains and Cultivation in Effluent
Theused in this study was provided by the Institution of Hydrobiology, Chinese Academy of Science.OUC1 was isolated from water samples, which were collected near Jimo hot springs, Qingdao, China (Meng., 2018). Two microalgae were pre- cultured to the exponential phase using BG11 medium (Stanier., 1971) under the continuous illumination at 60μmolphotonsm−2s−1on a rotatory shaker at 150rmin−1. ForOUC1 and, their temperatures were maintained at 35±1℃ and 25±1℃, respectively.
Experimental cultures were grown in 1000-mL Erlenmeyer flasks with the addition of 400 mL of effluent at different dilution ratios. Controls were performed in BG11 medium. The initial cell density was 1.3×106cellmL−1for both species of algae. Then, the microalgae were cultivated continuously to a stationary growth phase under the same conditions as pre-culture. All experiments were conducted in triplicate. Cell densities were measured daily. Algal biomass, lipid content, ultrastructure, and nutrient concentration in culture were analysed at the end of cultivation.
2.3 Measurement of Cell Density and Biomass
Algal cell density (104cellmL−1) was measured daily using a hemacytometer and an optical microscope (NikonYS2-H, Japan). Microalgal biomass (, gL−1) was determined gravimetrically (Ho, 2012) by filtering 10 mL of culture through 0.45μm filter membrane and over- drying at 50℃ for 8h.
2.4 Measurement of Lipid Contents
The lipid content of the algal biomass was measured by a modified version of chloroform-methanol method (Bligh and Dyer, 1959). For each group, a mixture consisting of 0.1g of dried algal biomass and 7.5mL of chloroform-methanol (2:1, v/v) was sonicated in an ice bath for 3 min with a sonicator (JY92-II, Scientz Biotechnology Co., Ltd, Ningbo, China). Then, 3mL of the chloroform-methanol (2:1, v/v) was added to the mixture and allowed to react for 12h. The chloroform-methanol layer containing the lipid was separated from the mixture by centrifugation at 5000for 10min. The extract was evaporated to dryness under nitrogen and weighed. Thereafter, the lipid content was calculated gravimetrically based on Eq. (1):
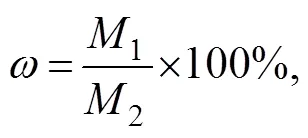
whereis the lipid content (%), and1and2represent the masses of the extracted lipid and the dry algal biomass, respectively.
The lipid productivity was calculated using Eq. (2):
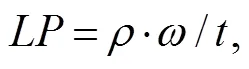
whereis the lipid productivity (mgL−1d−1),is the algal biomass,represents the lipid content (%),is the cultivation time (d).
2.5 Chemical Analysis
Before and after cultivation, an aliquot of the culture suspension was filtered through a filter (0.45μm) to remove cells and other particles. The filtrate was adopted to detect nutrients. The content of nitrite nitrogen (NO2−-N) was analysed by using a spectrophotometric method (MEP, 1987). The nitrate-nitrogen (NO3−-N) was identified according to an ultraviolet spectrophotometric method (MEP, 2007). The ammonia nitrogen (NH4+-N) was measured by Nessler’s reagent spectrophotometry (MEP, 2009). The orthophosphate (Pi) was quantitatively estimated by an ammonium molybdate spectrophotometric method (MEP, 1989). The nutrient removal amount,(mgL−1), was obtained by use of Eq. (3):

where0andare defined as the concentration of nutrient before and after cultivation, respectively.
The nutrient removal percentagewas calculated using Eq. (4):

2.6 Transmission Electron Microscopic Observation
After cultivation, algal suspensions in control, undiluted ETD and undiluted ELR were centrifuged at 10000for 15min. The cell pellets were fixed with 2.5% glutaraldehyde (1:50, v/v) for 4h, and rinsed three times with phosphate buffer (pH 7.8, 0.1molL−1). The cells were then fixed with 1% OsO4for 90min, and subsequently embedded in Spurr’s resin before being dehydrated using increasing acetone concentrations. Ultra-thin sections of cells were cut using an ultramicrotome, counterstained with aqueous uranyl acetate and lead citrate (Reynolds, 1963), and examined by transmission electron microscopy (TEM, JEM-2100, Japan).
2.7 Statistical Analysis
Results are presented in the form of mean ±standard deviation. Statistical analyses were conducted using SPSS 17.0 software (SPSS Co., USA). The correlations between biomass and dilution ratios, initial nutrient concentrations, and removal amount of nutrients were tested for significance using bivariate Pearson correlation analysis at significance levels of<0.05 and<0.01.
3 Results and Discussion
3.1 Effect of Effluents on the Growth of Microalgae
At various concentrations of ETD (Fig.1) and ELR (Fig.2), microalgal species had different responses. The growth ofOUC1 (Fig.1a) and(Fig.1b) was highly stimulated by ETD (except for theunder 1/5-3/5 ETD treatments). The cell densities at the stationary phase in undiluted ETD were 112% and 19% higher than that of the control, respectively. Compared with the control, the biomass concentrations ofOUC1 andcultivated in undiluted ETD were increased by 159% and 66%, respectively (Fig.1c). The cell density and biomass of the two microalgae in 1/5-4/5 ETD were increased with increasing ETD concentration, but they were lower than those in the corresponding undiluted ETD treatment group. This phenomenon was also found inOUC1 under ELR treatment. This may have been due to the declined concentrations of nutrients in the diluted effluents. In summary, two effluents can be directly used for microalgal cultivation without dilution.
The effect of the two effluents on the growth of microalgae indicated that the biomass concentrations ofOUC1 andin ETD were higher than in corresponding ELR treatment groups during the stationary phase (Figs.1c and 2c). This is because ETD is primarily derived from domestic sewage, which contains high concentrations of nutrients such as nitrogen and phosphorus (Table 1) for the rapid growth of microalgae. A previous study found thatsp.LX1 achieved a maximum biomass of 0.11gL−1after being cultured for 15days in secondary effluents (Li., 2010). In the present study,in undiluted ETD and ELR reached maximum biomass of 1.95 times and 1.36 times higher than that found in the aforementioned study, respectively. For these reasons, both undiluted ETD and ELR can be substituted for BG11 as a medium to promote the growth of microalgae, but undiluted ETD is more conducive to the growth of microalgae.
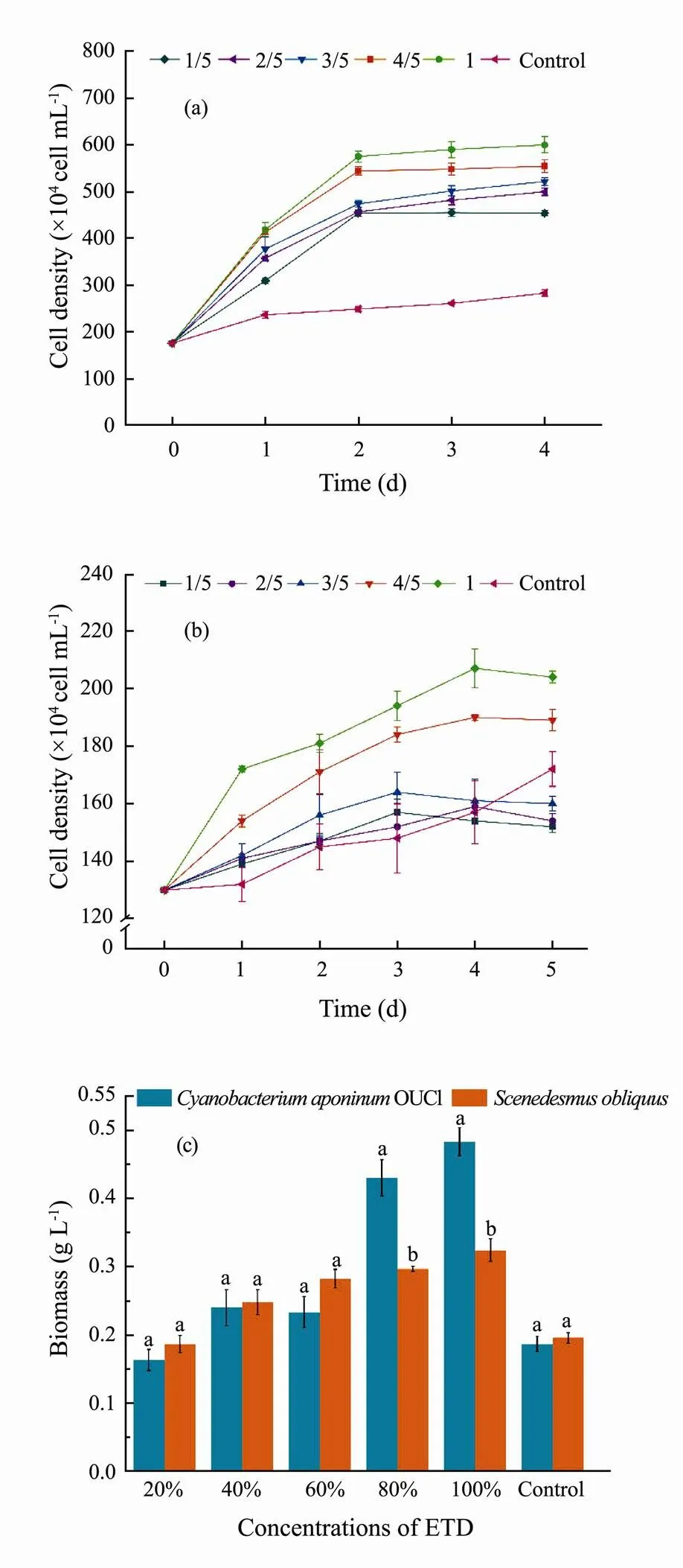
Fig.1 Algal cell densities of Cyanobacterium aponinum OUC1 (a) and Scenedesmus obliquus (b) cultivated in ETD with different dilution ratios and biomass concentrations (c) of two species of microalgae at the end of cultivation. Data are shown as mean ± standard deviation (SD) (n=3). Different letters above the bars represent significant differences (p<0.05).
3.2 Nutrient Removal from Effluents
Table 1 displays the water quality of the two effluents. It was found that the concentrations of various forms of nitrogen and phosphorus in ETD were higher than those in ELR. In the present study, microalgae were cultivated using an open system. CO2from the air could be used as a carbon source for algal growth, while nitrogen and phosphorus are entirely derived from the effluents or BG11 medium. The two microalgae in both the ETD and ELR treatment groups had the largest removal amount of NO3−-N, followed by NH4+-N and NO2−-N (Fig.3). Theremoval amount of NO3−-N increased with the ETD concentration in both microalgae, although a less pronounced increase was observed inwhen the ETD dilution ratio exceeded 4/5. At the end of culti- vation, the removal rates of NO3−-N forOUC1 andin undiluted ETD were 86.2% and 59% (Table 2), respectively. In the treatment of ELR, the removal amount of NO3−–N by microalgae still increased with the increase of ELR concentrations. Treatment of ETD and ELR causes a decrease in the concentration of NH4+-N, while a comparable trend was observed in the ETD treatment groups for the NO2−-N concentration. TheOUC1 in different concentrations of ELR did not decrease the concentration of NO2−-N. Some studies have reported that microalgae preferentially assimilated reduced nitrogen (NH4+-N) because the NO3−-N is first reduced to NO2−-N with the facilitation of nitrate reductase (NR) (Cai., 2013; Ge and Champagne, 2016), and the latter is transported into the chloroplast where it is transformed into NH4+-N by nitrite re- ductase (NiR) and ferredoxin (Fd). Ultimately, NH4+–N is incorporated into the amino acid glutamine by glutamine synthase (Cai., 2013). However, the assimilation of oxidised nitrogen by microalgal cells is also related to the activities of intracellular NR and NiR. Lomas and Gilbert (2000) found that the diatomsandsp. perform differently in intracellular assimilation of the same form of nitrogen. The accumulation content of NH4+-N insp. cells was larger than that of, while the assimilation rate of NO3−-N incells was several times higher (310fmol-Ncell−1h−124fmol-Ncell−1h−1) compared with thesp. cells. Further studies showed that the intracellular NiR and NR activities ofwere significantly higher than those ofsp. (<0.01) (Lomas and Gilbert, 2000). Above all, the considerable utilisation of NO3−-N by the two microalgae in the present study may be associated with the high activities of NiR and NR in the cells, but further research is required to verify this.
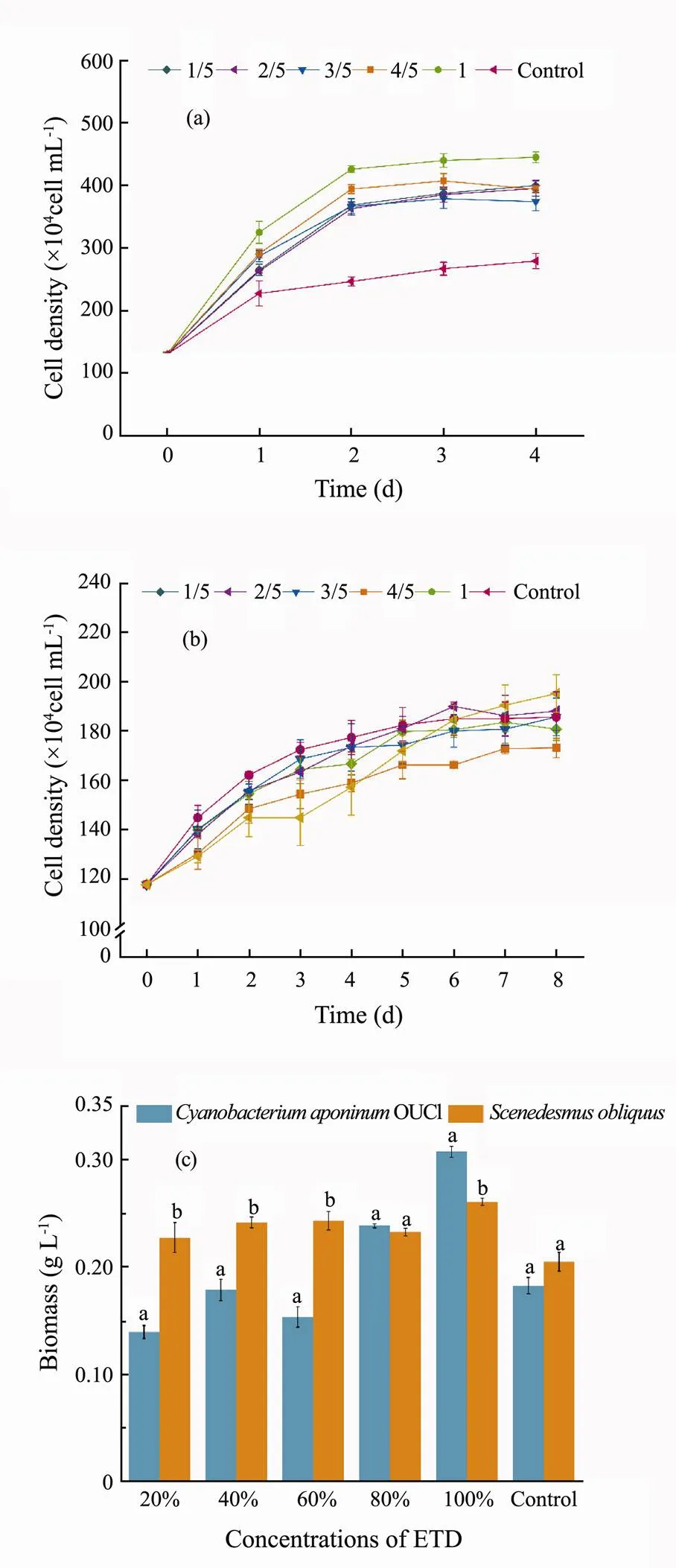
Fig.2 Algal cell densities of Cyanobacterium aponinum OUC1 (a) and Scenedesmus obliquus (b) cultivated in ELR with different dilution ratios and biomass concentrations (c) of two species of microalgae at the end of cultivation. Data are shown as mean ± standard deviation (SD) (n=3). Different letters above the bars represent significant differences (P<0.05).

Fig.3 Removal of NH4+-N, NO3−-N, NO2−-N and Pi in ETD and ELR at the end of algal cultivation. The blank bar means that no nutrients have been removed. Ca, Cyanobacterium aponinum OUC1; So, Scenedesmus obliquus. Data are shown as mean ± standard deviation (SD) (n=3).
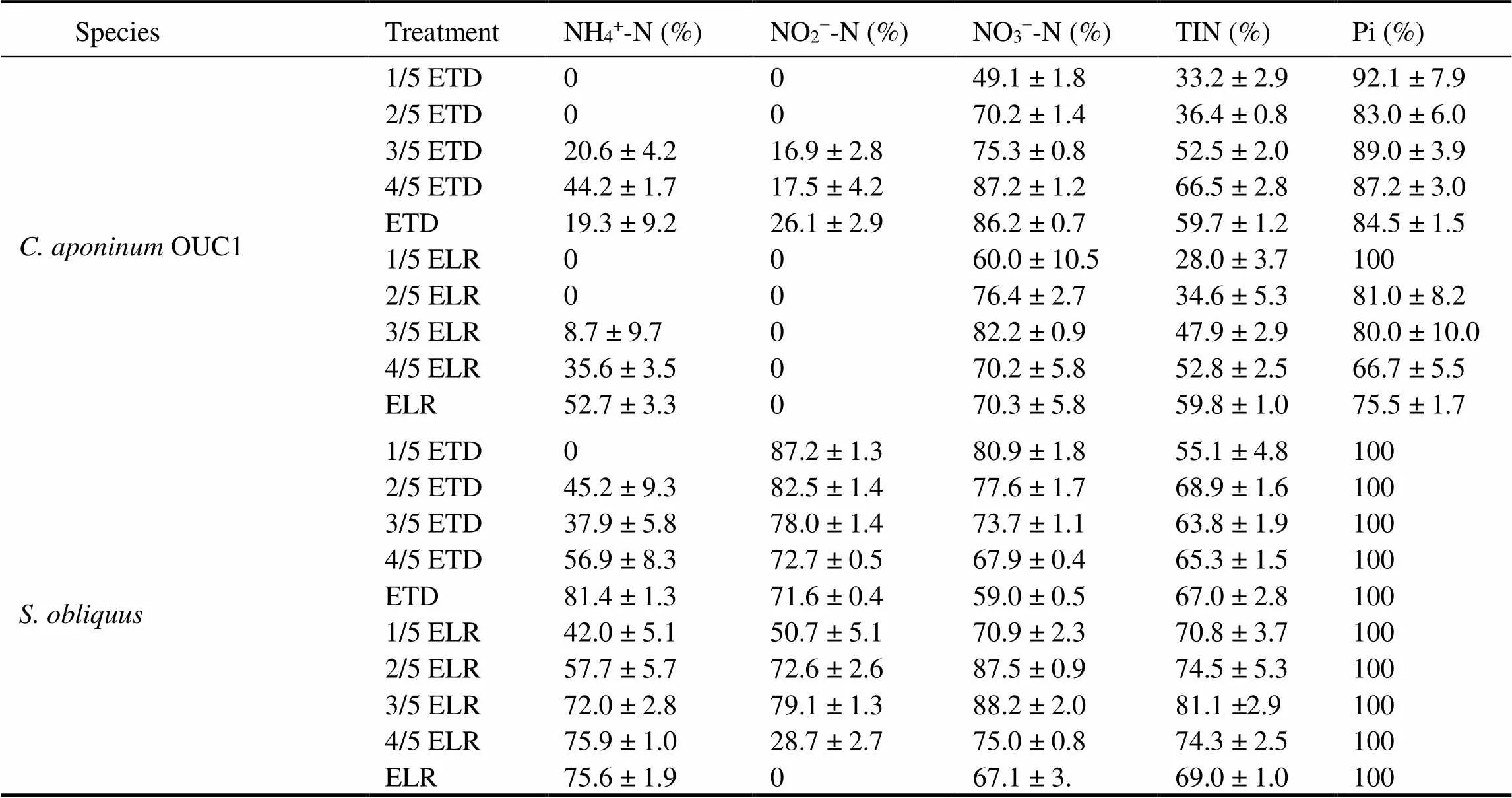
Table 2 Removal rates of nutrients in ETD and ELR by Cyanobacterium aponinum OUC1 and Scenedesmus obliquus
Notes: TIN, total inorganic nitrogen. Data are shown as mean ± standard deviation (SD) (= 3).
Phosphorus plays critical role in the conveyance of metabolic energy and as an essential macro-nutrient of nucleic acids, lipids, proteins, and phospholipid molecules in microalgae cells (Yao., 2015). Only the inorganic form of phosphorus can be utilised by microalgae (Markou and Georgakakis, 2011). In this study, both microalgae can eliminate Pi significant amount of in different concentrations of ETD and ELR.can remove Pi completely, while the Pi removal rates ofOUC1 in ETD and ELR were 84.5%-92.1% and 66.7%-81.0%, respectively (Fig.3). Several previous studies reported similar Pi removal efficiencies (exceeding 90%) by microalgae in municipal wastewater (Arbib., 2014; Tuantet., 2014). The optimum N/P ratio for the growth of microalgae was found to range from 6.8 to 10 (Martin., 1985; Wang., 2010). In the present study, ETD and ELR had an N/P ratio of 36.4 and 29.5, respectively, demonstrating an extreme phosphorus limitation to microalgae growth and subsequent nitrogen assimilation. This means that, when the Pi in an effluent is exhausted, inorganic nitrogen inevitably remains therein. The higher biomass and the greater assimilation of N and P obtained in a short period of time (4-6d) indicated the appropriateness ofOUC1 andfor ETD and ELR treatment, providing an effective method for removing nutrients from secondary effluent.
3.3 Correlation Analysis Between Nutrients and Biomass
According to Table 3, there was a significant and positive correlation (<0.05) between the initial concentrations () of nutrients and algal biomass () whenOUC1 was cultivated in ETD and ELR, while for, the significant correlation betweenandwas only found in ETD (<0.01). Similarly, the removal amounts () of nutrients, except for NO2−-N, were also positively correlated with the biomass of each alga (<0.05 or<0.01) (Table 4). This indicated that microalgal growth was highly dependent on the utilisation of nutrients from effluents. Forcultivated in ELR, the poor or even insignificant, correlations betweenorof nutrients and algal biomass, may be related to pollution stresses acting in this effluent. According to our investigation, most of the wastewater treated in Licun River WWTP comes from industrial activities, indicating that ELR may contain chemical compounds with higher concentrations than ETD which mainly receives domestic sewage. It has been reported cyanobacteria in general have higher tolerance to abiotic stresses including chemical pollutants (Ricci, 2017; Tiwari, 2018), conductive to uninhibited absorption of nutrients; however, other microalgae such asmay not grow well due to their sensitivity to pollutants in the effluent. Interestingly, both algae, cultivated in ELR, presented a negative correlation between the growth and NO2−-N removal (Table 4) because of the low rate of removal of NO2−-N. In fact, most ELR treatments with both algal species resulted in an increase of nitrite levels in the final effluent after treatment (Fig.3), which was attributed to nitrification of ammonia. Such increases were significantly higher withOUC1 and may be a consequence of the significant volume of oxygen released due to more rapid growth of this species thus oxidising ammonia to nitrite (Gupta., 2016). Other researchers have also observed this anomaly in NO2−-N (Lorenzen., 1998; Gupta., 2016). As for Pi, it is generally accepted that phosphorus can be eliminated from effluent in which microalgae grow through two ways: absorption into algal cells and formation of struvite precipitation by reaction with ammonium and magnesium ions (Cai., 2013; Ge and Champagne, 2016), however, no precipitate was observed in the algal cultures in the present study, which was consistent with the results of Diniz(2017). This was probably because of the low initial phosphate concentrations in effluents and the rapid algal uptake, therefore, it is speculated that Pi was mainly removed from effluent by microalgal assimilation, which was supported by the significant and positive correlations between theof Pi and algal biomass in all cases (Table 4).

Table 3 Correlations between the initial concentrations of nutrients (C) and microalgal biomass concentrations (ρ)
Notes:,OUC1;,;**<0.01,*<0.05.

Table 4 Correlations between the removal amounts of nutrients (C) and microalgal biomass (ρ)
Notes:,OUC1;,;**<0.01,*<0.05.
3.4 Lipid Content and Lipid Productivity
Ordinarily, microalgae accumulate lipids under nutrient limitations when light and CO2are available (Courchesne., 2009). In the present study, the lipid content ingenerally decreased with increasing ETD concentration, and the maximum lipid content was observed in 1/5 ETD, which was 28% higher than those in the control (Table 5).also reached its maximum lipid content in the 1/5 ELR group and was increased by 49% compared with the control. Other researchers have observed the highest lipid accumulation (28.36±2.02%) bycultivated in wastewater at a dilution ratio of 25% (Gupta., 2016). This is likely to have occurred because Pi was rapidly consumed by microalgae at lower concentrations of effluent, resulting in phosphorus limita- tion. Some studies have reported that lipid accumulation could be triggered under environmental stresses from, for example, nitrate or phosphate depletion (Courchesne., 2009; Zhu., 2014): however, the lipid contents inOUC1 did not show significant differences with the increase of effluent concentration in ELR groups and ETD groups (Table 6). All of them were lower than those in control, and the highest values of 13.7% and 11.2% were found, respectively, in the 4/5 ETD group and 4/5 ELR group. This was likely to have been related to the rapid growth of this species in ELR and ETD compared to that in control (Figs.1(a) and 2(a)). It has been reported that microalgae used starch instead of lipids as a primary carbon and energy storage under favourable growth conditions (Li., 2011).
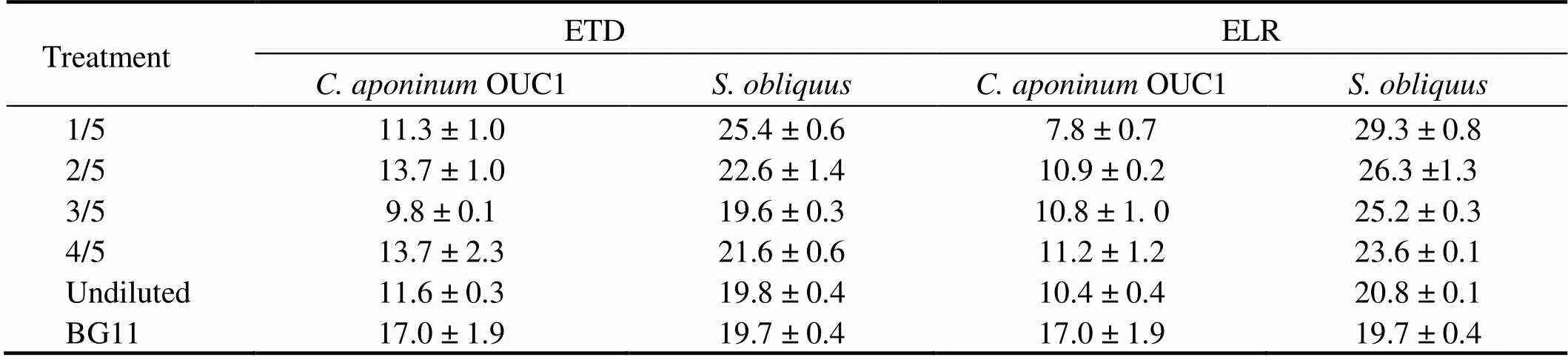
Table 5 Lipid content (%) in microalgae cultivated in different concentrations of ETD and ELR

Fig.4 Transmission electron micrographs of Scenedesmus obliquus(a–c)andCyanobacterium aponinum OUC1(d–f) cultivated in BG11 medium (control), undiluted ETD and undiluted ELR. Cw, cell wall; CHL, chloroplast; PP, polyphosphate; PY, pyrenoid; SS, starch sheath; S, starch granule; L, lipoidal globule; Gg, glycogen granule.

Table 6 Lipid productivity of two species of microalgae cultivated in undiluted effluents
The TEM images in Fig.4 are consistent with the aforementioned results. When exposed to ETD and ELR, larger, or more, lipoidal globules appeared incells compared with those in control (Figs.4a–c), however, formation of glycogen granules was stimulated and they occupied most of the intracellular space inOUC1 cells (Figs.4d–f), implying the generation of a little lipid. In cyanobacteria, glycogen, instead of starch, is the main carbon and energy storage polysaccharide. Its synthesis is controlled by the enzyme ADP-glucose pyrophosphorylase (AGPase; EC 2.7.7.27) (Quintana., 2011). This enzyme is inhibited by inorganic phosphorus. When the inorganic phosphorus content is low, the activity of AGPase is elevated and more carbohydrates are synthesised (Markou., 2012). In the present study, the inhibition of AGPase activity may be relieved by the onset of phosphorus limitation later in the cultivation period, leading to the massive synthesis of glycogen. On the contrary, there is no such mechanism in eukaryotes such as, and the organism will shift the fixed carbon into lipid as a secondary storage product under phos- phorus-limited conditions.
4 Conclusions
Disposal of wastewater frequently gives rise to high nutrient loading in aquatic environments, which may result in advantageous conditions for unwanted algae blooms. This research demonstrated that undiluted ETD significantly increased the cell densities, biomass concentrations, and lipid contents ofOUC1 and. Meanwhile, there was a significant reduction in the level of nitrogen and phosphorus in ETD and ELR afterOUC1 andutilisation. Following ETD and ELR cultivation, the microalgal cells exhibited ultrastructural modifications that were correlated with phosphorus limitation in effluent. The results of this research showed the potential of microalgae- wastewater coupling technology for techno-economical wastewater deep purification and microalgal cultivation, and further laid a foundation for sustainable microalgal-based biofuel production.
Acknowledgement
This work was supported by the National Marine Hazard Mitigation Service, Ministry of Natural Resources of the People’s Republic of China through its Commissioned Research Scheme (No. 2019005AC).
AlMomani, F. A., and Örmeci, B., 2016. Performance of, neochloris oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate., 95: 280-289, DOI: 10.1016/j.ecoleng.2016.06.038.
Arbib, Z., Ruiz, J., Álvarez-Díaz, P., Garrido-Pérez, C., and Perales, J. A., 2014. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2bio-fixation and low cost biofuels production., 49: 465-474, DOI: 10.1016/j.watres.2013.10. 036.
Arias, D. M., Rueda, E., Garcia-Galan, M. J., Uggetti, E., and Garcia, J., 2019. Selection of cyanobacteria over green algae in a photo-sequencing batch bioreactor fed with wastewater., 653: 485-495, DOI: 10. 1016/j.scitotenv.2018.10.342.
Bligh, E. G., and Dyer, W. J., 1959. A rapid method of total lipid extraction and purification., 37 (8): 911-917, DOI: 10.1139/o59-099.
Cai, T., Park, S. Y., and Li, Y., 2013. Nutrient recovery from wastewater streams by microalgae: Status and prospects., 19: 360-369, DOI: 10.1016/j.rser.2012.11.030.
Chen, G. Y., Zhao, L., and Qi, Y., 2015. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review., 137: 282-291, DOI: 10.1016/j.apenergy.2014.10.032.
Cheng, Q. L., Xu, L. G., Cheng, F. M., Pan, G., and Zhou, Q. F., 2018. Bicarbonate-rich wastewater as a carbon fertilizer for culture ofsp. of a giant pyrenoid., 202: 439-443, DOI: 10.1016/j.jclepro. 2018.08.066.
Courchesne, N. M. D., Parisien, A., Wang, B., and Lan, C. Q., 2009. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches., 141 (1): 31-41, DOI: 10.1016/j. jbiotec.2009.02.018.
Deegan, L. A., Johnson, D. S., Warren, R. S., Peterson, B. J., Fleeger, J. W., Fagherazzi, S., and Wollheim, W. M., 2012. Coastal eutrophication as a driver of salt marsh loss., 490 (7420): 388-392, DOI: 10.1038/nature11533.
Diniz, G. S., Silva, A. F., Araujo, O. Q. F., and Chaloub, R. M., 2017. The potential of microalgal biomass production for biotechnological purposes using wastewater resources., 29: 821-832, DOI: 10.1007/ s10811-016-0976-3.
Fujimoto, N., Sudo, R., Sugiura, N., and Inamori, Y., 1997. Nutrient-limited growth of microcystis aeruginosa and phormidium tenue and competition under various N:P supply ratios and temperatures., 42 (2): 250- 256, DOI: 10.4319/lo.1997.42.2.0250.
Ge, S. J., and Champagne, P., 2016. Nutrient removal, microalgal biomass growth, harvesting and lipid yield in response to centrate wastewater loadings., 88: 604-612, DOI: 10.1016/j.watres.2015.10.054.
Gojkovic, Z., Lindberg, R. H., Tysklind, M., and Funk, C., 2019. Northern green algae have the capacity to remove active pharmaceutical ingredients., 170: 644-656, DOI: 10.1016/j.ecoenv.2018.12. 032.
Gupta, S. K., Ansari, F. A., Shriwastav, A., Sahoo, N. K., Rawat, I., and Bux, F., 2016. Dual role ofandfor comprehensive wastewater treatment and biomass production for bio-fuels., 115: 255-264, DOI: 10.1016/j.jclepro. 2017.01.144.
Ho, S. H., Chen, C. Y., and Chang, J. S., 2012. Effect of light intensity and nitrogen starvation on CO2fixation and lipid/carbohydrate production of an indigenous microalgaCNW-N., 113: 244-252, DOI: 10.1016/j.biortech.2011.11.133.
Kulaev, I. S., and Vagabov, V. M., 1983. Polyphosphate metabolism in micro-organisms., 24: 83-171, DOI: 10.1016/S0065-2911(08)60385-9.
Li, X., Hu, H., and Yang, J., 2010. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga,sp. LX1, growing in secondary effluent., 27 (1): 59-63, DOI: 10.1016/j.nbt.2009. 11.006.
Li, Y., Han, D., Sommerfeld, M., and Hu, Q., 2011. Photosynthetic carbon partitioning and lipid production in the oleaginous microalgasp. (Chlorophyceae) under nitrogen-limited conditions., 102 (1): 123-129, DOI: 10.1016/j.biortech.2010.06.036.
Lomas, M. W., and Gilbert, P. M., 2000. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates., 36 (5): 903-913, DOI: 10.1046/ j.1529-8817.2000.99029.x.
Lorenzen, J., Larsen, L. H., Kjær, T., and Revsbech, N. P., 1998. Biosensor determination of the microscale distribution of nitrate, nitrate assimilation, nitrification, and denitrification in a diatom-inhabited freshwater sediment., 64: 3264-3269, DOI: 10.1002/(SICI) 1097-0290(19980905)59:5<651::AID-BIT17>3.0.CO;2-C.
Marella, T. K., Datta, A., Patil, M. D., Dixit, S., and Tiwari, A., 2019. Biodiesel production through algal cultivation in urban wastewater using algal floway., 280: 222-228, DOI: 10.1016/j.biortech.2019.02.031.
Markou, G., and Georgakakis, D., 2011. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review., 88 (10): 3389-3401, DOI: 10.1016/j.apenergy.2010.12.042.
Markou, G., Chatzipavlidis, I., and Georgakakis, D., 2012. Carbohydrates production and bio-flocculation characteristics in cultures of(): Improvements through phosphorus limitation process., 5 (4): 915-925, DOI: 10.1007/s12155-012-9205-3.
Martin, C., Noüe, J. D. L., and Picard, G., 1985. Intensive cultivation of freshwater microalgae on aerated pig manure., 7 (4): 245-259, DOI: 10.1016/0144-4565(85)90064-2.
Meng, F. P., Cui, H. W., Wang, Y. J., and Li, X. L., 2018. Responses of a new isolatedstrain to temperature, pH, CO2and light quality., 30 (3): 1525-1532, DOI: 10.1007/s10811-018- 1411-1418.
Ministry of Environmental Protection of China (MEP), 1987. Water quality-determination of nitrogen (nitrite)-spectro- photometric method (GB 7493-87), China Environmental Science Press, Beijing, China.
Ministry of Environmental Protection of China (MEP), 1989. Water quality-determination of total phosphorus-ammonium molybdate spectrophotometric method (GB 11893-89), China Environmental Science Press, Beijing, China.
Ministry of Environmental Protection of China (MEP), 2007. Water quality-determination of nitrate-nitrogen-ultraviolet spectrophotometry (HJ/T 346-2007), China Environmental Science Press, Beijing, China.
Ministry of Environmental Protection of China (MEP), 2009. Water quality-determination of ammonia nitrogen-nessler's reagent spectrophotometry (HJ 535-2009), China Environmental Science Press, Beijing, China.
Mirzaei, R., Mesdaghinia, A., Hoseini, S. S., and Yunesian, M., 2019. Antibiotics in urban wastewater and rivers of Tehran, Iran: Consumption, mass load, occurrence, and ecological risk., 221: 55-66, DOI: 10.1016/j.chemosphere. 2018.12.187.
Quintana, N., Kooy, F. V. D., Rhee, M. D., Voshol, G. P., and Verpoorte, R., 2011. Renewable energy from Cyanobacteria: Energy production optimization by metabolic pathway engineering., 91 (3): 471-490, DOI: 10.1007/s00253-011-3394-0.
Reynolds, E. S., 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy., 17 (1): 208, DOI: 10.1083/jcb.17.1.208.
Ricci, J. N., Morton, R., Kulkarni, G., Summers, M. L., and Newman, D. K., 2017. Hopanoids play a role in stress tolerance and nutrient storage in the cyanobacterium., 15: 173-183, DOI: 10.1111/gbi.12204.
Sakthivel, R., Ramesh, K., Purnachandran, R., and Mohamed, S. P., 2018. A review on the properties, performance and emission aspects of the third generation biodiesels., 82: 2970-2992, DOI: 10.1016/j. rser.2017.10.037.
Stanier, R. Y., Kuniawa, R., Mandel, M., and Cohen-Bazire, G., 1971. Purification and properties of unicellular blue-green algae (order Chroococcales)., 35 (2): 171-205.
Tang, D. Y. Y., Khoo, K. S., Chew, K. W., Tao, Y., Ho, S. H., and Show, P. L., 2020. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products., 304: 122997, DOI: 10.1016/j.bior- tech.2020.122997.
Tillberg, J. E., and Rowley, J. R., 1989. Physiological and structural effects of phosphorus starvation on the unicellular green alga., 75 (3): 315- 324, DOI: 10.1111/j.1399-3054.1989.tb04633.x.
Tiwari, B., Verma, E., Chakraborty, S., Srivastava, A. K., and Mishra, A. K., 2018. Tolerance strategies in cyanobacteriumsp. under pesticide stress and possible role of a carbohydrate-binding protein in the metabolism of methyl parathion (MP)., 127: 217-226, DOI: 10.1016/j.ibiod.2017.11.025.
Tripathi, R., Gupta, A., and Thakur, I. S., 2019. An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae,sp. Istga1., 135: 617-625, DOI: 10.1016/j. renene.2018.12.056.
Tuantet, K., Temmink, H., Zeeman, G., Janssen, M., Wijffels, R. H., and Buisman, C. J. N., 2014. Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor., 55: 162-174, DOI: 10.1016/j. watres.2014.02.027.
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., Wang, Y., and Ruan, R., 2010. Cultivation of green algaesp. in different wastewaters from municipal wastewater treatment plant., 162 (4): 1174-1186, DOI: 10.1007/s12010-009-8866-7.
Yao, L. L., Shi, J. Y., and Miao, X. L., 2015. Mixed wastewater coupled with CO2for microalgae culturing and nutrient removal., 10 (9): 16, DOI: 10.1371/journal.pone.013 9117.
Yu, C., Huang, X., Chen, H., Godfray, H. C. J., Wright, J. S., Hall, J. W., Gong, P., Ni, S., Qiao, S., Huang, G., Xiao, Y., Zhang, J., Feng, Z., Ju, X., Ciais, P., Stenseth, N. C., Hessen, D. O., Sun, Z., Yu, L., Cai, W., Fu, H., Huang, X., Zhang, C., Liu, H., and Taylor, J., 2019. Managing nitrogen to restore water quality in China., 567: 516-520, DOI: 10.1038/ s41586-019-1001-1.
Zhu, L., Hiltunen, E., Shu, Q., Zhou, W., Li, Z., and Wang, Z., 2014. Biodiesel production from algae cultivated in winter with artificial wastewater through pH regulation by acetic acid., 128: 103-110, DOI: 10.1016/j.apenergy. 2014.04.039.
. E-mail:mengfanping@ouc.edu.cn
February 11, 2020;
July 5, 2020;
September 30, 2020
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Numerical Simulation and Risk Analysis of Coastal Inundation in Land Reclamation Areas: A Case Study of the Pearl River Estuary
- Variation of Yellow River Runoff and Its Influence on Salinity in Laizhou Bay
- Cold Water in the Lee of the Batanes Islands in the Luzon Strait
- Preliminary Design of a Submerged Support Structure for Floating Wind Turbines
- Inversion of Oceanic Parameters Represented by CTD Utilizing Seismic Multi-Attributes Based on Convolutional Neural Network
- Trace-Norm Regularized Multi-Task Learning for Sea State Bias Estimation
