一个单核三价铬配合物的晶体结构、发光性质及热稳定性
2020-07-02刘荫秋
刘荫秋
(1. 井冈山大学化学化工学院,江西,吉安 343009;2. 江西省配位化学重点实验室,江西,吉安 343009)
1 INTRODUCTION
Metal-organic complex because of their structural diversity are potentially applied as material relating to gas storage, ion exchange, catalysis, luminescence, electric conductivity and so on[1-3]. We are interested in synthesizing compounds with perfect luminescent properties[4-6], due to their promising prospects to perform as medical probes in some disease checking and luminescent material in preparing high quality displays of new generation and so on[7-8]. The title Chromium(III) complex was first reported in 1951[9], but all carbon atoms in the structure were not resolved directly from the optic data at that time and their position parameters reported are probably not reliable, so it was synthesized again and by the help of today’s more advanced X-ray diffractometer and analysis methods we have found all atoms’ precise positions, and other crystal structure parameters, some of which have obvious deviation from what past reported, and at the same time we have carried out additional investigation on its’ thermal stability as well as its’ luminescent properties which were not reported in the past, herein we report our work here.
2 EXPERIMENTAL
2.1 Materials and methods
All chemicals used were analytical reagent and used without additional purification. Luminescent data were recorded on LS-55 Luminescence Spectrometer, X-ray diffraction tests were performed on a SuperNova area-detector diffractometer and thermal analysis test was carried on a TG209F3 thermogravimetric analyzer.
2.1 Synthesis of the title compounds
Potassium dichromate (1.0 g) and oxalic acid (5.0 g) were dissolved in 5 mL boiling water respectively, then the two solution were mixed together slowly while they were hot, after great amount of CO2were given off the residual solution was set in the air untouched, several days later single crystal of the title complex was obtained.
2.2 X-ray Structure determination
X-ray diffraction test were carried out at room temperature, intensity data were collected under a MoKα radiation (λ = 0.71073 Å). A total of 4113 reflections were collected, of which the unique reflections were 1434 [R(int) = 0.029]. Crystal structure were solved by direct methods using SHELXS-97 program and refined on F2with XSHELL6.2. All Hydrogen atoms of water molecules in the structure were found in electron density map and refined with O-H bond length fixed to 0.90 Å and with Uiso(H) = 1.2Ueq(O).
3 RESULTS AND DISCUSSION
Structure analysis basing on X-ray diffraction data shows the title complex crystallized in monoclinic, space group P2/n with the cell parameters being a = 7.8827 (4) Å, b = 5.7296(3) Å, c = 13.6447(7) Å, ß = 103.506(5)° (comparing with past reported a = 7.85 Å, b = 5.72 Å, c = 13.88 Å, ß= 109.30°, obvious deviation existing) (see Table 1), in every space unit there are two formula units: K[Cr(C204)2. (H2O)2]2.3H2O and each formula unit is consisted of one potassium ion, three free water molecules, a coordination anion composed of a Chromium(III) ion, the coordination center, two coordination water molecules,two oxalate ions which is bidentate ligand(see Fig.1). The Chromium(III) ions are six coordinated and adapts a approximate octahedral coordination configuration with the four carboxyl Oxygen atoms from two oxalate ions locating at the centre plane(coordination bonds length being1.9697(12) Å and 1.9572(12) Å respectively, comparing with past reported 1.93 Å and 1.92 Å for the same bonds length) and the other two Oxygen atoms from coordination water molecules residing at the two apexes(coordination bonds length being 2.0012(13) Å, the same bonds length past reported being 2.02 Å).
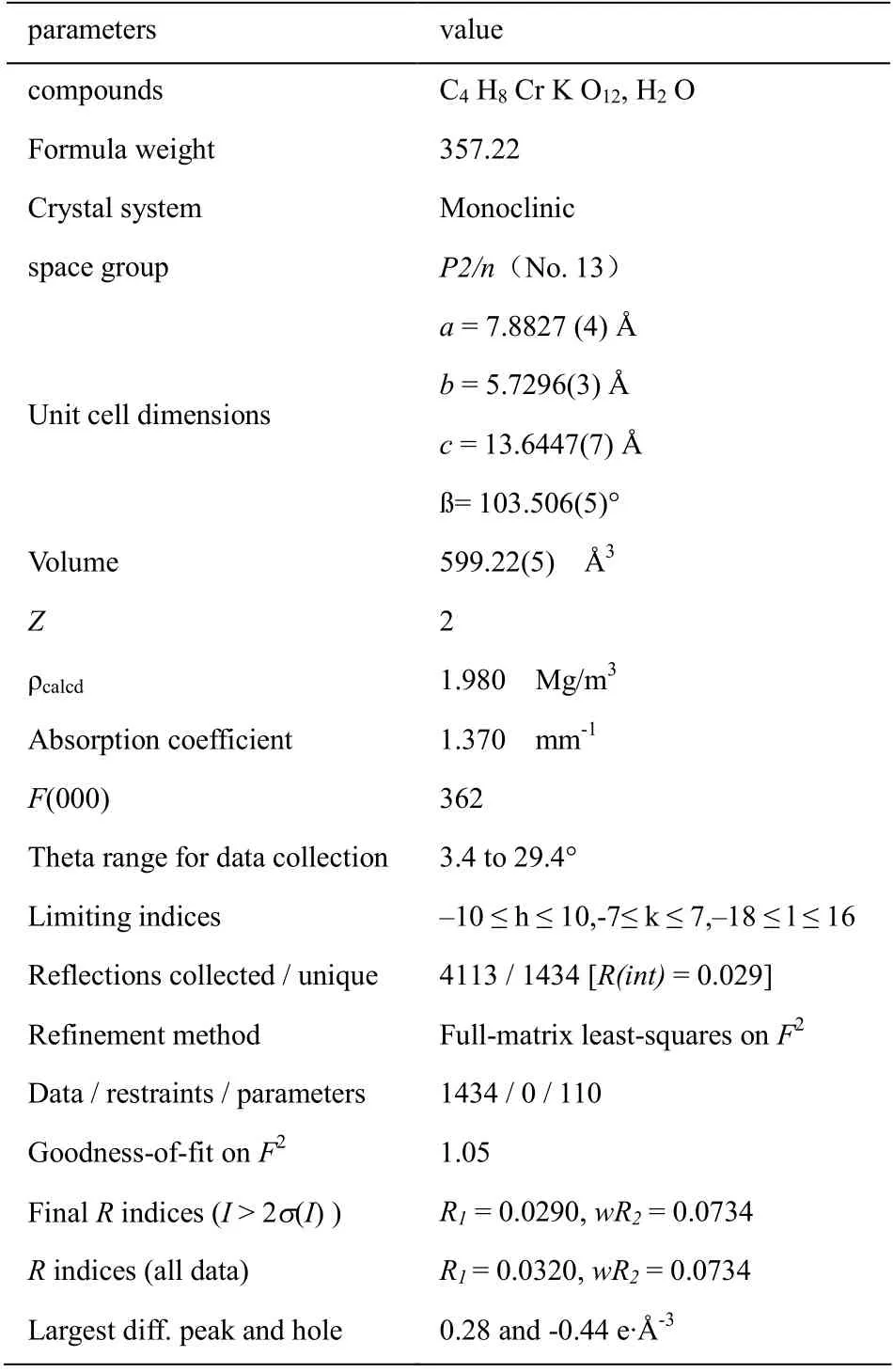
Table 1 Crystallographic data and experimental details for the title compound
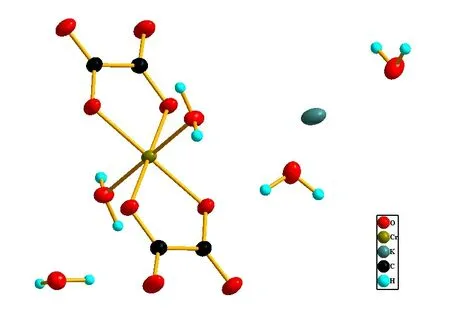
Fig.1 Coordination environment of Chromium(III) ions in the title complex, showing 50% probability displacement ellipsoids
Thermogravimetric experiment reveals the title complex keeps its thermal stability till about 357 K (see Fig.2). Along with the temperature increasing, the first step appears at about 357 K, which can be attributed to the lost of its three free water molecules in every formula unit(test data 85% and calculated data 84.88%), the second step due to the lost of the two coordination water molecules appears at about 486 K(test data 76% and calculated data 74.81%), the third step appears at about 573 K with the complex decomposing completely to leave its residual materials of Cr2O3.KO2(test data 42% and calculated data 42.30%).

Fig. 2 Spectrums of thermogravimetric test showing the thermal stability of the title complex
Luminescence test was performed at room temperature in solid state, Exposing to its the most effective ultraviolet radiations of 410 nm, the title complex shows its sharp emissions at about 610 nm(see Fig.3).
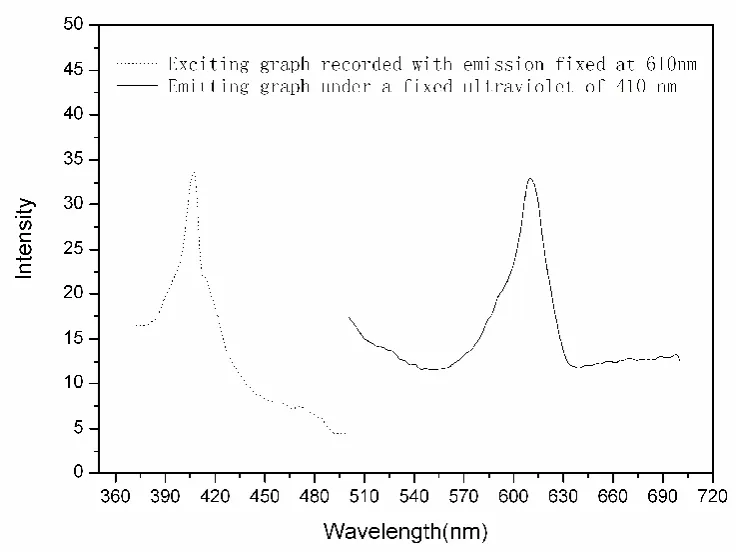
Fig.3 Spectrums showing the fluorescent study of the title complex
