A novel aeration strategy in repeated-batch fermentation for efficient ethanol production from sweet sorghum juice☆
2019-10-17NiphaphatPhukoetphimNaulchanKhongsayPattanaLaopaiboonLakkanaLaopaiboon
Niphaphat Phukoetphim ,Naulchan Khongsay ,Pattana Laopaiboon,3 ,Lakkana Laopaiboon,3,*
1 Department of Biotechnology,Faculty of Technology,Khon Kaen University,Khon Kaen 40002,Thailand
2 Graduate School,Khon Kaen University,Khon Kaen 40002,Thailand
3 Fermentation Research Center for Value Added Agricultural Products,Khon Kaen University,Khon Kaen 40002,Thailand
ABSTRACT To improve the efficiency of ethanol production in a batch fermentation from sweet sorghum juice under a very high gravity(VHG)condition(~290 g/L of total sugar)by Saccharomyces cerevisiae NP01,repeatedbatch fermentation under an aerated condition(2.5 vvm for the first 4 h during every cycle)was done in a 5-L fermenter.The average ethanol concentration(P),productivity(Qp)and yield(Yp/s)for five successive cycles were 112.31 g/L,1.55 g/L·h-1 and 0.44,respectively with 80.97% sugar consumption.To complete sugar consumption,the total sugar of the juice was reduced to a high gravity (HG) level (~240 g/L).The results showed that yeast extract was not necessary for ethanol production,and aeration during every other cycle i.e.,alternating cycles,was sufficient to promote both yeast growth and ethanol production.The average P,Qp and Yp/s values for eight successive cycles with aeration during alternating cycles were 97.58 g/L,1.98 g/L·h and 0.41,respectively with 91.21% sugar consumption.The total fatty acids in the yeast cells under the aerated condition were~50% higher than without aeration,irrespective the initial sugar concentration,whereas the ergosterol contents under aeration condition were~29%to 49%higher than those without aeration.
Keywords:Aeration Nitrogen supplement Repeated-batch fermentation Saccharomyces cerevisiae Sweet sorghum juice
1.Introduction
Ethanol is an alternative to fossil fuels as a source of energy.There has been much research into the use of ethanol as a motor fuel because in some ways,its combustion in an internal combustion engine is simpler than for gasoline.There are several ways to produce ethanol.One of the most attractive starting materials is biomass,which is highly available.Ethanol production is done from sugarcane,molasses and cassava.There is competition for these raw materials because they are used in other industries [1].In the 15 year plan and target of the Thai Government for biofuel development (2008-2022),ethanol production is projected to be 6.2 and 9.0 ml·d-1by 2016 and 2022,respectively [2].Shortages of sugarcane,molasses and cassava may result in this time frame.
Sweet sorghum[Sorghum bicolor(L.)Moench]is a possible feedstock for bioethanol production.High levels of fermentable sugars,such as sucrose,fructose and glucose,are contained in its stalks.Additionally,it can be cultivated in many areas since it is adaptable to temperature and soil conditions[3].The sweet sorghum cultivar KKU40 yields 15-25×10-4t·m-2after 90-100 days of growth[4].
Industrially,normal gravity (NG) to high gravity (HG) fermentations,with 16%-24% (w/w) of dissolved solids after two mashes,are used to produce ethanol [5].Numerous process improvements have been used to improve the productivity and cost effectiveness of ethanol fermentation.These include very high gravity (VHG) technology.VHG fermentation technology involves fermentation of a medium until its carbon sources are exhausted.These media contain 250 g·L-1or more of dissolved solids [6-8].However,high levels of dissolved solids and sugars in a medium can inhibit both yeast growth and fermentation.Several studies reported that S.cerevisiae could produce and tolerate high ethanol concentrations under appropriate nutritional and environmental conditions including aeration [9,10].
Repeated-batch processes show increased productivity over conventional batch fermentations because there is no new inoculum requirement for each batch and long-term productivity can be maintained [11].There is no requirement for cleaning and resterilization,so time and energy are saved.Operation in this mode is easier than for of a continuous fermentation [12].
It is well established that the presence of high ethanol levels leads to a loss of cell viability during fermentation followed by disruption of cell membranes.This leads to the impairment of some transport proteins in the cell membrane and nutrient limitations[13,14].However,ethanol tolerance is related to the lipid and ergosterol contents of yeast cell membranes [15,16].
Our previous study found a high ethanol concentration (P,132.82 g·L-1) and productivity (Qp,2.55 g·L-1·h-1) in batch ethanol fermentation from sweet sorghum juice with an agitation rate of 200 r·min-1,an aeration rate of 2.5 vvm and an aeration time of 4 h[10].In the current study,a novel aeration strategy in a repeatedbatch fermentation process was developed for highly efficient ethanol production under HG and VHG conditions.Additionally,the differences in fatty acid content and ergosterol in S.cerevisiae NP01 cells during the repeated-batch ethanol fermentation were investigated.
2.Materials and Methods
2.1.Microorganism and inoculum preparation
Active S.cerevisiae NP01 (accession number KP866701) grown in yeast extract malt extract medium for 18 h was transferred into sweet sorghum juice containing 150 g·L-1of total sugar and incubated at 200 r·min-1,30°C for 15 h,and used as an inoculum for ethanol production.
2.2.Raw material and ethanol production medium
Sweet sorghum juice was extracted from its stalks (cv.KKU 40,Faculty of Agriculture,Khon Kaen University,Thailand).The juice containing 18°Bx of total soluble solids was concentrated to 75°Bx and then stored at 4°C to prevent bacterial growth.
Ethanol production (EP) media were made from concentrated juice that was diluted to contain total sugar contents of 240 (HG condition) and 290 g·L-1(VHG condition).The diluted juices were then added to prepare media with and without 9 g·L-1of yeast extract.pH adjustment was not done in either case.
2.3.Repeated-batch fermentation
Yeast was inoculated into sterile EP medium in a 5-L fermenter(Biostat®B,B.Braun Biotech,Germany) to obtain an initial yeast cell concentration of~2×107cells·mL-1.A rotameter was connected to the fermenter to measure the aeration rate.The air was flowed through the fermentation broth via a sterile air filter with a pore size of 0.2 μm.The fermentation was first done in batch mode under an aerated condition (2.5 vvm during the first 4 h of fermentation) [10]until the level of total sugars in the broth had been reduced to~5% to 10% of its initial value.Then,75% of the working volume of the fermentation broth was removed [12]and immediately replaced with an equal volume of fresh sterile EP medium to start the next fermentation cycle.The aeration rate was measured during each cycle,and samples were withdrawn at regular time intervals for analysis.
2.4.Analytical methods
2.4.1.Residual sugar,ethanol,suspended cell and biomass concentrations
The fermentation broth was centrifuged.Then the level of residual sugars in the supernatant was determined in terms of total carbohydrate using a phenol sulfuric acid method [17].The ethanol concentration in the supernatant was analyzed using gas chromatography.The chromatograph conditions were solid phase:PEG-20M,carrier gas:nitrogen,150°C isothermal packed column,injection temperature:180°C,and flame ionization detector temperature:250°C [9].
Total and viable cell counts were determined with an optical microscope using a hemacytometer.The yeast cell viability was determined using a methylene blue staining method[18].Biomass concentration was determined with a standard curve that correlated cell concentrations (cells·mL-1) and cell dry weights(g·mL-1).
2.4.2.Fatty acid content
Fatty acid content of the yeast cells was determined by Central Laboratory (Thailand) Co.,Ltd.,Khon Kaen,Thailand using a Hewlett-Packard 5890 gas chromatograph(Hewlett-Packard Company,Germany) with a flame ionization detector.Their methyl esters were prepared according to AOAC [19].A capillary column(SP2560;Sigma Aldrich Co.LLC,USA),100 m long×1.25 mm with 0.2 m film,was used for separation of methyl ester.Chromatography was performed with an initial oven temperature of 100°C,which was maintained for 4 min,then heated to a final temperature of 240°C at a gradient of 3°C·min-1.The injector and detector temperatures were 225 and 285°C,respectively.Helium was used as the carrier gas at a flow rate of 0.75 mL·min-1.The results were expressed as μg·g-1(dry cell weight).
2.4.3.Ergosterol content
The ergosterol of yeast cells was extracted and determined as total ergosterol following the procedure described by Inoue et al.[20].Yeast cells cultivated under optimal and controlled conditions were harvested,washed with distilled water and freeze-dried.The freeze dried cells were suspended in 5 mL of 99.97% ethanol followed by addition of 30 mL of 99.97%methanol and 2.0 g of potassium hydroxide.Saponification was done by incubation of the mixture at 75°C for 30 min.After addition of 10 mL of distilled water and 10 mL of petroleum ether,the suspension was vigorously mixed.The layer of petroleum ether was transferred into a round bottomed flask.The ergosterol extraction procedure by petroleum ether was repeated twice.After the layer of petroleum ether was evaporated to dryness,the precipitate was dissolved in 2 mL of a chloroform:methanol solution (1:1).Ergosterol content was determined using an HPLC equipped with a UV-SPD-10A detector at λ 205 nm (Shimadzu,Japan).The separation was performed using a Water spherisorbs ODS2 C18 column(256 mm×4.6 mm;particle size 5 μm) with a methanol:water solution (94:1,v/v) as the mobile phase at a flow rate of 1 mL·min-1.The results were expressed as μg·g-1(dry cell weight).
2.5.Ethanol production efficiency
The ethanol yield(Yp/s)was calculated in terms of g ethanol produced per g total sugar utilized (g·g-1),whereas the ethanol productivity (Qp,g·L-1·h-1) was calculated as the ethanol concentration produced(P,g·L-1)divided by the fermentation time of each batch [9].
3.Results and Discussion
3.1.Repeated-batch ethanol fermentation under VHG conditions
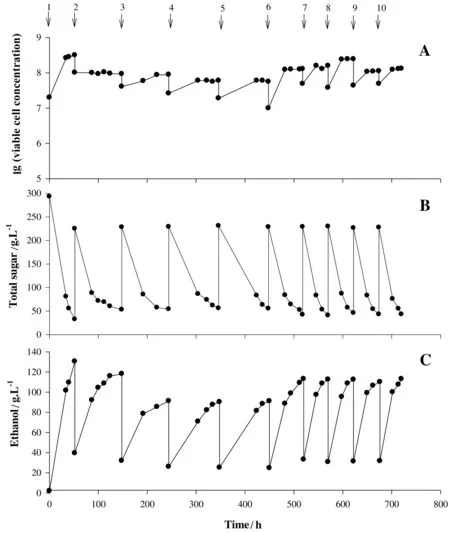
Fig.1.Repeated-batch ethanol fermentation from sweet sorghum juice at an initial sugar concentration of 290 g·L-1 by S.cerevisiae NP01.A:viable cell,B:total sugar and C:ethanol concentration.The arrows indicate the start of each batch.Batches 1 and 6-10 were performed with aeration at 2.5 vvm for 4 h,whereas batches 2-5 were performed with no aeration.

Table 1 Repeated-batch ethanol fermentation when the feeding medium was sweet sorghum juice containing 290 g·L-1 of total sugar and 9 g·L-1 of yeast extract
The time profiles of total sugar,ethanol and viable yeast cells during ten successive batches of ethanol fermentation from sweet sorghum juice supplemented with yeast extract are shown in Fig.1.In the first batch under an aeration rate at 2.5 vvm for the first 4 h,the cell viability increased during the first 12 h and remained constant thereafter.The sugar utilization,P,Qpand Yp/swere 88.91%,130.56 g·L-1,2.47 g·L-1·h-1and 0.50,respectively at 52 h(Table 1).The initial total sugar and viable cell concentrations in batches 2-10 were lower than those in batch 1,possibly due to the addition of fresh EP medium that was diluted by the remaining medium in the bioreactor(25%of total working volume).No aeration was supplied in batches 2 to 5 to investigate the effect of aeration in repeated-batch ethanol fermentation.The results showed that the viable cell concentrations in batches 2-5 were lower than in batch 1 (Fig.1A).This resulted in lower sugar consumption and ethanol production(Fig.1B and C).The sugar utilization,P,Qpand Yp/sdecreased to 75.28%,97.73 g·L-1,0.74 g·L-1·h-1and 0.41,respectively.The lower P values in batches 2-5 might have been due to the lower cell concentrations in the fermentation broth.This was supported by Chen et al.[21],who found that the initial cell concentration directly affected P and Qpin repeated-batch fermentations using S.cerevisiae.Thammasittirong et al.[22]reported that yeast cells with high viability are important to increase P values.The use of free cells in repeated-batch fermentation reduced yeast cell concentration,resulting in lower P in subsequent batches[12,23,24].Additionally,lower Yp/svalues in batches 2-5 were likely due to a higher glycerol content (the main by-product)production(Table 1).The final viable cell concentrations in batches 2-5 were relatively constant,ranging from 5.6×107to 9.6×107-cells·mL-1.Longer fermentation times(96 to 102 h)were observed in batches 2-5,which could have arisen from the lack of aeration during the fermentation.
To improve the efficiency of ethanol production,aeration(2.5 vvm for the first 4 h)was supplied during each cycle of batches 6-10.The average sugar utilization,P,Qpand Yp/svalues for five successive cycles increased to 80.97%,112.31 g·L-1,1.55 g·L-1·h-1and 0.44,respectively (Table 1).The initial sugar concentrations in the fermentation broths of batches 6-10 were approximately 230 g·L-1,resulting in lower final P values when compared to batch 1 (Fig.1B and C).Under this condition,the total sugars remaining in the fermented broths were 32.51 to 50.01 g·L-1.The initial viable yeast cell concentrations in batches 6-10 were higher than those in batches 2-5 (Fig.1A).The average sugar utilization,P,Qpand Yp/svalues with aeration (batches 6-10) were about 8,21,10 and 7%,respectively,higher than with no aeration (batches 2-5),and the fermentation time with aeration was 52 h shorter than without aeration.Deesuth et al.[16]reported that an aeration rate at 0.3 vvm for 12 h in a VHG fermentation(280 g·L-1of total sugar)gave P,Qpand Yp/svalues of 127.80 g·L-1,2.66 g·L-1·h-1and 0.49,respectively after 48 h.Arshad et al.[25]studied aeration(0.2 vvm)of a molasses medium in a VHG fermentation(sugar content of 27%),which yielded 12.2%(v/v) of ethanol using an S.cerevisiae mutant.The importance of aeration was also discussed by Brisha [26],who found that an aeration rate of 0.15 vvm for the first 12 h and agitation rate of 120 r·min-1led to an increase in the P value,up to 16% (v/v),from 350 g·L-1of sucrose.Optimum aeration for ethanol production is also yeast strain dependent.Jayus et al.[27]studied the ethanol production of two commercial strains (an alcohol yeast and a baker’s yeast) using sugarcane molasses (24°Bx) as a substrate.They found that an aeration rate of 0.3 vvm for 4 h and an agitation rate of 100 r·min-1did not affect the level of the P value of the alcohol yeast.However,the P value of the baker’s yeast under the same aeration condition increased by 17.5% (from 102.85 to 120.92 g·L-1),indicating the differences in oxygen sensitivity of the commercial yeast strains.
When comparing the ethanol production efficiency of a batch fermentation(batch 1)and repeated-batch fermentations(batches 6-10)in Table 1,the P,Qpand Yp/svalues of the repeated batch fermentations(batches 6-10)were lower than those of the batch fermentation (batch 1).However,inoculum preparation,requiring 33 h (primary seed in yeast extract malt extract medium for 18 h and secondary seed in sweet sorghum juice for 15 h),was not required and long-term productivity could be maintained in the repeated-batch fermentation.Additionally,there was no requirement for cleaning and re-sterilization,so time and energy were saved.Therefore,overall ethanol production efficiency of the repeated batch process was higher than that of the batch process.
The osmotic stress from high sugar concentrations in a fermented broth causes water outflow from yeast cells affecting in their growth[28].High osmotic conditions result in glycerol accumulation in the cytoplasm.This mitigates dehydration effects[29].This is pronounced under anaerobic conditions during ethanol production [30].Ethanol stress cannot be avoided,especially towards the end of fermentation.Osmotic stress can be mitigated through process engineering.Ethanol stress impedes the process in the form of stuck or sluggish fermentations.Residual sugars remain unfermented,reducing ethanol yield [28].In this study,glycerol concentration and its yield under aeration were lower than without aeration (Table 1).Similar results were reported by Alfenore et al.[31].They indicated that aeration affected by-product formation.Under full aeration,glycerol production was decreased from 12.2 to 4.0 g·L-1.Deesuth et al.[32]found that glycerol production in ethanol fermentation using dried spent yeast (DSY) addition at various aeration rates (0.05-0.35 vvm for 12 h) ranged from 9.8 to 9.9 g·L-1,which were lower than without aeration,12.4 g·L-1.These results clearly indicated that the optimal aeration condition improved the efficiency of ethanol production as well as decreasing by-product formation.
3.2.Repeated-batch ethanol fermentation under HG conditions
The initial total sugar level in the sweet sorghum juice medium was reduced to 240 g·L-1(HG condition),and the effects of yeast extract and aeration in repeated-batch ethanol production from the juice under this condition were then studied.In batch 1,the cell concentration increased and reached maximum value after 34 h (Fig.2A).The total sugar was almost consumed after 52 h,resulting in higher ethanol production (Fig.2B and C).The sugar utilization,P,Qpand Yp/svalues of the ethanol fermentation from the juice of batch 1 were 94.68%,101.11 g·L-1,2.97 g·L-1·h-1and 0.43,respectively (Table 2).A diminished Yp/simplied that sugar was,to a greater degree,converted to by-products such as glycerol as indicated by the YGly/svalue(Table 2).Under the unaerated conditions in batches 2-4,the viable cell count decreased compared to batch 1 (Fig.2A).Lower sugar consumption,P,Qpand Yp/svalues were observed and higher YGly/swas obtained in batches 2-4.Additionally,the fermentation time in the latter batches significantly increased from 36 h to 102 h.To increase the efficiency of ethanol production,aeration(2.5 vvm for the first 4 h)was supplied during each cycle in batches 5-8.It was found that yeast growth with aeration was higher than without aeration.The sugar utilization,P,Qpand Yp/svalues were improved and they were similar to those in batch 1 (Table 2).This again indicated that aeration was an essential factor promoting ethanol production efficiency under HG fermentations.
Laopaiboon and Laopaiboon [1]reported that substrate inhibition did not occur in ethanol fermentations of sweet sorghum juice at an initial total sugar concentration of 240 g·L-1using a free cell system.This was also supported by Ingledew [33],who reported that an initial sugar concentration of 240 g·L-1did not cause excessive osmotic pressure in the broth.Therefore,a nutrient supplement or 9 g·L-1of yeast extract in the juice might not be necessary under these conditions.When yeast extract was not used with juice in batches 9-11,the efficiency of ethanol production from the juice without yeast extract supplementation was not different from those with yeast extract supplementation(batches 5-8,Fig.2 and Table 2),indicating that yeast extract did not promote ethanol production from sweet sorghum juice in HG fermentations.
When aeration was supplied at every other cycle without yeast extract supplementation (batches 12-19) and under yeast extract supplementation(batches 20-23),the average sugar consumption,P,Qpand Yp/svalues were similar to those of batches 5-11 (Fig.2 and Table 2).These results indicated that aeration at every other cycle in repeated-batch ethanol fermentation from juice under HG conditions was sufficient for improvement of ethanol production efficiency irrespective of the presence of a nutrient supplement (i.e.,yeast extract).
The effect of yeast extract (removal of yeast extract from the fermentation medium) on ethanol production under VHG conditions was studied by Laopaiboon et al.[9],Deesuth et al.[16]and Khongsay et al.[34].They found that nitrogen supplementation was extremely required for improvement of ethanol production from sweet sorghum juice in VHG fermentations.When the repeated-batch ethanol fermentation under VHG and HG conditions were compared (Tables 1 and 2),the P values under the HG condition were 12% lower than those under the VHG condition.However,the Qpvalues under the HG condition were 29% higher than under the VHG condition.

Fig.2.Repeated-batch ethanol fermentation from sweet sorghum juice at an initial sugar concentration of 240 g·L-1 by S.cerevisiae NP01.A:viable cell,B:total sugar and C:ethanol concentration.The arrows indicate the start of each batch.

Table 2 Repeated-batch ethanol fermentation when the feeding medium was sweet sorghum juice at an initial sugar concentration of 240 g·L-1.
A comparison of the ethanol production efficiencies in the repeated-batch fermentation to other studies is presented in Table 3.Fermentations that integrated aeration in the early stage showed superiority in overall ethanol production.This might have been due to increased intracellular contents of saturated fatty acids,unsaturated fatty acids,total fatty acids and ergosterol in the yeast cells [35].Under HG conditions with aeration,increased P and Qpvalues using sweet sorghum juice in repeated-batch fermentations were obtained,whereas diminished P and Qpvalues were observed in the conventional repeatedbatch operation (unaerated).This demonstrated the importance of aeration in repeated-batch fermentations to achieve high ethanol production efficiencies from sweet sorghum juice.Therefore,the efficiency of ethanol production in conventional repeatedbatch fermentation can be improved through optimized aeration strategies.

Table 3 Repeated-batch ethanol fermentation from sweet sorghum juice under HG conditions
3.3.Effects of aeration on the levels of storage fatty acids and ergosterol
Ethanol is a stressor for S.cerevisiae cells growing under ethanolic conditions [36].It modifies the polarity of membranes.Hydration of polar head groups changes membrane surfaces (plasma membrane and organelles),altering membrane functions,e.g.,nutrient uptake and excretion of ethanol [37,38].Previous reports clearly showed that aeration was a crucial factor for growth and ethanol production of S.cerevisiae NP01.Various researchers reported that an appropriate aeration rate promoted synthesis of plasma membrane,fatty acids [39]and ergosterol [40]to protect the integrity of cell membranes at high ethanol concentrations(more than 15%) [41].Lin et al.[42]found that a small amount of aeration during the initial stage of yeast growth could improve the efficiency of ethanol production.Arshad et al.[25]indicated that yeast cells need some aeration at the initial stage to overcome osmotic stress and ethanol induced oxidative stress at the end of fermentation.
To evaluate the differences and changes in the intracellular composition of yeast cells during repeated-batch fermentation of sweet sorghum juice containing 290 g·L-1of total sugars and 9 g·L-1of yeast extract(VHG condition),the concentrations of saturated fatty acids(SFAs),unsaturated fatty acids(UFAs),total fatty acids (TFAs) and ergosterol contents were determined for various ethanol fermentations(Table 4).Under aeration in batch 1(Condition I),the TFA content was (2637±88)μg·(g DCW)-1,and the most abundant fatty acid was linoleic acid (C18:2n6),which was approximately 23.98%of TFAs.This was followed by palmitic acid,C16:0 (20.04%),oleic acid,C18:1 (12.62%),palmitoleic acid and C16:1 (10.24%),respectively.The levels of other fatty acids were less than 10%.When aeration was not supplied in batches 2-5(Condition II),significantly diminished C16:0,C18:2n6 and C18:3n3 was observed,and the TFA content remained only(1285±22) μg·(g DCW)-1.When the aeration was re-introduced in batches 6-10(Condition III),the levels of these three fatty acids markedly increased,and the TFA content was increased by approximately a factor of two to (2506±62)μg·(g DCW)-1.These results confirm that dissolved oxygen is required for yeast to facilitate synthesis of these fatty acids[43].The ergosterol content of S.cerevisiae NP01 cells under Conditions I and III(aerated)was similar at(282±20) and (279±11)μg·(g DCW)-1,respectively;whereas it was only(198±8)μg·(g DCW)-1in Condition II(unaerated).These results indicated that aeration promoted fatty acid(primarily C:16 and C:18)and ergosterol formation in yeast cells(Table 4),increasing the efficiency of ethanol production (Table 1).
Variation in the intracellular composition of yeast cells in repeated-batch fermentation from the sweet sorghum juice containing 240 g·L-1of total sugar with and without 9 g·L-1of yeast extract (HG condition) was investigated (Table 5).With aeration and yeast extract supplementation (batches 1,5-8 or Condition I),the most abundant fatty acid in the yeast cells was linoleic acid(C18:2n6).It accounted for approximately 32.62%of TFAs,followed by palmitic acid,C16:0 (20.14%),cis-8,11,14-eicosatrienoic acid and C20:3n6 (11.40%).With no aeration and with yeast extractsupplementation in batches 2-4(Condition II),the TFA content significantly decreased from(2743±102)μg·(g DCW)-1(Condition I)to (1141±81)μg·(g DCW)-1.The fatty acids that were most decreased were C16:0,C18:0,C18:1,C18:2n6 and C18:3n3.

Table 4 Fatty acid and ergosterol contents of S.cerevisiae NP01 cells under VHG conditions in repeated-batch ethanol fermentation
The effects of yeast extract supplementation on the intracellular composition in repeated-batch ethanol fermentation were observed in Conditions III,IV and V (Table 5).Under Condition III(aeration without yeast extract),Condition IV(aeration every other cycle with no yeast extract)and Condition V (aeration every other cycle with yeast extract),the TFA contents were similar (ranging from (2118±98) to (2289±112)μg·(g DCW)-1).These values were slightly lower than for Condition I ((2743±102)μg·(g DCW)-1).The ergosterol contents of the four conditions (I,III,IVand V)were not significantly different((311±14)to(364±18)μg·(g DCW)-1),but they were significantly higher than that of Condition II ((172±5)μg·(g DCW)-1).These results indicated that yeast extract supplementation under a HG condition did not affect TFA and ergosterol formation.This might be one of the reasons that no difference in the efficiency of ethanol production was observed under Conditions III,IV and V (Table 2).

Table 5 Fatty acid and ergosterol contents of S.cerevisiae NP01 cells under HG conditions in repeated-batch ethanol fermentation
4.Conclusions
Sweet sorghum juice is proved to be an excellent raw material for ethanol production.The requirement for a nitrogen supplement and aeration supply is related to the initial sugar concentration of the juice.Repeated-batch ethanol production from sweet sorghum juice at a VHG condition (290 g·L-1of total sugar) required both nitrogen supplement (9 g·L-1of yeast extract) and aeration(2.5 vvm for the first 4 h) during every cycle to improve the efficiency of ethanol fermentation.In repeated-batch fermentation from sweet sorghum juice under a HG condition(240 g·L-1of total sugar),no nitrogen supplementation was required,only aeration every other cycle was sufficient to promote efficient ethanol production.A proper aeration during the initial stage of the fermentation not only stimulated cellular growth but also allowed yeast to synthesize essential components (lipids and ergosterol) irrespective of the initial sugar concentration in the medium,resulting in an increase in the efficiency of ethanol production.
Acknowledgements
The authors would like to thank the National Research Council of Thailand (NRTC) and Center for Alternative Energy Research and Development (AERD),KKU,Thailand for the financial support;and Associate Prof.Dr.Prasit Jaisil,Faculty of Agriculture,KKU for providing sweet sorghum juice.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene☆
- Distributed control and optimization of process system networks:A review and perspective☆
- Heat exchanger network synthesis integrated with flexibility and controllability☆
- Synthesis of flexible heat exchanger networks:A review☆
- Simulation and heat exchanger network designs for a novel single-column cryogenic air separation process☆
- A review of extractive distillation from an azeotropic phenomenon for dynamic control☆
