Recent strategies on targeted delivery of thrombolytics
2019-09-11TingHungNiLiJinqingGo
Ting Hung,Ni Li,b,Jinqing Go,*
aCollege of Pharmaceutical Sciences,Zhejiang University,Hangzhou 310058,China
bDepartment of Cardiothoracic Surgery,Ningbo Medical Centre Lihuili Hospital,Ningbo University,Ningbo 315041,China
Keywords:Thrombus Thrombolysis Targeted therapy Drug delivery system
ABSTRACT Thrombus formed in blood vessel is a progressive process, which would lead to lifethreatening thrombotic diseases such as ischemic stroke.Unlike other diseases,the recognition of thrombus is usually in the late stage where blood vessels are largely blocked.So acute thrombotic diseases have a narrow therapeutic window,and remain leading causes of morbidity and mortality,whereas current thrombolysis therapy has limited therapeutic effects and bleeding complications. Thrombolytic agents in unwanted sites would cause hemorrhage due to the activation of plasminogen.Moreover,untargeted thrombolysis therapy require large amounts of thrombolytic agents, which in return would enhance hemorrhage risk.To improve the efficiency while minimizing the adverse effects of traditional thrombolysis therapy,novel drug delivery systems have been investigated.Various targeting strategies including ultrasound and magnetic field directed targeting,and specific binding,have been designed to deliver thrombolytic drugs to the thrombotic sites.These strategies demonstrate promising results in reducing bleeding risk as well as allowing less dosage of thrombolytic drugs with lowered clot lysis time.In this review,we discuss recent progress on targeted delivery of thrombolytics,and summarize treatment advantages and shortcomings,potentially helping to further promote the development of targeted thrombolysis.©2019 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Thrombus is localized clotting of blood caused by activated coagulation system under physiological or pathological conditions.Blood clot grows bigger with the aggregation of platelets as the pathological state progresses,and further forms partial or complete blockage of vascular lumen [1]. Early stage of thrombosis may be neglected as no obvious symptoms appear on patients. However, when blood vessels are nearly blocked,blood flow stasis would cause necrosis of involved organs and even result in death.Cerebrovascular disease caused by thromboembolic vessels is becoming one of the leading causes of mortality globally[2].Therefore,quick and effective thrombolysis is urgently needed.

Fig.1-Illustration of platelets guided process of thrombus.The process of thrombosis involve with adhesion of platelets to damaged endothelium,aggregation of activated platelets to form a prothrombotic surface,and finally the formation ofinsoluble blood clots.

Table 1-A comparison of artificial DDSs and biological DDSs.
The pathophysiology of thrombosis has been extensively investigated and the underlying mechanism is being updated.Three important factors leading to the development of thrombosis were first proposed by Rudolph Virchow.The three components were referred to alternations in blood flow, vessel wall, and blood composition. Modern research updates the three components to abnormalities of hemorheology, abnormalities in the endothelium/endocardium,and abnormalities in platelet and the coagulation and fibrinolytic pathways[3].
Antithrombotic drugs applied in clinic are generally classified into antiplatelet and anticoagulant drugs which work upstream to prevent thrombogenesis,and thrombolytic drugs which work downstream to dissolve the thrombus or slower or prevent its formation [1,4]. Among them, thrombolytic drugs, namely plasminogen activators (PA), such as streptokinase (SK), urokinase plasminogen activator (uPA), tissue plasminogen activator (tPA), recombinant tPA(rtPA) et al. are used as the main treatment for thromboembolic diseases [5].However, as complex protein macromolecules, thrombolytics suffer from various activations within the bloodstream,leading to allergic reactions and short half life. And their nonspecific effects result in bleeding complications. These drawbacks limit their further applications. Therefore, the development of targeted thrombolysis therapy is of great importance.
Targeted drug delivery has been extensively researched due to its tremendous potential to revolutionize medicine by delivering drugs to the specific regions within the body.For the most threatening diseases such as cancer,targeted drug delivery has shown promising therapeutic outcomes.Our previous study showed that targeted drug carriers vary from synthetic carriers such as nanoparticles[6,7]and liposomes[8,9]to biological carriers such as stem cells [10-14], with different targeting strategies based on specific pathological characteristics. Likewise, current targeting strategies on thrombolysis mainly involve applying ultrasound or magnetic field as external stimulus to guide drug carriers to thrombus site,and specific binding of drug carriers to thrombus components.Moreover, novel theranostic approaches which can diagnose and treat diseases simultaneously have attracted major attention because of their capability to monitor therapeutic outcomes.Understanding the particular mechanism of diseases helps to find efficient targets, and develop effective targeted therapy.The process of thrombosis can be briefly described as the sequence of adhesion of platelets to damaged endothelium,aggregation of platelets to form a prothrombotic surface, release reaction of platelets to promote clot formation,then the initiation of coagulation,and finally the formation of insoluble blood clots [15,16]. (Fig. 1) So platelets serve as major targets for thrombolysis.

Fig.2-Different targeting strategies of artificial DDSs.(A)Ultrasound triggered thrombolysis.(B)Magnetic field induced thrombolysis.(C)Ligand binding directed thrombolysis.
2. Targeted drug delivery system for thrombolytics
Traditionally applied thrombolytics have some drawbacks such as short half-life, bleeding complications, and allergic reaction, which limit their clinical applications. Targeted delivery of thrombolytics specifically to the wanted site is required to enhance the therapeutic outcomes as well as to lower adverse effects. Several drug delivery systems(DDSs) have been designed to solve the problem. Artificial DDSs which load drugs into nano/micro-carriers or designed as prodrug construct can accomplish targeted drug delivery through physical targeting using ultrasound and magnetic field or modifying carriers with specific binding moiety(Fig. 2). Contrary to artificial DDSs, biological DDSs are composed of cells and their fragments, and possess the advantages of intrinsic responsiveness to related microenvironment in the body, which offer inherent targeting and great biocompatibility [17-19] (Fig. 3). The advantages and drawbacks of artificial DDSs and biological DDSs are compared in Table 1.
However, the studies on biological thrombolytic DDSs are few compared with artificial thrombolytic DDSs.This may be attributed to the difficulties of manipulating cells, and requires advanced techniques and updated information on the standard of source,processing,storage,et al.Inspired from biological DDSs, synthetic carriers designed to mimic cell derived natural carriers can also be used as efficient carriers for targeted thrombolysis, which may represent an alternative strategy [20].Various targeted DDSs of thrombolytics are summarized in Table 2.

Fig.3-Different drug carriers for biological DDSs.Cell,cell membrane,and extracellular vesicle can be used asbiological drug delivery carriers.
3. Artificial thrombolytic DDSs
Artificial thrombolytic DDSs include synthetic carriers sensitive to ultrasound or magnetic field, synthetic carriers modified with specific binding moiety and camouflaged prodrug construct. External stimuli including ultrasound [21-26] and magnetic field[27-29]at the thrombus site can trigger or guide thrombolytic agents to the expected location,reduce the unwanted activation of systemic plasminogen,and thus providean efficient targeted thrombolysis approach.As the main constituents of thrombus are activated platelets and fibrin,many artificial DDSs have achieved targeted thrombolytics delivery to thrombus using platelet-specific or fibrin-specific ligands as targeting moiety.The frequently used targeting ligands are antifibrin or antiplatelet monoclonal antibodies[30-32]and Arg-Gly-Asp(RGD)motifs[33-35].Other components of thrombus such as activated factor XIII can also serve as effective targets[36,37].Besides,prodrug constructs of PA triggered at thrombus site can also offer localized thrombolysis therapy with less systemic side effect. The rational design, diverse constructs,and controllable management of artificial thrombolytic DDSs make them feasible for efficient targeting of thrombus.

Table 2-A summary of different targeted delivery of thrombolytics.

Table 2(continued)
3.1. Ultrasound triggered targeting
Ultrasound was first discovered to increase enzymatic thrombolysis over 40 years ago.Several mechanisms for ultrasound enhanced thrombolysis including acoustic streaming, radiation force,and cavitation of ultrasound have been postulated[38-40].Nevertheless,the underlying mechanism remains unclear.
However,using ultrasound alone has some disadvantages such as inducement of embolization caused by the fragmentation of clots, mechanical vascular impairment, and reocclusion due to activation of platelets [41]. Therefore, ultrasound is mainly applied as an adjuvant thrombolysis therapy,i.e.,sonothrombolysis.Besides the thrombolysis ability,ultrasound serves as an attractive imaging modality in respect of its convenience,safety and cost,and is widely applied in clinical diagnosis.The required ultrasound frequencies and peak negative pressures for diagnosis are similar to those used for inducing thrombus dissolution.So a theranostic strategy can be achieved by diagnostic ultrasound.To achieve better imaging and delivery of thrombolytic drugs, contrast agents such as microbubbles (MBs) and echogenic liposomes (ELIPs) have been extensively studied.
Ultrasound plus MBs was proved capable of reducing clot diameter, but unable to induce fibrinolysis in human blood clots. Only when in combination with PA, would the fibrin degradation occur.Therefore,the combination of ultrasound,MBs and PA exerts a synergistic action between the thrombolytic activity of PA and contrast agent enhanced sonothrombolysis [42]. In vivo study also confirmed the synergistic effect on ischemic arterial thrombolysis of ultrasound plus MBs with addition of PA [43]. Arterial embolization rats were divided into 5 groups: untreated control group,rtPA group, ultrasound+MBs group, ultrasound+MBs+rtPA group and ultrasound+MBs+1/2 rtPA+group. The results indicated that ultrasound+MBs had minimal thrombolytic effect, while addition of half dose of rtPA significantly improved recanalization rate and recanalization grade.However,ultrasound+MBs+rtPA group and ultrasound+MBs+1/2 rtPA group exerted no difference on therapeutic outcomes.Histopathological changes in thrombosed vessels also confirmed structural disintegration of thrombus and breakdown of fibrous network within the thrombus in these two groups.
The PA loading of MBs didn’t change MBs’good ultrasound contrast imaging properties, and the combination with low frequency ultrasound demonstrated promoted thrombolysis,which is dose-dependent[44].Moreover,several clinical trials have been conducted to assess safety, efficacy and technical feasibility of therapeutic MBs in thrombolysis treatment.The promising results proved the combination of MBs and ultrasound an efficient approach to improve recanalization rates,increasing the thrombolytic effect[21-26].A pilot study by Perren et al. designed a non-randomized trial to compare acute ischemic stroke patients who underwent intravenous rtPA thrombolysis and sonothrombolysis over 60 min.The patients were treated with or without additional continuous MBs.The results showed that the combination of ultrasound and MBs achieved a better flow signal, indicating greater immediate clinical improvement [45]. Randomized trials also confirmed the therapeutic benefits of contrast enhanced sonothrombolysis, suggesting sonothrombolysis a promising noninvasive treatment of acute ischemic stroke [22,23]. For ST-segment elevation myocardial infarction which requires immediate restoration of coronary blood flow, sonothrombolysis offered an easy applicable therapeutic strategy. Randomized trails proved that high mechanical index ultrasound impulses along with contrast agent infusion could safely improve early epicardial patency rates and recovery in ST-segment elevation myocardial infarction [25,26]. The contrast agents used in these clinical trials were almost commercialized MBs,generally the more stable second-generation MBs such as sulphur hexafluoride filled MBs(Sonovue).All marketed contrast agents comprise MBs with a stabilizing shell and stable gases. So these microbubbles have high molecular weight and very low solubility in water,which can dissolve more slowly in blood circulation and thus offer longer persistence.Due to their properties of sensitivity, safety, low cost and portable nature, the manufacturing of contrast agents has now reached the stage of maturity[46].Whether their different characteristics influence sonothrombolysis remains to be investigated[47].
Entrapment of PA into ELIPs also displayed efficient drug release and clot lysis when exposed to ultrasound irradiation in vitro [48]. PA released from the PA-ELIPs remained similar thrombolytic activity as the free form, and showed substantially increases of lysis rate compared with either PA or ELIPs alone in an in vitro human clot model [49,50].And PA-loaded ELIPs exhibited comparable thrombolytic efficiency to other preclinically or clinically protocols including free PA alone and free PA co-administered with MBs in a rabbit thrombus model[51]. Since insoluble gas plays an important role in promoting more sustained cavitation activity[52],loading octafluoropropane gas within liposomes rather than air enhanced stable cavitation activity mediated by ultrasound,and increased thrombolysis efficacy[53].
Nano carriers possess advantages like an internal volume for encapsulating drug and an abundant surface area for modifying with ligands, which is beneficial for designing an ultrasound-responsive drug delivery system. tPA-cationized gelatin complex modified with PEG showed a nearly half suppressed thrombolytic activity and prolonged half life of tPA by 3 times in the systemic circulation, but recovered the activity only when under ultrasound. Administration of tPA-cationized gelatin complexes intravenously followed by ultrasound exposure resulted in thorough recanalization in a rabbit embolic model, in significantly contrast to single administration of the complex [54]. Another ultrasoundresponsive nanoparticle (NP) comprising tPA, zinc ions, and basic gelatin showed suppressed tPA activity by 50% while achieved complete recovery by ultrasound in vitro. Such NP achieved occluded artery recanalization in 9 of 10 acute myocardial infarction swine models within 30 min under the irradiation of ultrasound,while treatment with the same dosage of PA alone recanalized only 1 of 10 swine [55]. Similarly,uPA loaded in hollow nanogels was able to be triggered to release in faster rate under diagnostic ultrasound in vitro[56],circulating longer than nude uPA in vivo,and increase the effectiveness of thrombolysis in an acute ischemic stroke rat model[57].
Different from MBs and ELIPs,those nano carriers take ultrasound as a‘switch’.They remain inhibited thrombolytic activity in systemic circulation due to the designed construction,and restore the ability when exposed to the force of ultrasound,resulting in localized drug release.As contrast agents,ultrasound exerts an interaction effect on MBs and ELIPs.Ultrasound trigger their drug release,while they enhance ultrasound’s thrombolytic capability. The large size and transient cavitation effect of contrast agents may limit their permeation into thrombus in micro circulation.To design a smaller contrast agent with continuous cavitation effect, dodecafluoropentane NPs were prepared and showed phase change to MBs under ultrasound irradiation. The phase-change NPs exhibited sustaining cavitation effect, and increased thrombolytic efficiency as compared to SonoVue MBs in vitro artificial vascular system. This technology provides a novel approach for sonothrombolysis in micro circulation.But it needs further in vivo research to verify its thrombolytic efficiency,and whether it would be an alternative contrast agent requires much more investigation[58].
And as ultrasound imaging is the second-most applied imaging method in clinical setting, ultrasound based theranostics show great potential for diagnosing and treating thrombosis simultaneously [59]. To further improve the targeting ability,some targeting ligands have been introduced to the contrast agents, achieving both active targeting and ultrasound triggered targeting. The examples are described before. What makes the treatment regimen of sonothrombolysis complicated is that the therapeutic outcomes from different experiments have varied greatly [60,61], primarily because the optimal acoustic conditions have not been determined. The ultrasound parameters such as ultrasound frequency, pressure and waveform cycle length are of great importance in manipulating clot dissolution [25,62,63]. Besides,properties of the contrast agents also play a crucial part in the efficiency of sonothrombolysis [64,65]. There are generally two approaches for sonothrombolysis, low-frequency(20-30 kHz) and high-intensity (>10 W/cm2) ultrasound, and high-frequency (1-3 MHz) and low-intensity (0.5-5 W/cm2)ultrasound to the thrombus site [66].The former approach is able to destroy atherosclerotic plaques and thrombus even in absence of enzymatic thrombolysis, but its safety issues should be verified. The latter approach is more promising for clinical sonothrombolysis applications such as ischemic stroke due to its ability to focus at depth,but its potential heating of the surrounding tissue is the main concern [67]. Nevertheless, the optimal ultrasound parameters need further investigation.
3.2. Magnetic field induced targeting
Magnetic targeting is a physical targeting strategy using external magnetic field to localize magnetic carriers. Magnetic carriers usually consist of a magnetic core and an organic or inorganic shell. The shell can prevent magnetic carriers from aggregation and provide biocompatible, high-capacity functionalized surfaces. The strong magnetic properties and biocompatibility of magnetic carriers facilitate their applications in magnetically guided drug targeting, magnetic resonance imaging as contrast-enhancing agents, and magnetic diagnosis.
Iron oxide nanoparticles (IONPs) are extensively investigated magnetic carriers owning to their biodegradability of known iron metabolism pathways [68].PA loaded IONPs performed effective local thrombolysis under the guidance of magnet field. Organic polymers such as dextran [69], polyacrylic acid (PAA) [27] and chitosan [70] as well as inorganic silica shell [29] have been used to stabilize IONPs and provide IONPs with abundant conjugation with PA.Biocompatible tPA conjugated dextran-IONPs showed efficient drug targeting while not losing the enzyme in thrombus-mimicking gels by external magnetic field [69]. rtPA conjugated PAA-IONPs displayed superior improvement on hemodynamics than that of equivalent amount of free rtPA in a rat iliac arterial thrombosis model. Hind limb skin tissue perfusion of the rats measured by a laser Doppler perfusion imager illustrated quick reversion of perfusion within 30 min by administration of PAA-IONPsrtPA under the guide of magnetic field,while the blood flow of free rtPA and IONPs didn’t recovered even after 2 h(Fig.4).The way of how to apply magnetic field is the most critical part.PAA-IONPs-rtPA under static magnetic field didn’t reverse the hemodynamics, while an alternative magnetic field seemed essential for target thrombolysis due to the aggregation and dissipation movement of IONPs.Moreover,it is likely that mechanical dragging force created by alternative magnetic field also facilitated rtPA penetration and thus enhanced thrombolysis. Administrating PAA-IONPs-rtPA under alternative magnetic field may accomplish effective and reproducible target thrombolysis with less than 20%of a routine dose of rtPA under magnetic guidance[27].
As a high positive charge polymer,chitosan was able to facilitate the penetration of the tPA conjugated chitosan-IONPs at physiological pH into thrombus which contains negatively charged fibrin. Both ex vivo and in vivo rat thrombus model showed its effective thrombolysis with reduced blood clot lysis time and lower drug dose[70].To improve the amount of PA loaded per carrier,a shell of poly[aniline-co-N-(1-one-butyric acid) aniline] was used to compose high drug-conjugated IONPs. The binding capacity was much improved, and thus accelerated thrombolysis,restoring blood flow within half an hour of treatment at 20%of the regular rtPA in a rat embolism model[28].Besides,SiO2shell functionalized with NH2groups can also provide IONPs with abundant conjugation with tPA.The conjugated tPA showed improved storage, high activity retention, and manipulation stability. An ex vivo thrombolysis model demonstrated increased thrombolytic effect with SiO2-IONPs-tPA under the guidance of magnetic field compared with the same samples without magnetic targeting and with the same drug dosage of free tPA[29].In addition,thrombolytic drugs can also be used as the coating material.Surfacefunctionalized IONPs by uPA coating displayed about 50% increase in thrombolysis capacity than pure uPA solution, and removed nearly complete thrombus in a microfluidic channel under the control of oscillating magnetic field [71]. Various coating materials have been developed for IONPs so far,which makes IONPs excellent drug carriers with the advantages of good biocompability and specific localization.These modified IONPs hold great potential in clinical thrombolysis therapy.
In addition to iron oxide,other metallic nanoparticle such as nickel magnetic nanoparticles can also be used as drug carriers. Tadayon et al. synthesized SiO2coated Cu0.5Ni0.5Fe2O4NPs for immobilizing SK and tPA with a high drug loading efficiency and activity retention,which showed reduced bleeding complication[72].Later,they synthesized biological nanoparticles (CuNPs) by Streptococcus equi, and developed a targeted therapeutic delivery system with the two thrombolytic agents immobilized to CuNPs. The magnet-guided SiO2-CuNP-tPASK showed effective thrombolysis with no statistically significant difference form chemical synthesis in the rat embolism model.The biosynthesis process possesses benefits including low energy requirement,well dispersed in aqueous solutions,nontoxicity, ect, and shows potential as targeted carrier for thrombolytics delivery[73].
Moreover,encapsulation of thrombolytic drugs and IONPs in biodegradable vehicles can also be beneficial for targeted thrombolysis. Torno et al. demonstrated the improved lysis efficiency of magnetically-guided, ultrasound-responsive and non-medicated magnetic spheres by fabricating magnetic carriers with FDA-approved polymer poly(lactic acid)-poly(ethylene glycol) (PLA-PEG) [74]. Later, they developed medicated microspheres by co-encapsulating tPA and IONPs into PLA-PEG microcarriers to protect tPA during systemic circulation while concentrating the thrombolytics at the thrombus site and activating its release focally. External magnetic fields were employed to concentrate the carriers at the target site, and ultrasound was used to enhance permeation of thrombolytics into the inner of thrombus [75].Likewise,liposomes loaded with thrombolytic drugs and IONPs formed magnetic liposomes, and showed thermosensitive property.The induction heating of IONPs in an alternating magnetic field was proved to trigger drug release after magnetic guidance of thermosensitive magnetic liposomes to the thrombus site[76].
Magnetically guided carriers have been studied extensively due to their benefits of controlled drug release,reduced drug toxicity and feasibility of application. The delivery efficiency of thrombolytic agents in magnetic carriers have been evaluated in animal models, showing higher thrombolytic activity and lower hemorrhage risk. However, their application in clinical thrombolysis requires thorough research on preparation process and therapeutic regimen.Moreover,their systemic clearance and potential long term toxicity remain obstacles to clinical application[77].
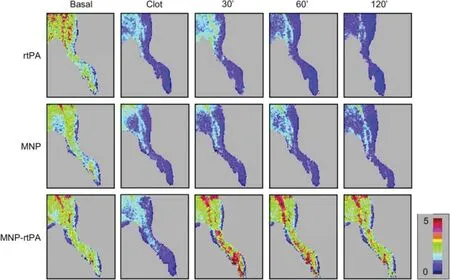
Fig.4-MNP-rtPA improved tissue perfusion in a rat embolic model.Hind limb skin tissue perfusion of the rat was measured by a laser Doppler perfusion imager.After clot lodging into the left iliac artery,rtPA(0.2 mg/kg;0.27 U/kg),rtPA(0.2 mg/kg;0.22 U/kg)covalently bound to PAA-coated MNP or equivalent MNP(2.5 mg/kg)was administered from the right iliac arterial 5 min after introducing the clot.(Reproduced with permission from[27].Copyright 2009 Elsevier B.V.)
3.3. Antibody-binding targeting
Direct conjugation of antibodies to thrombolytic drugs can achieve obvious targeting.However,with the emergence of recombinant thrombolytics which possess fibrin binding sites comparable to high-affinity antibodies, the development of antibody thrombolytic conjugates has not continued.Instead,antibody-directed thrombolytic carriers have been extensively explored to provide both targeting ability and protection from systemic clearance[78].
Antifibrin antibody modified tPA NPs showed merely slightly less activity than free tPA in vitro fibrinolysis assay,whereas displayed over 3 time less tPA activity than free tPA in the absence of thrombus.This property is critical for reducing systemic plasmin,and thus lowering the risk of hemorrhage[79]. Another fibrin-specific uPA formulation coupled by antifibrin antibodies showed improved thrombolysis in vitro,and retain high binding affinity in intravenous administration in vivo,offering a high vascular constraint and low dose thrombolysis approach[80].
Platelet membrane glycoprotein IIb/IIIa (GPIIb/IIIa) receptors participate in the pathway of platelet aggregation and thrombosis. So antibodies against GPIIb/IIIa receptors allow specific binding to thrombus. Abciximab (a Fab fragment of a humanized monoclonal antibody against GPIIb/IIIa) bound microbubbles (MBs) induced thrombolysis in the absence of thrombolytics, and showed superior thrombolysis effect to non-specific MBs in rat thrombotic occlusion models,indicating the effectiveness of ligand targeting[81].
However, anti-GP IIb/IIIa antibodies that target all the receptors regardless of the activation status would cause excess bleeding. To enhance the specificity, conformation-specific anti-GP IIb/IIIa single-chain antibodies (scFvs) which bound specifically to a Ligand-Induced Binding Site (LIBS) on activated GP IIb/IIIa have been generated [30-32]. A scFv fused,thrombin-activatable,low-molecular-weight pro-uPA was activated specifically by thrombin, and prevented the development of nascent thrombus in murine injury models. Such PA construct can be applied for thromboprophylactic agents[82].By conjugating scFv to MBs,a noninvasive approach was demonstrated for sensitive detection of thrombus and screening the therapeutic outcomes of thrombolysis in the carotid artery of mice[32].Besides,fusing scFvs to single-chain urokinase plasminogen activators (scuPA) provided a targeted fibrinolytic drug which was capable of targeting and inhibiting thrombus formation effectively at low systemic concentration.Its high specificity towards activated platelets potentially solve the problem of bleeding complications in thrombolysis therapy [83]. Furthermore, dual coupling of both scuPA and scFv to microbubbles demonstrated an innovative theranostic approach with scFvs to visualize thrombosis and scuPA to lyse thrombi.The thrombolytic ability of this targeted theranostic microbubbles (TT-MBs) was compared with platelet-targeted ultrasound contrast (LIBS-MBs) plus saline or high/low dose of commercial uPA by measuring thrombus size (Fig. 5). The results showed that TT-MBs and LIBS-MB plus a high dose of commercial uPA had the highest thrombolytic ability, while TT-MBs minimized bleeding time over LIBS-MB plus a high dose of commercial uPA .This technology concurrently used activated-platelet-targeted and scuPA-loaded MBs, and had potential for developing bleeding-free thrombolysis therapy with rapid diagnosis[84].
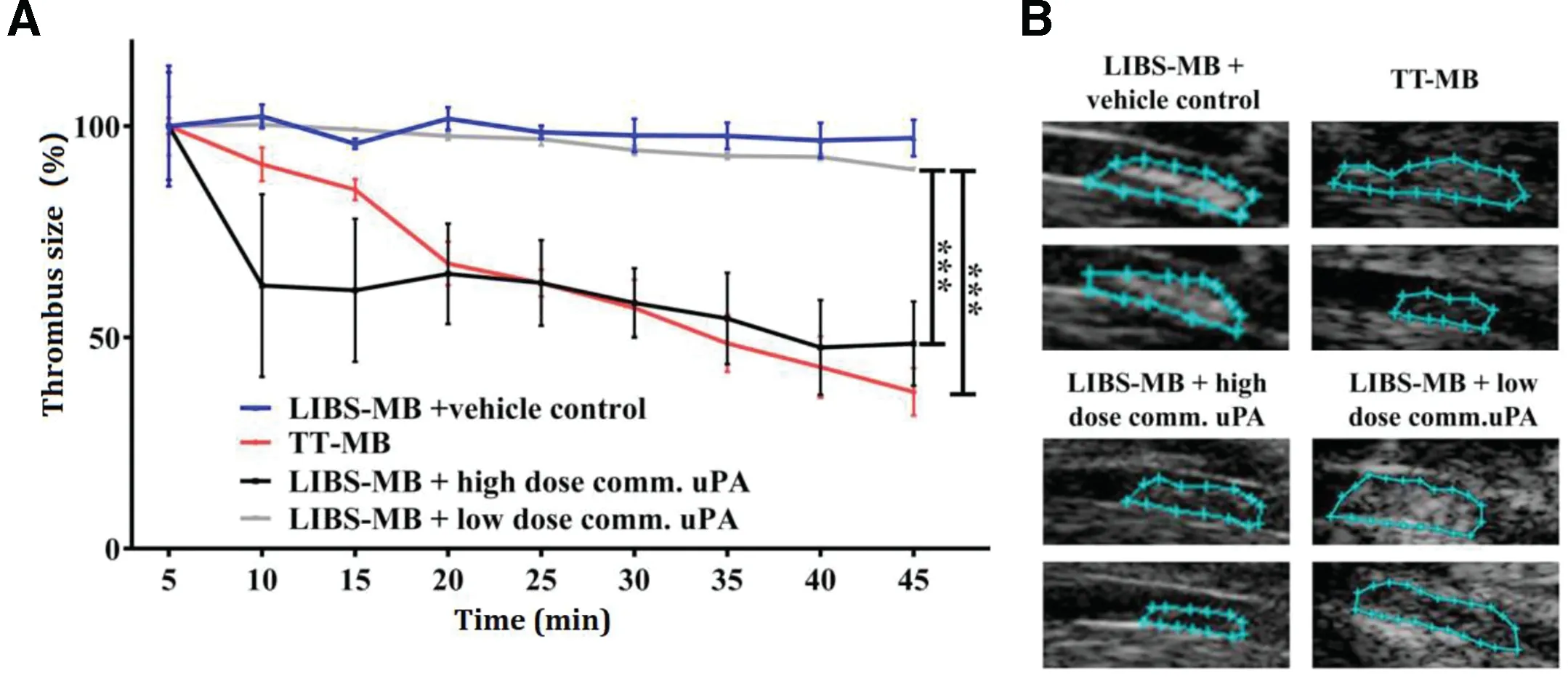
Fig.5-Monitoring of thrombolysis via molecular ultrasound imaging showed a theranostic effect and a reduction of thrombus size after the injection of TT-MB.A.A reduction of thrombus size was observed for animals administered with LIBS-MB and high dose of commercial uPA at 500 U/g BW(black line and B)as compared to LIBS-MB and saline(blue line and C)as vehicle control.A reduction of thrombus size was also observed with TT-MB(red line and D)as compared to LIBS-MB and low dose of commercial uPA at 75 U/g BW(light grey line and E).Baseline area was set to 100%and areas were calculated every 5 min for 45 min.Thrombus size was traced and calculated using VisualSonics software.Treatment groups were compared by use of repeated measures ANOVA over time with Bonferroni post tests at each time point(Mean%±SEM;**P <0.01, ***P <0.001,n ≥3 each).(Reproduced with permission from[84].Copyright 2016 Ivyspring International Publisher.)
3.4. Peptide-binding targeting
RGD sequence is a frequently used ligand in thrombus targeting for its selective recognition and binding site of platelet membrane GPIIb/IIIa receptor. RGD peptides conjugated thrombolytics carriers displayed target sensitive properties,and revealed the improved thrombolytic activity in vitro[33-35]. Thrombus-targeted tPA-MBs carrying RGD motifs under ultrasound trigger had higher thrombolysis with lower dose than non targeted tPA-MBs [33]. And RGD peptides modified SK-liposomes were found to decrease the clot lysis time,and increase the total clot dissolution[34].
In vivo studies also demonstrated promising results in specific drug delivery,and thus were able to reduce drug dose and decrease hemorrhagic risk[61,85-88].The thrombus-targeted tPA-MBs and SK-liposomes mentioned above further showed remarkable thrombolysis efficacy in vivo with the benefits of much reduced drug dosage [85,86,89].It’s worth noticing that the combination of ultrasound enhanced thrombolysis with active targeting exhibited satisfactory thrombolytic efficacy[61,88].Moreover,the combination can provide both the diagnosis and treatment of thromboembolic diseases[87].
To improve the binding rates to active platelets,cyclic RGD(cRGD) ligands which are conformationally constrained, and provide high affinity and selectivity for GPIIb/IIIa have been introduced to drug carries [90-92]. In vitro and in vivo experiments showed that cRGD modified liposomes bound activated platelets remarkably higher than linear RGD modified liposomes [91]. In another recent study, cRGD modified uPAliposomes was demonstrated to reduce uPA dosage by 75%while maintaining the equal thrombolytic effect as free uPA in the mouse thrombus model[92].
In addition to RGD peptides,other targeting peptides have also been introduced to direct against thrombus components[20,93-95].The combination of different targeting ligands can achieve higher selectivity towards activated thrombus. As P-selectin also mediates inter-platelet interactions, peptide targeted to P-selectin combined with RGD peptide showed enhanced site-specificity and stability of binding compared to single receptor targeting under dynamic flow,and thus can assure effective drug delivery in the vascular lumen [94].Based on this multivalent targeting strategy,applying secreted phospholipase A2(produced from activated platelets in thrombotic milieu) cleavable glycerophospholipids as lipid vesicles can permit selective thrombolytics delivery as well as thrombusselective SK release[20].
Prodrug-based delivery systems of thrombolytics modified with targeting peptide demonstrated effective thrombolysis while significantly reduced hemorrhagic risk due to its localized drug release. Absar et al. have developed a series of tPA prodrug constructs by camouflaging tPA with different shielding molecules and applying different triggering agents.Conjugating tPA with low-molecular weight heparin followed by complexation with albumin-protamine conjugate can achieve remarkable supressed enzymatic activity, which was totally recovered upon heparin trigger [96].To avoid any potential adverse effects of low-molecular weight heparin,relatively inert negatively charged Polyglutamate was used to synthesize oligoanion-modified tPA, which demonstrated good feasibility [97]. In consideration of the extent of accumulation of the prodrug construct on the thrombus ahead of heparin addition, they engineered a covert construct of t-PA which was conjugated with albumin by a peptide linker and was cleavable by thrombin.This strategy demonstrated equivalent thrombolytic activity with significantly reduced hemorrhagic risk in a rat thrombosis model[98].
The aforementioned ligand binding directed targeting strategies including antibody and peptide-binding targeting perform better therapeutic outcomes in vitro and in vivo experiments than conventional PA solutions.On the other hand,the intrinsic binding ability of PA to fibrin can also be potentially used to target thrombus. tPA loaded liposomes [99] and rtPA loaded NPs[100]can serve as self-targeted agents without further modifications.But their targeting ability hasn’t been compared with ligand modified formulations.It is notable that the current ligands used in artificial DDSs may suffer from limited specificity.Besides,the high cost and potential immunogenicity of antibody hinder more versatile uses. These drawbacks prevent active targeting from being a stand-alone targeting method. Therefore, researchers tend to apply ligand binding targeting in conjunction with other targeting methods such as ultrasound triggered drug delivery to target very specific locations[61,85-88].
4. Biological thrombolytic DDSs
Biological DDSs provide advantages of biocompatibility and natural mechanisms for homing.However,studies on biological thrombolytic DDSs are few compared to artificial ones.And almost all the biological thrombolytic DDSs refer to red blood cells (RBCs),which may be attributed to RBC being the most abundant,long circulating cells in body and representing promising drug carriers for biological DDSs[101-105].
RBCs based thrombolysis therapy are mostly performed by one team.Muzykantov et al.initially developed RBC-SK complex by coupling SK to RBCs using avidin-biotin interaction,which provided local lysis of a fibrin clot in vitro [106]. Later,they designed RBC-tPA complex by using streptavidin-biotin as a crosslinker.This tPA carrying RBCs altered the fibrinolytic profile of tPA,allowing dissolving nascent clots from within as well as exerting lowest effects on preexisting thrombus or extravascular tissue.RBC-tPA possessed a 17 time improvement in lysing nascent versus preexisting clots in vitro.In vivo venous thrombus and arterial thrombus were dissolved only when the RBC-tPA were administrated ahead of thrombosis,but not after.RBC-tPA was found 10 and 20 times more specific than free tPA in lysing nascent over preexisting pulmonary and arterial thrombus,respectively[107].The large size of RBCs precludes them from entering and lysing preexisting clots,which makes RBCs desired carriers for thromboprophylaxis rather than thrombolysis. The drug delivery of RBCs is driven by passive inclusion of drug-loaded RBC into nascent thrombus rather than fibrin affinity, offering their accelerated dissolution from within.This feature helps to reduce morbidity and mortality in thromboprophylaxis conditions where the risk of both thrombosis and bleeding is high. In a cerebral thromboemboli model,preinjecting RBC-tPA provided rapid nascent thrombus lysis,rapid and durable reperfusion without aggravating brain damage or causing postthrombotic hemorrhage.As a contrast,preinjection of tPA couldn’t achieve such therapeutic effects even at a 10 time higher dosage, which increased death [108]. Further, by fusing a mutant tPA with a scFv which has specificity for glycophorin A on RBCs,circulating RBCs were endowed with high fibrinolytic activity without altering their behaviors. The development of a recombinant PA variant which bound to circulating RBCs provided thromboprophylaxis by using a clinically relevant approach[109].
RBCs conjugated PA can also be applied as prodrug.Single chain uPA (scuPA) and its receptor,suPAR act as an excellent candidate pairing in prodrug construct since plasma downregulated the activity of scuPA-suPAR complex while fibrin restored it.By coupling scuPA to RBCs-suPAR,a fibrin-dependent prodrug was composed for safe and effective thromboprophylaxis[110].However,practical limitations concerned with this approach such as danger of blood-transmitted infections and limited drug storage life are recognized.To solve this concern,a more practical alternative for clinical administration has been designed.RBCs were endowed with scuPA binding ability by injecting scFv fused scuPA(scFv/uPA-T)which can be activated specifically by thrombin and recognize circulating RBC.In vitro results showed that scFv/uPA-T bound selectively to RBCs without changing their biological activity and retained its zymogenic properties until transformed by thrombin.And a single intravenous injection of scFv/uPA-T demonstrated effective prophylaxis against venous and arterial thrombosis for up to 24 h [111]. Such strategy is also efficient for prevention of intravascular microclot formation after experimental subarachnoid hemorrhage[112].
Pharmacokinetic study showed that conjugation to RBC significantly prolonged the circulation time of PA [113]. The blood clearance differed between RBC-tPA and RBC-rtPA.RBCtPA had a faster initial blood clearance than RBC-rtPA, while RBC-tPA got a higher lysis effect than RBC-rPA. This was explained by suppressed rtPA’s ability to be activated by fibrin in RBC-rtPA complex and unchanged fibrin activation of tPA in RBC-tPA complex. Besides, high fibrin affinity of RBC-tPA endured hemodynamic stress,which improved fibrinolysis of RBC-tPA over RBC-rPA both in vitro and in vivo [114]. Therefore, the functional profile of RBC-PA is affected by pharmacokinetic features of carrier RBC such as prolonged circulation time,interior PA characteristics including clearance rate and adhesion to clots,and alternations exerted by coupling to RBC, for example, altered resistance to inhibitors. These factors should be taken into consideration when exploiting rational design of RBC-PA.
Using biological DDSs to target thrombus has been developed since over 30 years ago [107]. But recent studies on this strategy are rare,and lack of updated developments.This may be due to the booming progress in artificial DDSs on thrombolytics, the difficulties in manipulating cells, and cell carriers’inability to lyse preexisting thrombus. However, the extracellular vesicle [115] derived from related cells or cell membrane modified vectors [116] as drug carries may provide benefits of non-immunogenicity, intrinsic targeting and sufficient permeation into thrombus, which may provide a more promising strategy for targeted thrombolysis therapy.Moreover,artificial thrombolytic DDSs mimicking specific properties of cell[117]or cell derived fragments[20]have been developed for efficient thrombolysis. These artificial thrombolytic DDSs may be easier to manipulated than biological ones.But a conclusion on which DDS outperforms the others in therapeutic outcomes hasn’t been reached yet.
5. Future prospect
Targeted thrombolysis therapy exhibits promising results in improving thrombolytic efficiency as well as reducing the side effects.Various carriers are used for drug delivery,and the targeting strategies can vary from ligand binding mediated targeting by surface modification or inherent homing ability of biological DDSs to physical targeting by external stimulus.Of all the strategies,ultrasound triggered targeting seems more feasible for clinical application. The equipment needed for sonothrombolysis is highly portable,and is already present in most hospitals.It’s worth noticing that sonothrombolysis has demonstrated promising results in improving drug action in clinical trials. The addition of contrast agents demonstrated increased recanalization rates in embolism region, and thus performed better therapeutic outcomes without safety issue.Sonothrombolysis possesses the advantages of being noninvasive, highly portable and efficient. However, mechanical stress induced by ultrasound might increase the risk of hemorrhagic. There are many studies on optimizing ultrasound parameters, but the results lack of consistency. Further research is needed to determine the best ultrasound parameters for facilitating thrombus dissolution, which is important for designing safe sonothrombolysis system. Besides, like RBCs,the large size of contrast agents may impede them from penetrating into the clot.Clinical sonothrombolysis trials mainly focus on MBs as contrast agents in enhancing thrombolysis,but MBs as drug carriers are rare.The drug loading efficiency,stability and targeting ability of drug-loaded MBs require further evaluation.
Current clinical thrombolysis trials are almost designed on sonothrombolysis, and magnetic field guided and ligand binding directed thrombolysis studies only involve with animal experiments.As magnetic field induced the migration of magnetic carriers,the management of magnetic field should be optimized.Likewise,the drug loading capability and body tolerance of magnetic carriers need to be assessed. To improve the specificity of ligand binding directed targeting,various targeting moieties including conformation-specific antibodies and peptides have been developed.However,the specificity, cost and possible immunogenicity of these ligands are the main challenges for further development.
Extracellular vesicles like exosomes or cell membrane coated nano carriers may provide a more favorable size as well as intrinsic targeting property.Their potential treatment efficacy lack of investigation, and may be a new research direction for targeted thrombolysis. Ultrasound-guided and magnetic resonance imaging are being developed for simultaneously thrombolysis and visualizing the antithrombotic surface, which is beneficial for minimizing risks of bleeding.Such theranostic approaches are helpful for managing therapeutic outcomes of thrombolysis. Further study is needed to promote the translation from experiments to clinical application.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81620108028) and National Key R&D Program of China(2017YFE0102200).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Cellulose based polymers in development of amorphous solid dispersions
- Phyto-phospholipid complexes(phytosomes):A novel strategy to improve the bioavailability of active constituents
- MSNCs and MgO-MSNCs as drug delivery systems to control the adsorption kinetics and release rate of indometacin
- Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis
- Percutaneous absorption and brain distribution facilitation of borneol on tetramethylpyrazine in a microemulsion-based transdermal therapeutic system
- Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule(Avodart®):Effect of γ-cyclodextrin and solubilizers
