Percutaneous absorption and brain distribution facilitation of borneol on tetramethylpyrazine in a microemulsion-based transdermal therapeutic system
2019-09-11XiaogeHuNingChengJihuiZhaoXianghuaPiaoYuluYanQiboZhangKuanZhouYongtaiZhangNianpingFeng
Xiaoge Hu,Ning Cheng,Jihui Zhao,Xianghua Piao,Yulu Yan,Qibo Zhang,Kuan Zhou,Yongtai Zhang,Nianping Feng
School of Pharmacy,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
Keywords:Tetramethylpyrazine Borneol Microemulsion Percutaneous absorption Brain distribution
ABSTRACT In this study,we show that the percutaneous absorption and brain distribution of tetramethylpyrazine (TMP) is enhanced when combined with borneol (BN) in a microemulsionbased transdermal therapeutic system (ME-TTS). The formulation of the TMP and BN microemulsion (TEM-BN-ME) was optimized in skin permeation studies in vitro following a uniform experimental design. Male Sprague-Dawley rats were used for the in vivo pharmacokinetic and tissue distribution studies of TMP-BN-ME-TTS. In the pharmacokinetic study,the TMP-BN-ME-TTS treated rats had significantly higher(P <0.05)Cmax and AUC of TMP than the TMP-ME-TTS treated rats,indicating that BN improves the rate and extent of TMP percutaneous absorption.In the tissue distribution study,the AUC of TMP in brain was significantly higher in the TMP-BN-ME-TTS group (P <0.05), indicating that BN facilitates the distribution of TMP in brain. In summary, BN enhanced the percutaneous absorption and brain distribution of TMP in a microemulsion-based transdermal therapeutic system.©2018 Published by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Stroke is a heterogeneous vascular disease that occurs at increasing frequency with age. Characterized by high mortality and morbidity, stroke severely reduces the quality of life in the victims and has become a challenging global public health problem because of aging populations [1,2].2,3,5,6-tetramethylpyrazone (TMP), an active ingredient isolated from the traditional medicinal herb Ligusticum wallichii Franch, has been widely used in China for the treatment of ischemic stroke in both the acute and recovery stages[3-6]. Numerous mechanisms have been proposed for TMP’s neuroprotective activity, including the reduction of calcium overload, inhibition of the inflammatory response, antioxidation, anti-excitotoxicity, anti-apoptosis, thrombolytic effects, the enhancement of neurogenesis and cell differentiation, and the enhancement of dendritic plasticity [7-11].Due to its therapeutic effects, TMP has been included in the Chinese Pharmacopoeia for the treatment of cardiovascular and cerebrovascular diseases.
Borneol (BN), a bicyclic terpene extracted from Dryobalanops aramatica C.F. Gaertn., has been used in combination with Ligusticum wallichii Franch and other traditional herbs for treating stroke in China for over 1000 years.The Chuanxiong Longnao Pill, containing Ligusticum wallichii Franch and borneol as its main ingredients,was first recorded as a treatment for stroke in Shengji Zonglu, an encyclopedia of Chinese medical prescriptions compiled during the Song Dynasty(960-1279 CE). Xiongbing compound (XBC) microemulsion, a modern Chinese prescription containing TMP and BN, has been used for several decades at the First Affiliated Hospital of Guangzhou University of Chinese Medicine to treat stroke with obvious therapeutic benefits[12].Pharmacological studies suggest that BN reduces brain injury after ischemic stroke by directly reducing the content of malondialdehyde (MDA)and increasing lactate dehydrogenase (LHD) and superoxide dismutase (SODase) activities in brain tissue. BN also enhances the bioavailability of TMP and facilitates its brain distribution by increasing its absorption through the blood brain barrier (BBB) after oral or intranasal administration[13-16].These may underlie the synergistic effects of TMP and BN when they are used together in the treatment of stroke.
When TMP is used to treat ischemic stroke,a long course of treatment is usually required, ranging from several weeks in the acute stage to several years on the recovery stage. Intravenous (IV) injection and oral administration are typically used [3,17]. However, TMP exhibits low bioavailability and a very short biological half-life,if be administered orally,and it is therefore necessary to administer frequently (3 or 4 times per day). Since the IV and oral routes also suffer from poor patient compliance,especially in elder patients,transdermal administration may be superior alternative.
As reported in an earlier study, we developed a microemulsion-based transdermal therapeutic system(TMP-ME-TTS),and demonstrated that the bioavailability and brain distribution of TMP was enhanced in comparison with a TMP transdermal patch[18].In the work presented here,TMP and BN were incorporated together into a microemulsion simultaneously. The TMP-BN-ME formulation was optimized in skin penetration experiments in vitro using the skin flux of TMP and BN as indices.After optimization,a microemulsion based transdermal therapeutic system, incorporating TMP and BN (TMP-BN-ME-TTS) was prepared and used in vivo in rats to evaluate the influence of BN on the pharmacokinetic and tissue distribution of TMP.
2. Materials and methods
2.1. Materials
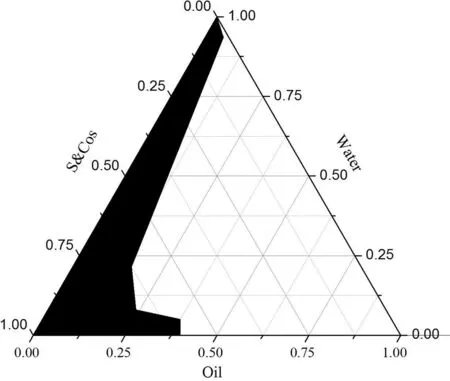
Fig.1-The pseudo-ternary phase diagram of TMP-BN-ME.Notes:S&Cos represent ethyl oleate,Labrasol® (surfactant)and Transcutol® P(cosurfactant);the shadow area represents microemulsion zone.
2,3,5,6-tetramethylpyrazone (TMP)and borneol (BN)were obtained from Nanjing Zelang Medical Technology Co., LTD(China). Caprylocaproyl macrogol-8 glycerides (Labrasol®)and highly purified diethylene glycol monoethyl ether(Transcutol®P)were purchased from Gattefossè(France).Ethyl oleate was purchased from Shanghai Chemical Reagent Corporation(China).Ethylene vinyl acetate(EVA)membrane with 19% VA content (3 M CoTran 9728) was obtained from 3 M Pharmaceuticals (USA). Acrylic pressure sensitive adhesive(DURO-TAK®87-2677) was provided by Henkel AG&Co. (Germany).Other chemicals are HPLC or analytical grade.
2.2. Formulation optimization and validation of TMP-BN-ME
TMP-BN-ME was formulated using ethyl oleate,Labrasol®and Transcutol®P as the oil phase,surfactant(S)and co-surfactant(Cos),respectively.To determine the microemulsion zone the weight ratio of S to Cos (Km) was fixed at 3:1,and the weight ratio of oil to S&Cos was varied(9:1,8:2,7:3,6:4,5:5,4:6,3:7,2:8,1:9).Mixtures were gently stirred at room temperature,while distilled water was added drop-wise.The critical points separating microemulsion from other phase zones were recorded when the appearance of the system changed from clear to turbid,and back again,the data were used to construct a pseudoternary phase diagram.
Using the pseudo-ternary phase diagram for guidance,nine formulations were selected to optimize the TMP-BN-ME using a uniform experimental design.The weight contents of oil(X1)and S&Cos(X2)in the microemulsion served as factors,and the in vitro rat skin flux of TMP(JTMP)and BN(JBN),viscosity and mean particle size served as indices.The constructed pseudo-ternary phase diagram is shown in Fig.1.
In vitro skin permeation experiments of the abovementioned TMP-BN-MEs (n = 5) were conducted using Franz type diffusion cells.Fresh excised abdominal skin from male Sprague-Dawley rats was used as a barrier,and 30% ethanol,20%PEG-400 in normal saline(v/v)was the receptor medium.The effective permeation area and receptor compartment volume were approximately 2 cm2and 12.5 ml,respectively.1 ml of TMP-BN-ME (30 mg of TMP and 60 mg of BN) was added to each donor compartment.At 1,2,4,6,8,12 and 24 h after the addition of TMP-BN-ME,a 0.5 ml of sample was withdrawn from each receptor compartment,and replaced with the same volume of blank medium at 32°C.The levels of TMP and BN in the collected samples were determined simultaneously using GC-FID method. The cumulative amounts of TMP or BN permeating the skin barrier were calculated using the equation:
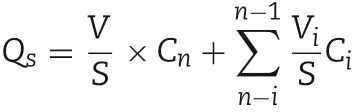
Where Cnis the TMP or BN concentration in the receptor medium at each time point,Ciis the TMP or BN concentration of the sample, and V and Viare the volumes of the receptor medium and the sample,respectively.S is the effective diffusion area.The skin flux of TMP(JTMP)and BN(JBN)were calculated from the slope of the linear portion of the plot showing cumulative amount per unit area versus time.
Viscosity, mean particle size and polydispersity indices(PDIs) of the TMP-BN-ME formulations were measured using a rheometer (MCR 101, Anton Paar, Germany) and Zetasizer(Nano ZS90 Malvern Instruments Ltd.,UK),respectively.
The correlation between the indices and factors in the formulation optimization experiments was estimated using multiple linear regression. The independent variables,namely the weight contents of oil (X1) and S&Cos (X2) in the microemulsion, were fitted using linear equations to the dependent variables JTMP, JBN, viscosity, and TMP-BN-ME mean particle size. Both enter (simultaneous) and stepwise methods were employed. Based on the best fit models (expressed as function), the formulation of TMP-BN-ME was optimized as follows: JTMPand JBNwere not less than 630 μg/cm2/h and 240 μg/cm2/h, respectively. Viscosity and mean particle size of TMP-BN-ME were not more than 30 cPs and 45 nm,respectively.The optimal formulation was predicted to contain(w/w)oil(7.9%),S(37.5%),Cos(12.5%),water(42.1%),TMP(3%)and BN(6%). After this formulation was prepared, the mean particle size and viscosity of TMP-BN-ME were measured and compared with those predicted values to confirm model validity.
2.3. Preparation and in vitro evaluation of TMP-BN-ME-TTS
TMP-BN-ME was sealed within a reservoir in which ethylene vinyl acetate (EVA) membrane (3 M CotranTM9728) was the release liner,and aluminum-plastic film was the backing layer. Pressure-sensitive adhesive (DURO-TAK®87-2677) was dissolved in 0.1 ml of ethyl acetate, and painted evenly as a film on the surface of the EVA membrane. The organic solvent was allowed to evaporate for 24 h,and the surface of EVA membrane was covered with a layer of non-adhesive material.The TMP-BN-ME-TTS contained approximately 1.5 g of TMPBN-ME and had an effective permeation area of approximately 6 cm2.After preparation,the TMP-BN-ME-TTS was stored in a desiccator until use.
The effect of storage on the skin permeation behavior of TMP and BN was investigated using in vitro skin permeation experiments.Freshly prepared TMP-BN-ME-TTS (0 h storage)was compared with TMP-BN-ME-TTS stored for 12, 24 and 36 h.
2.4. Pharmacokinetics and tissue distribution of TMP-BN-ME-TTS
Male Sprague-Dawley rats (8-10 weeks, 250 ± 10 g) were provided by the Animal Center of Shanghai University of Traditional Chinese Medicine.Hair in the abdominal region was removed carefully using an electric clipper. After 24 h, rats were visually inspected and those without skin damage were enrolled randomly in the TMP-BN-ME-TTS group and the TMP-ME-TTS group(TMP loaded ME-TTS),n=5 in each group.Rats in each group received a single dose of TMP-BN-ME-TTS(45 mg of TMP and 90 mg of BN) or TMP-ME-TTS (45 mg of TMP).Before administration and at 1,2,4,6,8,12,24 and 36 h after the administration,300 μl of blood was taken from each rat,and plasma samples were obtained by centrifuging blood at 3000 rpm for 10 min.TMP and BN levels in plasma samples were determined simultaneously using a sensitive GC-MS/MS method.
Male Sprague-Dawley rats were also used as animal model for the tissue distribution study of TMP-BN-ME-TTS in comparison with TMP-ME-TTS. Animal handing, grouping and dosing were the same as in the pharmacokinetic study. Before administration and at 1,2,4,6,8,12,24 and 36 h after the administration,rats were sacrificed (n = 3 animals per group at each time point) and the major tissue and organs were isolated and cleaned. Samples were weighed, cut into small pieces, diluted with two volumes of normal saline, and homogenized using a portable homogenizer.Homogenates were centrifuged at 12 000 rpm,4°C for 10 min.TMP and BN levels in the supernatants were determined simultaneously using the GC-MS/MS method described above.
To estimate the pharmacokinetic parameters for the control and experimental groups, noncompartmental pharmacokinetic analysis was conducted using BAPP 2.0(Bioavailability program package 2.0, Center for Metabolism and Pharmacokinetics,China Pharmaceutical University,Nanjing,China).
2.5. Evaluation of skin irritation by TMP-BN-ME-TTS
To determine if TMP-BN-ME-TTS causes skin irritation, New Zealand rabbits (2.0-2.5 kg) were divided randomly into four treatment groups:the blank microemulsion(Blank ME)group,the TMP-BN-ME group,the blank microemulsion-based transdermal therapeutic system (Blank ME-TTS) and the TMP-BNME-TTS group (n=4 animals per group). A small region on the left flank of each rabbit(approximately 3 cm×3 cm)was shaved with an electric clipper.After the rabbits had recovered for 24 h,they were visually inspected for skin damage.Rabbits without skin damage were enrolled in the experiments.Rabbits were treated with Blank ME, TMP-BN-ME, Blank ME-TTS or TMP-BN-ME-TTS, once daily for 3 consecutive days, each treatment lasted four hours. After the preparations were removed,and the treated skin was cleaned with warm distilledwater and inspected visually for skin irritation. Images were recorded before each treatment, 1 h after the removal of the preparations following the first and second treatments,and 1,24,48 and 72 h after removal of the preparations following the final treatment.
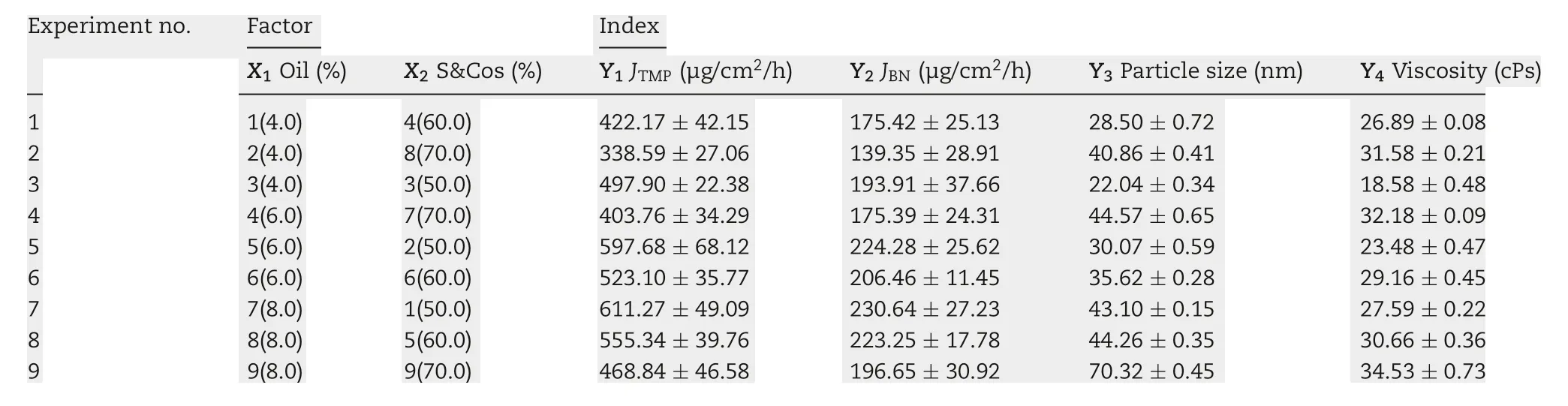
Table 1-TMP-BN-ME composition characteristics for formulation optimization.
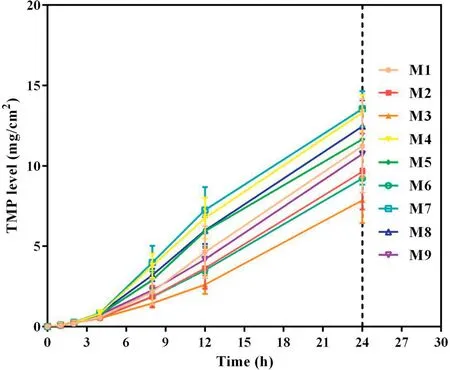
Fig.2-Cumulative skin permeation of TMP versus time for tested formulations of TMP-BN-ME(n=5).Note:M1-M9 represent the 9 formulations tested to optimize the TMP-BN-ME formulation.The weight ratios of oil from M1 to M9 are 4%,4%,4%,6%,6%,6%,8%,8%and 8%,respectively;the weight ratios of surfactant and co-surfactant from M1 to M9 are 60%,70%,50%,70%,50%,60%,50%,60%and 70%,respectively.Loading of TMP and BN are 3%and 6%in all the microemulsions.
3. Results and discussion
3.1. Optimization of TMP-BN-ME composition
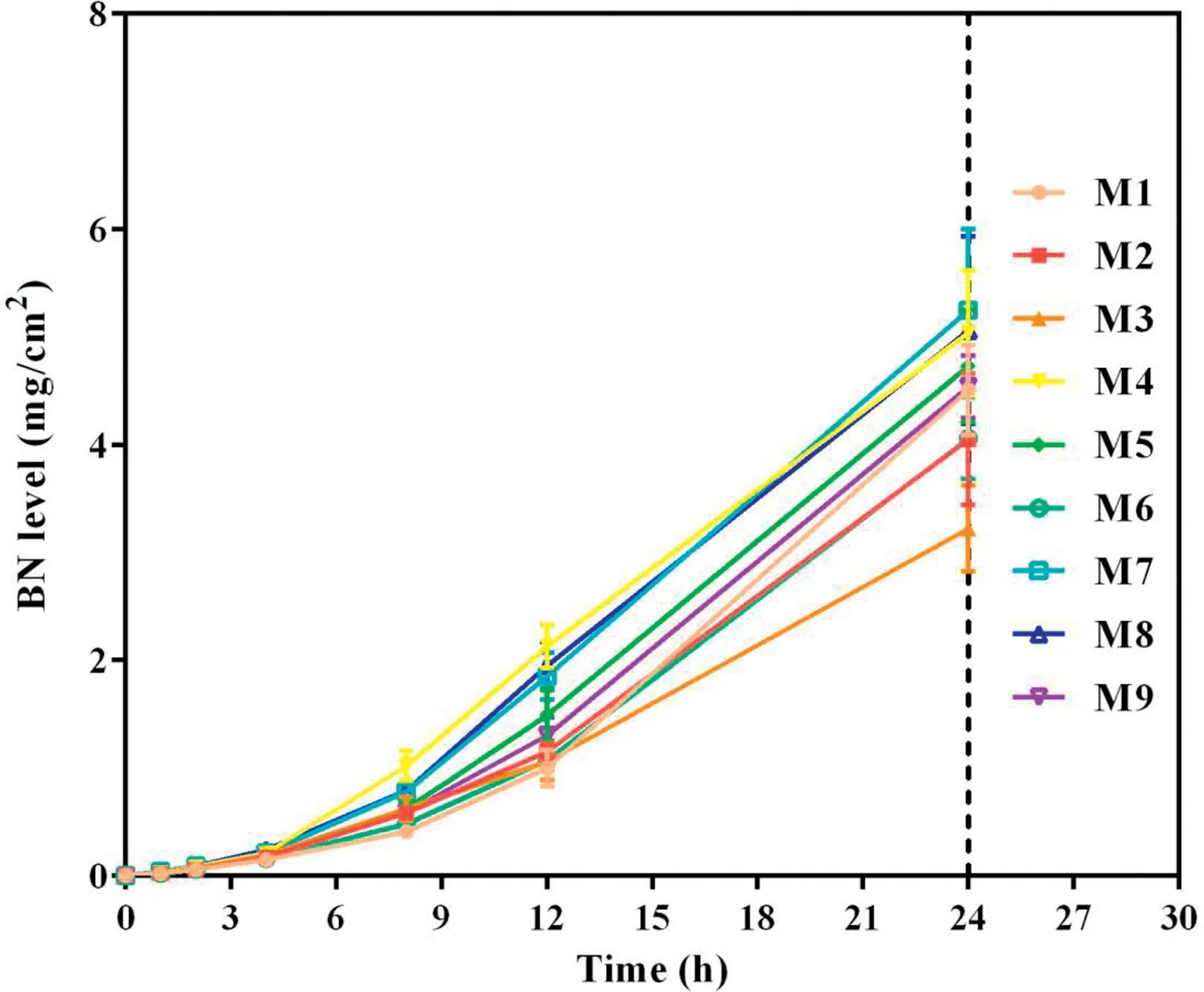
Fig.3-Cumulative skin permeation of BN versus time for tested formulations of TMP-BN-ME(n=5).Note:M1-M9 represent the 9 formulations tested to optimize the TMP-BN-ME formulation.The weight ratios of oil from M1 to M9 are 4%,4%,4%,6%,6%,6%,8%,8%and 8%,respectively;the weight ratios of surfactant and co-surfactant from M1 to M9 are 60%,70%,50%,70%,50%,60%,50%,60%and 70%,respectively.Loading of TMP and BN are 3%and 6%in all the microemulsions.
To optimize the formulation of TMP-BN-ME,the composition of the microemulsion was varied, using nine combinations of oil (X1) and surfactant plus co-surfactant (S&Cos; X2).TMP(JTMP)and BN(JBN),viscosity,and mean particle size were measured for all formulations and are shown in Table 1. Figs. 2 and 3 show cumulative skin permeation as a function of time for TMP and BN, respectively. The best fit multiple linear regression functions reflecting the relationship between content by weight of the oil phase(X1)and S&Cos(X2)in TMP-ME,versus the JTMP(Y1), JBN(Y2), particle size (Y3), and viscosity (Y4),are shown below.
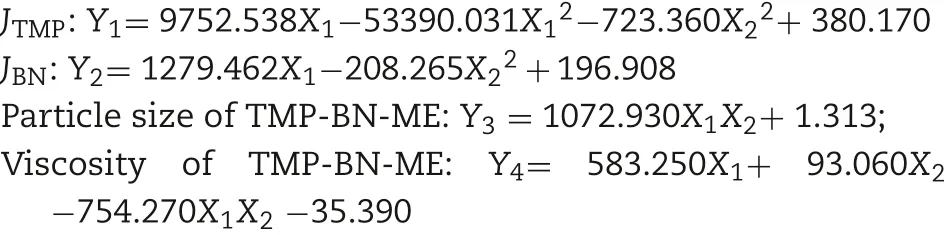
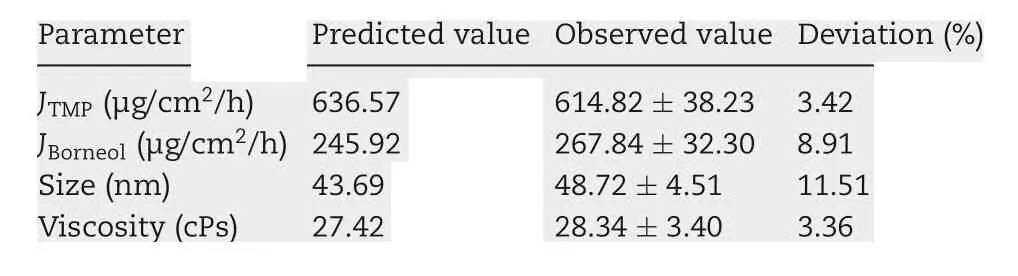
Table 2-Validation of TMP-BN-ME optimum formulation.
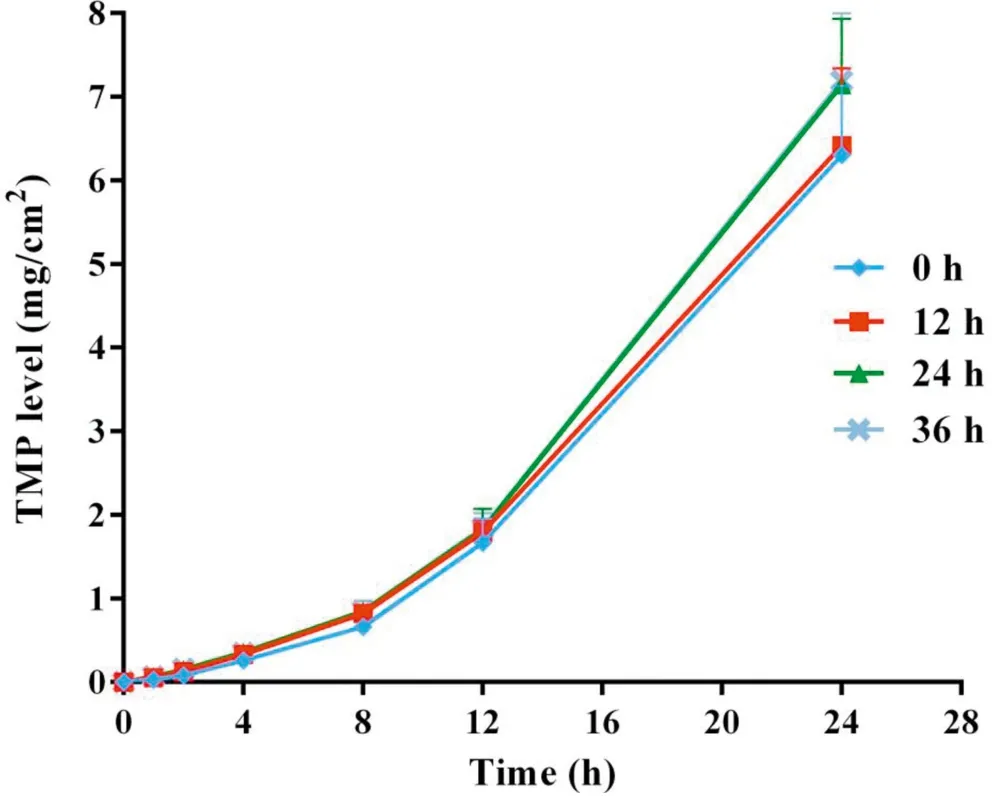
Fig.4-Cumulative skin permeation of TMP versus time for freshly prepared TMP-BN-ME-TTS(0 h)and TMP-BN-ME-TTS stored for 12 h,24 h and 36 h(n=5).
Using these functions, an optimal TMP-BN-TTS formulation was prepared using (w/w) oil (7.9%), S&Cos (50%), water(42.1%), TMP (3%), and BN(6%). Mean particle size, viscosity,JTMP, and JBNwere then measured and compared with predicted values (Table 2). Except for particle size, the deviation between the predicted and observed parameters was less than 10%,suggesting that the model effectively predicts JTMP,JBNand viscosity.In the optimum formulation of TMP-BN-ME,the weight content of oil phase was close to the highest value tested, whereas the weight content of S&Cos was the lowest value tested,suggesting that higher JTMPand JBNvalues might be obtained by increasing oil phase content and decreasing the S&Cos content in TMP-BN-ME. This result is consistent with previous reported results [19]. A possible explanation may be the higher viscosity of TMP-BN-ME,when the weight content of S&Cos increases, exerts a negative effect on JTMPand JBN.
3.2. Preparation and in vitro evaluation of TMP-BN-ME-TTS
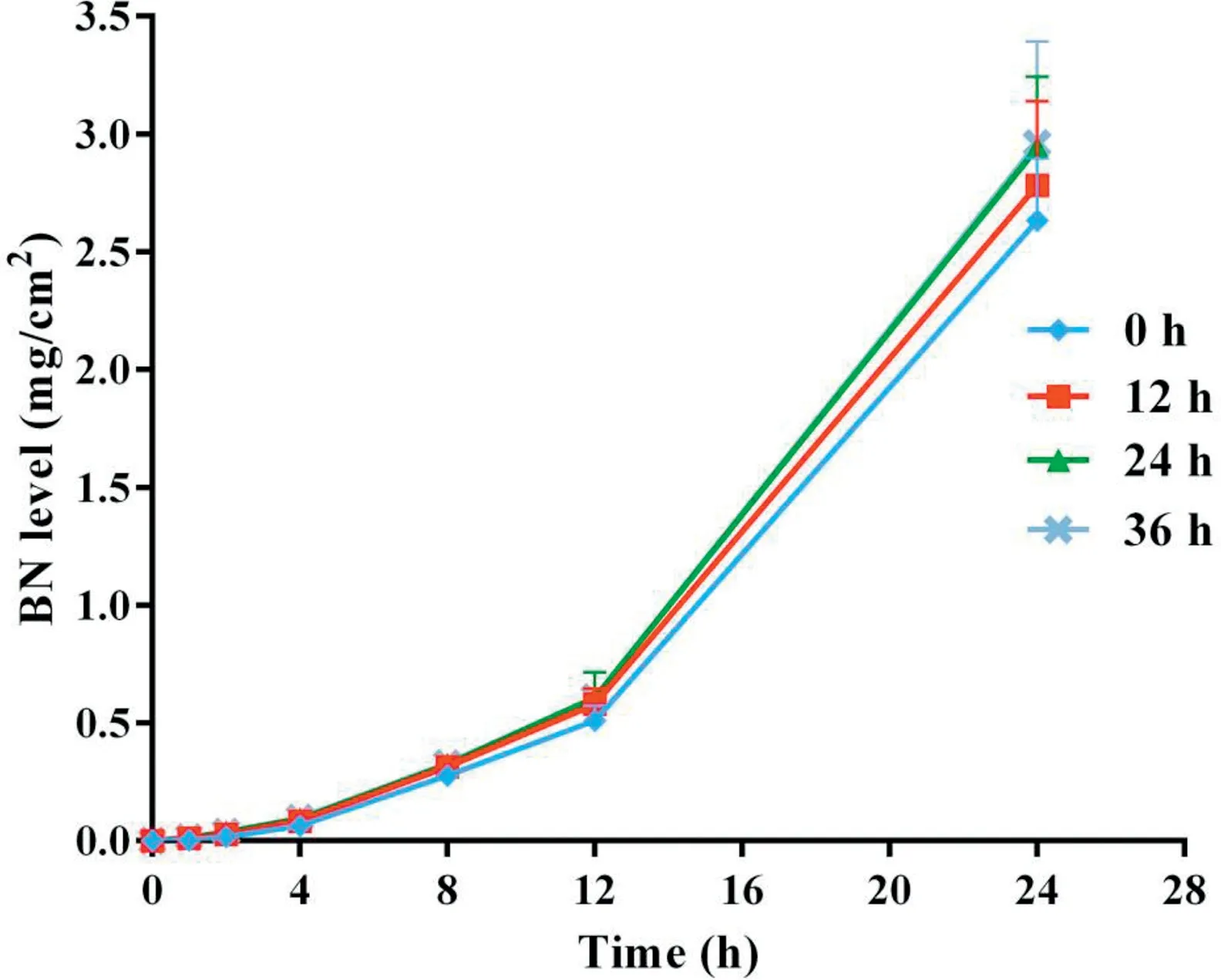
Fig.5-Cumulative skin permeation of BN versus time for freshly prepared TMP-BN-ME-TTS(0 h)andTMP-BN-ME-TTS stored for 12 h,24 h and 36 h(n=5).
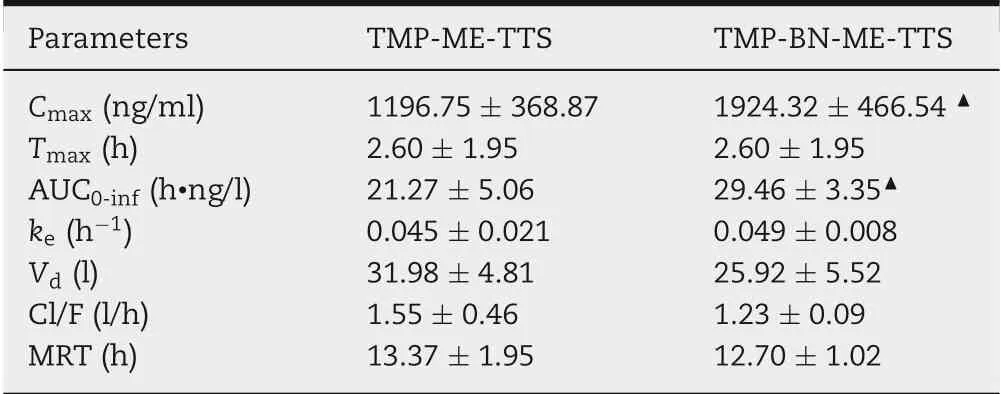
Table 3-The parameters of TMP in the pharmacokinetic study of TMP-BN-ME-TTS and TMP-ME-TTS (n = 5 animals for each group).
Figs. 4 and 5 show cumulative skin permeation of TMP and BN as a function of TMP-BN-ME-TTS storage time. Freshly prepared TMP-BN-ME-TTS was compared with preparations stored for 12,24 and 36 h.Skin permeation increase for both TMP and BN, as storage time increases to 24 h, suggesting that the diffusion balance for TMP and BN between the MEreservoir and pressure sensitive adhesive (PSA) was reached during this period. The time required for diffusion balance was much shorter than that reported for another reservoirtype transdermal therapeutic system [20]. However, the two systems are not directly comparable,because they contain different active pharmaceutical ingredients (TMP and BN versus fentanyl),different reservoirs (microemulsion versus gel) and have different release liners (3 M CoTran 9728 versus 3 M Co-Tran 9702).
3.3. Pharmacokinetics and tissue distribution of TMP-BN-ME-TTS
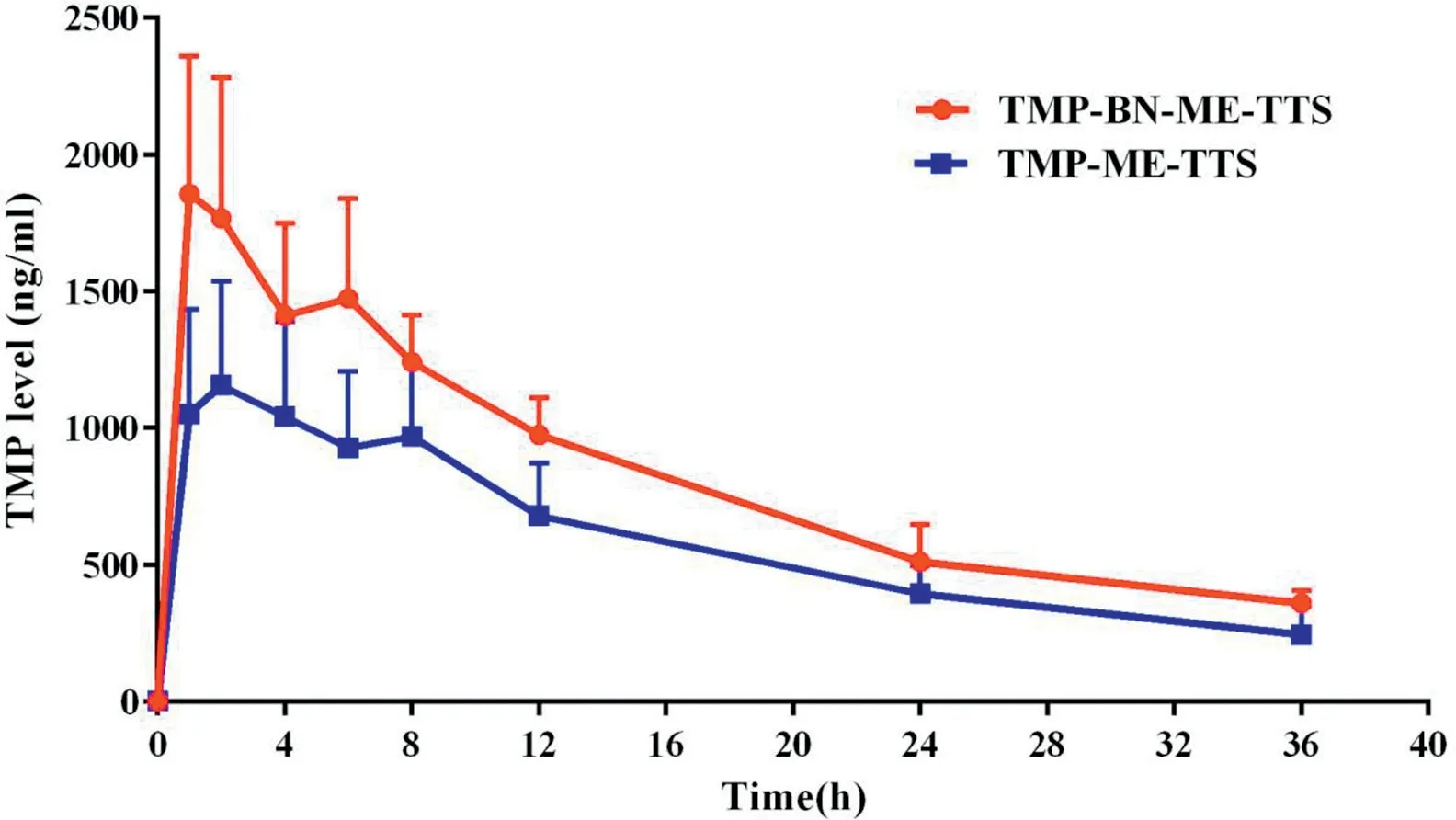
Fig.6-Mean TMP levels in rat plasma versus time after the single dose administration of TMP-BN-ME-TTS orTMP-ME-TTS(n=5).
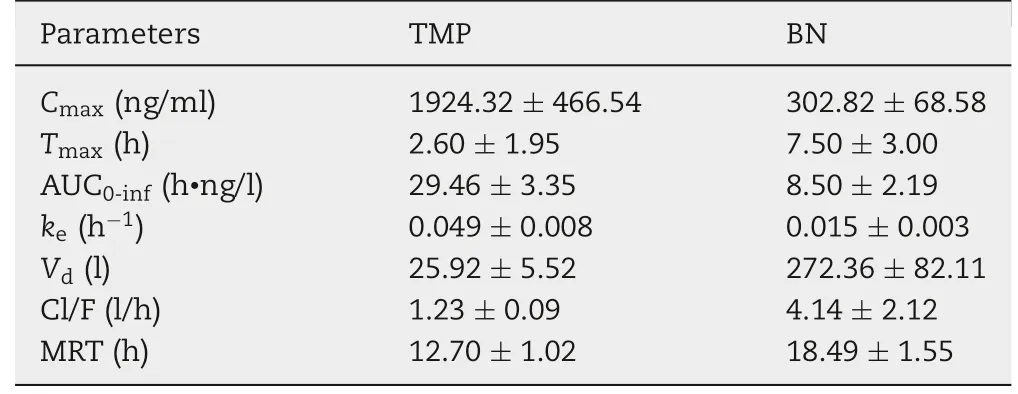
Table 4 - The parameters of TMP and BN in the pharmacokinetic study of TMP-BN-ME-TTS(n=5).
The pharmacokinetic parameters for TMP in the presence and absence of BN are listed in Table 3. The time course of the mean TMP concentrations in rat plasma after transdermal administration, using TMP-BN-ME-TTS or TMP-ME-TTS, are presented in Fig.6.These data show that significantly higher Cmaxand AUC0-infvalues for TMP were seen in the TMP-BNME-TTS group (P <0.05). These results are consistent with previous studies reporting BN facilitates nasal and oral absorption of TMP, probably due to skin penetration enhancement effect of BN [13-15]. However, no significant difference in Vdfor TMP between the TMP-BN-ME-TTS group and TMPME-TTS group was seen,suggesting that,generally speaking,the addition of BN showed no obvious influence on TMP’s tissue distribution behavior.
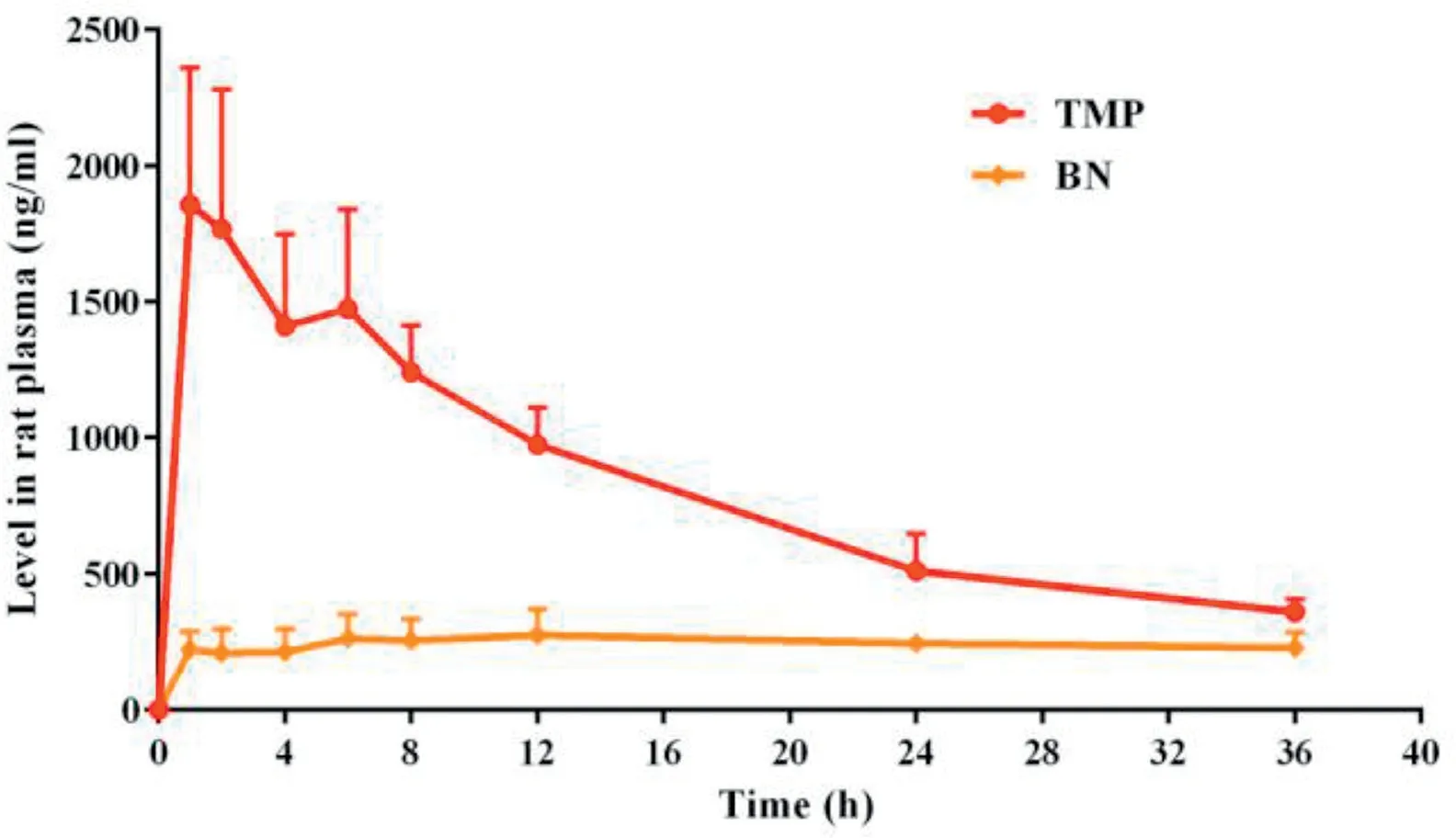
Fig.7-Mean TMP or BN levels in rat plasma versus time after the single dose administration of TMP-BN-ME-TTS(n=5).
The pharmacokinetic parameters for TMP and BN (when present together in TMP-BN-ME-TTS)are listed in Table 4,and the mean TMP and BN concentrations in rat plasma versus time after transdermal administration are shown in Fig. 7.These data show that TMP and BN exhibit different pharmacokinetic behavior after transdermal administration. Mainly,TMP penetrated through the skin and reached the circulation system quickly (AUC0-inf= 29.46 ± 3.35 h·ng/l for TMP and 8.50±2.19 h·ng/l for BN),whereas BN distributed more extensively into tissue and organs after percutaneous absorption(Vd=25.92±5.52 l for TMP and 273.36±82.11 l for BN).
AUC0-infvalues are compared for the same tissue after the transdermal administration of TMP-BN-ME-TTS and TMP-ME-TTS in Fig. 8. No significant difference in TMP AUC0-infin most of the main tissue and organs between the TMP-BN-ME-TTS group and TMP-ME-TTS group was seen, suggesting the addition of BN showed no obvious influence on TMP’s tissue distribution behavior, which was in accordance with our results of pharmacokinetic studies.However, TMP’s brain distribution is facilitated in the presence of BN,as indicated by significantly a higher TMP AUC0-inf(P <0.05). This result is consistent with recent study, which demonstrated reversible blood brain barrier (BBB)-opening ability of BN, and suggests that the modulation of multiple ATP-binding cassette transporters, including P-glycoprotein,tight junction protein,and the predominant enhancement of vasodilatory neurotransmitters may be involved[21].

Fig.8-AUCs of TMP in rat tissue and organs after the single dose administration of TMP-BN-ME-TTS or TMP-ME-TTS(n=5).
Fig.9-Response of intact rabbit skin to multiple-dose delivery of TMP-BN-ME-TTS.
3.4. Evaluation of skin irritation by TMP-BN-ME-TTS
The potential for TMP-BN-ME-TTS to provoke skin irritation was investigated using rabbits. Animals were treated with TMP-BN-ME-TTS and controls once daily for three consecutive days and then visually monitored, and photographed(Fig.9).No irritation reaction was observed for either TMP-BNME or TMP-BN-ME-TTS,and both were indistinguishable from the controls.These results demonstrate that TMP-BN-ME-TTS elicits no adverse effects on the skin.
4. Conclusion
The percutaneous absorption and distribution of TMP to the brain are enhanced significantly when TMP and BN are combined and incorporated into a microemulsion-based transdermal therapeutic system.This approach offers a promising alternative for TMP administration via a transdermal route and may also improve the therapeutic effects of TMP against ischemic stroke.Studying the influence of BN on the pharmacokinetics and tissue distribution behavior of TMP and the underlying mechanisms, is necessary for better understanding the potential clinical applications of the TMP-BN combination in particular,and for BN-enhanced drug delivery in general.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by the Program from Shanghai University of Traditional Chinese Medicine(B201725).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.06.003.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Recent strategies on targeted delivery of thrombolytics
- Cellulose based polymers in development of amorphous solid dispersions
- Phyto-phospholipid complexes(phytosomes):A novel strategy to improve the bioavailability of active constituents
- MSNCs and MgO-MSNCs as drug delivery systems to control the adsorption kinetics and release rate of indometacin
- Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis
- Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule(Avodart®):Effect of γ-cyclodextrin and solubilizers
