A Sb-bridging tetra-TmIII encapsulated tetrameric antimonotungstate and its luminescence properties
2020-06-19XIEYingsongLIUYaxuanXUJiapengXUXinCHENLijuan
XIE Yingsong, LIU Yaxuan, XU Jiapeng, XU Xin, CHEN Lijuan*
(1. College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China; 2. Anhui China Tobacco Industry Co. LTD, Hefei 230031, Anhui, China)
Abstract: A Sb-bridging tetra-TmIII encapsulated tetrameric antimonotungstate [H2N(CH3)2]8Na6 H8{[Tm4(H2O)6Sb6O4](B-α-SbW10O37)2(B-α-SbW8O31)2}·24H2O (1) was triumphantly isolated by reaction of Na9[B-α-SbW9O33]·19.5H2O, SbCl3, Tm(NO3)3·6H2O and dimethylamine hydrochloride in acidic aqueous solution. Its tetrameric polyoxoanion consists of two divacant [B-α-SbW10O37]11- segments, two tetravacant [B-α-SbW8O31]11- segments and two [Tm2(H2O)3Sb3O2]11+ clusters. The luminescence property of 1 demonstrates that the characteristic emission peak at 450 nm can be assigned to the1D2→3F4 transition of the Tm3+ ion under excitation of the UV light of 353 nm. Its relevant luminescence performance parameters such as the chromaticity coordinate, the color purity, the dominant wavelength and the correlated color temperature are (0.184 3, 0.135 0), 71.69%, 470 nm and 4 471 K, respectively. This observation indicates that 1 may be a blue emission emitter for the application of white LEDs.
Keywords: polyoxometalate; antimonotungstate; photoluminescence
In the past two decades, heteropolyoxotungstates (HPOTs) have attracted considerable attention because of their complex ingredients, beautiful structures and interesting applications in catalysis[1], magnetism[2], electrochemistry[3], biomedicine[4], optics and so forth[5]. Furthermore, lacunary HPOTs can exhibit various sizes, shapes, bond modes, and flexible coordination sites[6-7], which can usually function as superior building blocks to construct novel transition-metal (TM)/lanthanide (Ln)/main-group (MG)-containing HPOT aggregates[8]. Relative to TM ions, Ln ions not only have high coordination numbers and strong oxophilicity but also display excellent luminescence performances[9]. As a result, synthesis and discovery of novel Ln-containing HPOTs based on the building block strategy have been a remarkable research hotspot. For instance, NIU’s group reported a 2D graphite-like framework constructed from high-nuclear Ce10cluster with the investigation of magnetic property[10]. ZHAO et al. communicated an unprecedented 3D porous framework derived from aggregation of giant cerium-bismuth tungstate clusters and investigated its high proton conductivity[11]. SU and coworkers prepared a serious of nanoscale Ln-containing selenotungstates by reaction of the {Se6W39} precursor and Ln ions under acidic aqueous conditions[12].
In the family of Ln-containing HPOTs, the research on Ln-containing antimonotungstates (Ln-AMTs) has developed as a significant subclass recently. It should be noted that the main-group SbIIIcations with the lone-pair electrons and empty 5s and 5p orbitals can also work as effective metal linkers. In the paper, we utilized the building block strategy to synthesize a TmIII-SbIII-substituted tetrameric AMT [H2N(CH3)2]8Na6H8{[Tm4(H2O)6Sb6O4](B-α-SbW10O37)2(B-α-SbW8O31)2}·24H2O (1) (CCDC 1984008) by introducing extra SbCl3in the reaction process. It should be pointed out that the polyoxoanion of1is constructed from two divacant Keggin [B-α-SbW10O37]11-fragments and two tetravacant Keggin [B-α-SbW8O31]11-fragments connected by two [Tm2(H2O)3Sb3O2]11+clusters. To the best of our knowledge, such phenomenon that an AMT includes two types of different lacunary Keggin HPOT fragments and one type of heterometallic clusters is extremely rare. Moreover, the luminescence properties of1were also investigated, illustrating that1may be a blue emission emitter, which has a certain potential for the application of white light-emitting diodes (W-LEDs).
1 Experimental
1.1 Physical measurements
Na9[B-α-SbW9O33]·19.5H2O was prepared according to the synthetic method recorded by the literature[13]. All other reagents were purchased directly without further purification. The IR spectrum was collected on Perkin-Elmer FT-IR spectrometer in the range of 400-4 000 cm-1. The C, H and N elements were measured on a Vario EL Cube CHNS analyzer. TG analysis was measured under the N2atmosphere with a heating rate of 10 ℃/min from 25 to 1 000 ℃. Luminescence emission and excitation spectra were measured on a FLS 980 Edinburgh Analytical Instrument under a 450 W xenon lamp. Decay curves were obtained under the excitation of a μF900H high-energy microsecond flash lamp.
1.2 Preparation of the SbCl3 solution and 1
Preparation of the SbCl3solution: The solid-state SbCl3(4.566 g,0.020 mol) was absolutely dissolved in hydrochloric acid (HCl) solution (6 mol/L, 20 mL, 0.120 mol). The concentration of Sb3+ions is 1.0 mol/L.
Preparation of1: Na9[SbW9O33]·19.5H2O (2.600 g, 0.908 mmol) and dimethylamine hydrochloride (1.000 g, 12.262 mmol) were dissolved in 15 mL water and then a solution of SbCl3(0.25 mL, 0.25 mmol) was added under stirring. The pH value of the resulting solution was adjusted to 5.60 by using HCl (6 mol/L). Then, Tm(NO3)3·6H2O (0.200 g, 0.432 mmol) was added and the pH value was adjusted to 5.60 again by using NaOH (6 mol/L). The mixture was stirred for another 30 min and then was kept in the 90℃ water bath for 1 h. After being taken out, the mixture was filtered and left for slow evaporation at room temperature. Yellowish needle crystals of1were obtained for about one week. Yield: 0.18 g (36.7%) (based on SbCl3). The SbCl3solution was prepared by dissolving solid-state SbCl3(4.564 g, 0.020 mol) in HCl solution (6 mol/L, 20 mL). Elemental analysis (%) calcd: C, 1.63; H, 1.13; N, 0.95. Found: C, 1.51; H, 1.25; N, 0.85.
1.3 X-ray crystallography
Single-crystal X-ray diffraction data of1were collected on a Bruker Apex II diffractometer equipped with CCD two-dimensional detector by using monochromated Mo Kαradiation (λ= 0.071 073 nm) at 296(2) K. Absorption corrections were applied using multi-scan technique and performed by using the SADABS program. The structure was solved by direct methods and refined onF2by full-matrix least squares methods using the HELXTL-97 program package[14-15]. The remaining atoms were found by Fourier syntheses refined by the continuous full-matrix least square methods onF2[16]. Hydrogen atoms attached to C and N atoms were geometrically placed and hydrogen atoms linking lattice water molecules were not located. Crystallographic data of1are shown in Table 1.

Table 1 Crystal data and structural refinements of 1
2 Results and discussion
2.1 Crystal structure
The X-ray single crystal diffraction indicates that1crystallizes in the monoclinic space groupP2(1)/n. The molecular unit of1includes a tetrameric {[Tm4(H2O)6Sb6O4](SbW10O37)2(B-α- SbW8O31)2}22-polyoxoanion, eight [H2N(CH3)2]+counter cations, six Na+ions, six H+protons and twenty four lattice water molecules. As shown in Fig. 1a, the tetrameric polyoxoanion of1consists of two [Tm2(H2O)3Sb3O2]11+clusters connecting two divacant Keggin [B-α-SbW10O37]11-segments and two tetravacant Keggin [B-α-SbW8O31]11-segments through 28 μ2-O atoms. The [B-α-SbW10O37]11-segment is derived from the rearrangement of a {WO6} octahedra on the [B-α-SbW9O33]9-segment whereas the [B-α-SbW8O31]11-segment stems from the removal of a {WO6} octahedra from the [B-α-SbW9O33]9-segment during the reaction. Two same [Tm2(H2O)3Sb3O2]11+clusters ({Tm2Sb3}) are centrosymmetric (Fig. 1b). Each {Tm2Sb3} cluster possesses one [Sb3O2]5+fragment, one [Tm1(H2O)2]3+ion and one [Tm2(H2O)]3+ion (Fig. 1c). Four Tm3+cations exhibit a parallelogram distribution and are situated on four vertexes of the parallelogram. The distances of Tm1…Tm2 and Tm1…Tm2A are 0.598 3-0.650 8 nm (Fig. 1b). In the {Tm2Sb3} cluster, two crystallographically independent Tm3+ions (Tm13+and Tm23+) reside in the octa-coordinate distorted square antiprismatic geometries. However, the coordinate environment of the Tm13+cation is somewhat different from that of the Tm23+ion. The square antiprism geometry of the Tm13+cation is defined by two μ2-O atoms from two {WO6} octahedra of the neighboring divacant Keggin [B-α-SbW10O37]11-segment [Tm1-O4: 0.229 8(12) nm, Tm1-O20: 0.240 0(12) nm], three μ2-O atoms from three {WO6} octahedra of two opposite tetravacant Keggin [B-α-SbW8O31]11-segments [Tm1-O31: 0.232 0(12) nm, Tm1-O58A: 0.228 1(15) nm, Tm1-O59: 0.2264(12) nm], one μ2-O atom connecting Sb43+with Sb53+ions [Tm1-O30: 0.245 9(13) nm] and two coordinate water molecules [Tm1-O1W: 0.240 6(13) nm, Tm1-O2W: 0.235 0(15) nm] (Fig. 1d). In contrast, the square antiprism geometry of the Tm23+cation is built by six μ2-O atoms from five {WO6} octahedra of two tetravacant Keggin [B-α-SbW8O31]11-segments [Tm2-O45A: 0.229 6(13) nm, Tm2-O60A: 0.263 3(13) nm, Tm2-O61A: 0.228 2(13) nm, Tm2-O68A: 0.225 9(14) nm, Tm2-O17: 0.228 5(12) nm, Tm2-O33: 0.238 1(13) nm], one μ2-O atom that bridges Sb33+and Sb43+ions [Tm2-O12: 0.244 2(13) nm] and one water ligand [Tm2-O3W: 0.238 4(15) nm] (Fig. 1e). Notably, each {Tm2Sb3} group is surrounded by two tetravacant Keggin [B-α-SbW8O31]11-segments and one divacant Keggin [B-α-SbW10O37]11-segment (Fig. 1f).

Fig.1 (a) Tetrameric polyoxoanion {[Tm4(H2O)6Sb6O4](SbW10O37)2(B-α-SbW8O31)2}22- in 1, (b) View of two {Tm2Sb3} groups, (c) View of a {Tm2Sb3} group, (d) The distorted square antiprismatic geometry of the Tm13+ cation, (e) The distorted square antiprismatic geometry of the Tm23+ cation, (f) Combination of two tetravacant Keggin [B-α-SbW8O31]11- segments, one divacant Keggin [B-α-SbW10O37]11- segment and a {Tm2Sb3} cluster. Symmetry code: A: -2-x, -y, -2-z. {WO6}: purple octahedra, O: rose red spheres, W: green spheres, Sb: blue spheres, Tm: bright yellow spheres
Furthermore, the 3 × 3 × 3 supramolecular 3-D stacking reveals that tetrameric polyoxoanions of1are tidily arranged in the -ABAB- pattern along theaandcaxis (Fig.2). Obviously, although neighboring tetrameric polyoxoanions are parallel to each other along thecdirection, they arrange oppositely in a staggered mode along thebdirection. This -ABAB- arrangement can effectively enhance the space utilization and adopt the closest packing of tetrameric polyoxoanions.
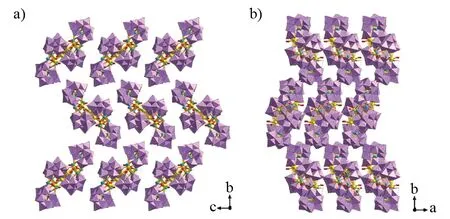
Fig.2 (a) 3 × 3 × 3 supramolecular 3-D stacking of 1 viewed along the a axis, (b) The 3×3×3 supramolecular 3-D stacking of 1 viewed along the c axis
2.2 IR spectrum
The IR spectrum of1was measured in the range of 4 000-400 cm-1(Fig. 3a). In the low wavenumber region, four main typical vibration peaks from lacunary AMT segments appear at 933, 884, 778 and 716 cm-1, which respectively originate from the asymmetrical stretching vibrations of terminal W-Ot, corner-sharing W-Ob, edge-sharing W-Ocand Sb-Oabonds[17]. In the middle wavenumber region, one sharp absorption band at 1 629 cm-1is attributed to the O-H bending vibration of lattice and coordination water molecules while the weak band at 1 464 cm-1originates from the bending vibration of the C-H bands of [H2N(CH3)2]+cations. In the high wavenumber region, it can be seen that the very strong and wide absorption band at 3 468 cm-1is assigned to the O-H stretching vibration of lattice and coordination water molecules while the weak absorption band at 3 162 cm-1and the weak absorption band at 2 804 cm-1respectively correspond to the stretching vibrations of N-H andC-H bonds from [H2N(CH3)2]+cations[18].

Fig.3 (a) IR spectrum of 1, (b) TG curve of 1
2.3 Thermogravimetric (TG) analysis
The TG curve was measured to verify the thermostability of1in the temperature range of 25-1 000 °C under a N2atmosphere with a heating rate of 10 ℃ min-1(Fig. 3b). The TG analysis suggests that1has undergone a two-step weight loss. The first weight loss of 4.85% (calcd. 4.57%) from 25 to 167 ℃ is ascribed to the liberation of twenty-four lattice water molecules and six coordination water molecules. Upon heating to 1 000 ℃, the second weight loss of 9.96% (calcd. 10.22%) is due to the removal of eight dimethylamine groups, the dehydration of six H+protons and the sublimation of four WO3groups.
2.4 Luminescence properties
The solid-state emission spectrum and lifetime decay behavior of1were measured at room temperature. Under irradiation of the UV lamp at 362 nm, the emission spectrum of1displays a strong emission peak at 453 nm (Fig. 4a), which is ascribed to the f-f1D2→3F4transition of the Tm3+ions[19]. The lifetime decay curve of1(Fig. 4b) can be fitted with a second-order exponential equationI=A1exp(-t/τ1)+A2exp(-t/τ2), whereτ1andτ2are the short and long components of luminescence lifetime,A1andA2are pre-exponential factors. The fitting results areτ1=33.53 μs,τ2=200.14 μs,A1=613.67 andA2=69.18.


Fig.4 (a) Solid-state emission spectrum of 1 (400-600 nm) upon λex=362 nm at room temperature, (b) Decay lifetime of 1 upon λex=362 nm, (c) CIE 1931 diagram of 1
3 Conclusion
In summary, we have successfully synthesized a Sb-bridging tetra-TmIIIencapsulated tetrameric AMT1simultaneously containing divacant Keggin and tetravacant Keggin HPOT units. Moreover, the luminescence properties of1were studied and relevant luminescence performance parameters were also provided. This finding can offer meaningful guidance for the further development of novel luminescent multi-Ln incorporated HPOTs. Our following work will go ahead with exploiting valuable luminescent HPOTs with multicolor-tuning optical behaviors.
