Exosomes in esophageal cancer: A review on tumorigenesis,diagnosis and therapeutic potential
2019-08-14LinLinSuXiaoJingChangHuanDiZhouLiuBingHouXiaoYingXue
Lin-Lin Su, Xiao-Jing Chang, Huan-Di Zhou, Liu-Bing Hou, Xiao-Ying Xue
Abstract
Key words: Exosome; Esophageal cancer; Tumorigenesis; Biomarker; Therapeutic potential
INTRODUCTION
Exosomes were first discovered by Johnstone and Pan while studying the transformation from reticulocyte to mature red blood cells in 1987. Originally,exosomes were considered a form of waste discharge in the process of reticulocytes differentiating into mature red blood cells. Interestingly, further studies found that exosomes from intracellular multivesicular bodies that fuse with cell membranes and then released into the extracellular matrix are membrane nanoscale vesicles with a diameter of 50-150 nm and have a lipid bilayer membrane structure[1]. Moreover,studies have found that exosomes can be secreted from a variety of cells including red blood cells, T cells, B cells, dendritic cells and tumor cells[2-5]and are widely distributed in various body fluids, such as blood, urine, saliva and milk.
Exosomes contain various types of biologically active substances, including proteins, lipids, DNA fragments and RNA, such as microRNA (miR), circular RNAs(circRNAs), long non-coding RNA and mRNA[6]. Researchers found that these biologically active substances contained within the exosomes may participate in the immune response, antigen presentation, intercellular communication, transport of proteins and RNA and many other physiological processes[7-9]. It suggested that exosomes are a crucial intercellular organelle and communication media. Thus,exosomes may play a pivotal role in the diagnosis and therapy of various diseases including tumors.
There were 455800 newly diagnosed esophageal cancer (EC) patients and 400200 deaths worldwide in 2012[10]. Just in China, there were approximately 477900 new cases and 375000 deaths in 2015[11]. Recent studies have shown that exosomes secreted from various cells including tumor cells, irradiated T cells[12]or tumor associated fibroblasts[13]and exosomal contents may play important roles in EC development.Therefore, exosomes may be of value as a diagnostic/prognostic tool and as a therapeutic target in EC (Figure 1).
PubMed, MEDLINE advanced search builder and Geenmedical was used for this review’s literature search. The terms searched were “exosome” and “esophageal cancer,” and the time period was restricted from 2008 to 2018. Then we further screened the retrieved articles according to (1) the formation and regulation of exosomes and (2) the research progress of exosomes in EC. Cross references were also screened for papers relevant to this paper. Here we reviewed relevant articles from the last 10 years with the aim of summarizing the function of exosomes in tumorigenesis, diagnosis and treatment of EC (Table 1).
EXOSOME AND ESOPHAGEAL CANCER TUMORIGENESIS
Recent studies found that exosomes derived from many cancer cell types, including glioblastoma[14], tongue cancer[15], thyroid carcinoma[15], breast cancer[17], EC[18],etc, may play a crucial part in the course of tumor formation and cancer metastasis by transfer of exo-miRNA, exo-circRNA, exo-DNA and exo-protein.
Promotion of tumor proliferation
Researchers have found that exosomes are involved in tumor proliferation by transferring exo-microRNA. MiR-93-5p[18]can be transferred between EC9706 cells by exosomes and may downregulate the expression of p21 and Cyclin D1 through the PTEN/PI3K/Akt pathway to promote the proliferation of EC cells. Keet al[19]found that the overexpression of miR-25 and miR-210 in EV-Co-Cultured (EV: the extracellular vesicle secreted from esophageal adenocarcinoma (EA) cells) gastroids decreased the mRNA levels of PTEN and AIFM3, respectively, which are known tumor suppressor genes[20,21].
Promotion of invasion and metastasis of tumors

Figure 1 The role of exosomes in esophageal cancer. CTLs: Cytotoxic T lymphocytes; MVB: Intracellular multivesicular body. Red line and cross bar: Inhibit;Green arrow: Promote.
Exosomes and epithelial-mesenchymal transition:A large number of studies have indicated that epithelial-mesenchymal transition (EMT) is closely related to cancer metastasis[22-26]. Recent studies found that exosomes may be involved in the course of tumor metastasis by EMT. Minet al[12]found that exosomes derived from irradiated T cells showed the potential role of promoting metastasis of TE13 cellsin vitro, possibly by promoting EMT through regulating β-catenin and the NF-κB/snail pathway. A recent study demonstrated that overexpression of exosomal circPRMT5 in urothelial carcinoma of the bladder tissues could also promote the EMT processin vivoandin vitrothrough the miR-30c sponge[27]. However, exosomal circRNA has not been reported in the pathogenesis of EC. Thus, further studies on the relationship of exocircRNA and EMT in tumors is needed.
MiR-21:A study has shown a high expression of miR-21 in EC cells and the exosomes secreted by them, and the expression of miR-21 is higher in exosomes than in their donor cells[28,29]. Exosome-shuttling miR-21 overexpression from parental cells could target programmed cell death 4 protein thus activating its downstream c-Jun Nterminal kinase signaling pathway and distinctly promote the migration and invasion of recipient cells.
Stathmin-1:Stathmin-1 is a microtubule-destabilizing cytosolic phosphoprotein that plays an important role in tumor cell proliferation and migration and may be regulated by miR-34a[30,31], miR-223[32]and miR-193b[33]. It is associated with cancer metastasis and poor prognosis in osteosarcoma[31,34], prostate cancer[35,36], head and neck squamous cell carcinoma[37], hepatocellular carcinoma[32], colorectal cancer[33,38],gallbladder carcinoma[39]and non-small-cell lung cancer[40]. In esophageal squamous cell carcinoma (ESCC), stathmin-1 is also vital for ESCC invasiveness and predicts a poor prognosis[41]. Knockdown stathmin-1 expression enhanced the sensitivity of ESCC cell line to docetaxel and radiation[42]. Stathmin-1 can be delivered by exosomes and then promote the division, growth, migration, and invasion of esophageal squamous cells[43].
Others:In addition, studies demonstrated that the downregulation of theECRG4gene in serum exosomes of oral squamous cell carcinoma patients was closely related to tumor growthin vitroandin vivo[44]. MiR-221-3p from cervical squamous cell carcinoma exosomes transferred into human lymphatic endothelial cells to accelerate lymphangiogenesis and lymphatic metastasisviadownregulating vasohibin-1[45], but their relationship in EC requires further research.
EXOSOMES AS BIOMARKERS FOR EARLY DIAGNOSIS AND PROGNOSTIC PREDICTION OF ESOPHAGEAL CANCER
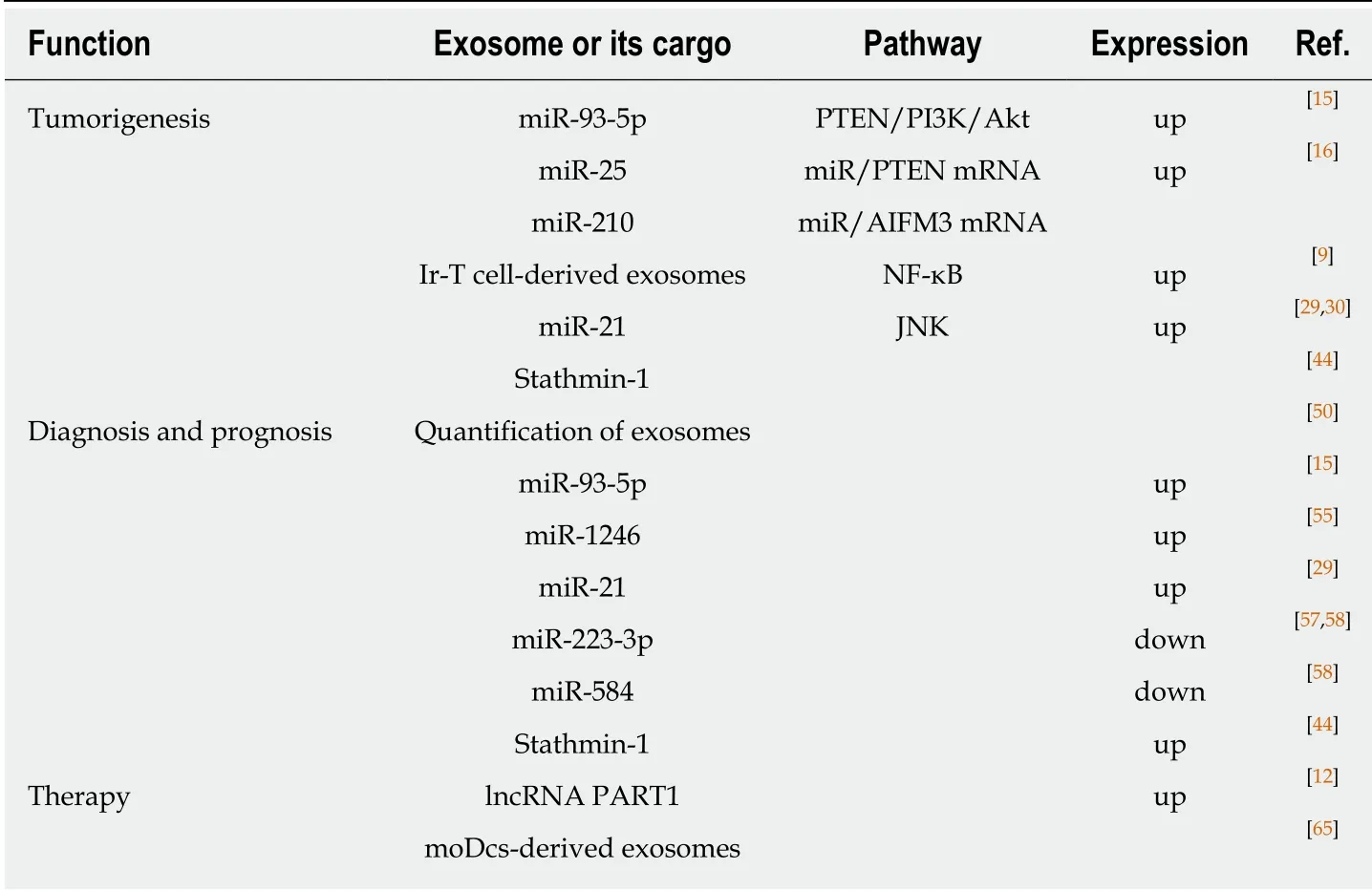
Table 1 The function of exosomes in esophageal cancer
Studies have confirmed that the number or contents (including RNA and proteins) of exosomes in the circulating blood are of use as a biomarker for cancers, such as lung cancer[46], prostate cancer[47], colorectal cancer[48], as well as EC[18,28,43,49].
Exosome quantification
Matsumotoet al[49]established mouse subcutaneous tumor models by injecting human ESCC cell line (TE2-CD63-GFP) and detected tumor-derived exosomes from the plasma in the mouse model. They also recruited 66 ESCC patients and 20 nonmalignant patients to measure exosomes separated from 100 µL of each individual’s plasma and analyzed the relationship between the prognosis of ESCC cases and the plasma exosome amount. Results showed that ESCC patients had more exosomes than in non-malignant patients. The 3-year overall survival rate of patients with higher levels of exosomes was 79.2%. However, the 3-year overall survival rate of patients with lower levels of exosomes was 47.2%. The molecular mechanism and the major donor cell require further research.
Exosome microRNA
MiRNAs, a large family of small, noncoding RNAs, are enriched in exosomes and could regulate the expression of their target genes. MiRNAs are relatively stable,which can in part represent the level of miRNAs in its donor cell. Numerous studies have indicated that aberrant expression levels of exosomal miRNAs are closely related to the onset of multiple diseases, including cancer. MiR-21, miR-25, miR-93, miR-192,and miR-210 are widely studied oncogenic miRNAs, and have been studied as biomarkers for some kinds of human cancers[50,51].
MiR-93-5p:MicroRNA-93-5p can be transferred between EC9706 cells by exosomes and promote the proliferation of recipient EC9706 cells. Liuet al[18]also found that the expression of miR-93-5p was statistically different (P= 0.035) between EC patients and healthy participants (the former being 1.39 times higher than the latter). The upregulation of plasma miR-93-5p in ESCC patients increased the risk of EC. The Cancer Genome Atlas data analysis also showed that miR-93-5p expression differs in tissues and is associated with patient survival. Therefore, miR-93-5p may be a plasma biomarker for the diagnosis and prognosis of EC.
MiR-1246:MiR-1246 is another cancer associated miRNA dysregulated in many malignant tumors[52,53]. Takeshitaet al[54]used ESCC cell lines (including TE1, TE2, TE4,TE6 and TE11) to evaluate the diagnostic and prognostic values of the exo-miR-1246 and estimated the miR-1246 level in peripheral blood of ESCC patients. In the serum samples of ESCC patients, the sensitivity and specificity of miR-1246 were 57.4% and 67.4%, respectively. The area under the curve of receiver operating characteristic curve was 0.665 when setting the optimal cut-off value to 1.15 for squamous cell carcinoma (SCC)-Ag. Furthermore, the miR-1246 expression level of the lymph nodes in adjacent stations was apparently higher than that of the distant lymph nodes.
MiR-21:One microarray data analysis[28]showed that a total of 15 miRNAs were upregulated in the plasma of ESCC patients compared with healthy control participants. They were hsa-miR-16-5p, hsa-miR-130a-3p, hsa-miR-15a-5p, hsa-miR-144-3p, hsa-miR-19b-3p, hsa-miR-5196-5p, hsa-miR-25a-3p, hsa-miR1914-3p, hsa-miR-93-5p, hsa-miR-107, hsa-miR-3911, hsa-miR-21-5p, hsa-let-7d-3p, hsa-let7i-5p and hsamiR-1290. In contrast, four miRNAs including hsa-miR-1238-3p, hsa-miR-6069, hsamiR-191-3p, hsa-miR-4665-3p and hsa-miR-937-5p were downregulated. One casecontrol study on the correlation between exosome-shuttling miR-21 and EC morbidity indicated that the relative expression of miR-21 was 2.95 times higher in EC patient plasma compared to healthy controls. Furthermore, conditional logistic regression analysis showed that the higher miR-21 was expressed, the higher the EC incidence risk was (odds ratio: 1.107; 95% confidence interval: 1.012-1.21;P= 0.026). The area under the curve value was 0.60 to show the diagnostic value of exosome-shuttling miR-21 in ESCC patients.
MiR-223-3p:Exo-miRNAs are also important biomarkers for EA diagnosis and progression[55]. Warnecke-Eberzet al[56]first isolated exosomes from serum of EA patients and compared exosomal miRNA profiles in matching primary tumors with adjacent tissues. Results showed that a total of eight miRNAs (miR-126-5p, miR-146a-5p, miR-192-5p, miR-196b-5p, miR-223-3p, miR-223-5p, miR-409-3p and miR-483-5p)were significantly overexpressed. Conversely, ten miRNAs (miR-22-3p, miR-23b-5p,miR-27b-3p, miR-149-5p, miR-203-5p, miR-224-5p, miR-452-5p, miR-671-3p, miR-944-5p and miR-1201-5p) were significantly downregulated. They also detected that miR-223-3p was overexpressed in T2-staged adenocarcinoma patients and was higher than that in T3 tumors. There was no statistical difference in the overexpression of miR-223-5p and miR-483-5p in EA and ESCC. This result is consistent with the research of Zhouet al[57].
MiR-584:A four-stage study[57]including screening, training, testing and validating identified that miR-106a, miR-18a, miR-20b, miR-486-5p and miR-584 were upregulated, but miR-223-3p was downregulated in the plasma of patients with ESCC. MiR-584 was also overexpressed in ESCC tissues. This result is consistent with the data in The Cancer Genome Atlas database. Furthermore, exosome miR-584 in plasma was consistently dysregulated. Therefore, miR-584 could play an important role in the early diagnosis of ESCC.
Exosome proteins
Stathmin-1 was abundant in exosomes and could enter peripheral blood loaded by exosomes[43]. Yanet al[43]found that the serum levels of stathmin-1 in ESCC patients with lymph node metastasis were significantly higher compared with the cases without lymph node metastasis. As the stage increased, its sensitivity improved accordingly. They also measured the serum levels of stathmin-1 in patients with various cancers. When setting the diagnostic critical value to 4.47ng/mL, the positive rates of stathmin-1 were 81.0% (295/364) for ESCC, 68.8% (33/48) for head and neck squamous cell carcinoma, 71.2% (37/52) for lung squamous cell carcinoma, 50.9%(27/53) for lung adenocarcinoma, 27.1% (16/59) for gastric cancer, 43.9% (25/57) for colorectal cancer and 45.3% (24/53) for hepatocellular carcinoma. The area under the curve value of stathmin-1 for SCC was over 0.8, but it was lower than 0.7 for other types of cancer. Thus, stathmin-1 showed excellent diagnostic capability for SCC and may be a serology biomarker for SCC in the clinic, especially for ESCC.
EXOSOMES AND ESOPHAGEAL CANCER THERAPEUTIC POTENTIAL
Chemotherapy
Kanget al[15]demonstrated that long non-coding RNA PART1, as a competitive endogenous RNA, regulated and transported by exosomes, took part in the formation of drug resistance in ESCC patientsviathe STAT1-long non-coding RNA PART-Bcl-2 pathway in a gefitinib drug-resistant cell line. We hypothesize that the level of PART1 in exosomes is a promising diagnostic serological biomarker to evaluate the clinical benefits of gefitinib therapy in ESCC patients.
Radiation therapy
Radiotherapy is one of the main treatment methods for EC. Several studies have shown that exosomes derived from the exposed cells in the microenvironment could increase the curative effect of radiotherapy through the bystander effect and abscopal effect[58-61]. For example, exosomes derived from mesenchymal stem cells are the main determinant in enhancing radiation effects in the metastatic spread of melanoma cells.More often than not the reason might be that exosome-derived factors could be involved in the bystander and abscopal effects when combining radiotherapy with mesenchymal stem cells[62]. Recently, Brutonet al[63]presented an unusual case of the abscopal effect in EA with distant metastasis. After palliative radiation therapy to this patient, they observed a complete response of the irradiated tissues, the primary tumor and adjacent lymph nodes, as well as non-irradiated distant lymph nodes. One year later, the patient is still cancer-free. This case inspires the hope that advanced understanding of the abscopal effect of radiation therapy increases the effect of EA and improves patient outcomes.
Immunological therapy
Anin vitrostudy[64]revealed that immunotherapy, which was based on dendritic cells,could generate monocyte-derived dendritic cells (moDcs). The moDcs were powerful enough to induce cytotoxic T lymphocytes. Advanced study demonstrated that SART1 peptide-specific cytotoxic T lymphocytes could be induced by moDcs-derived exosomes, which had antigen presenting ability. Therefore, exosomes may play a potent part in the immunotherapy of EC.
Targeted therapy
Targeted therapy, also known as bio-missile, is a treatment for established cancercausing sites at the cellular and molecular level. As is well known, exosomes are cellderived natural nanometric vesicles, widely existing in body fluids and participate in the processes of many diseases, including cancer. There are many advantages of exosome-based nanometric vehicles: non-toxic, safe, non-immunogenic and programmable to have a strong delivery capacity and targeting specificity. Therefore,some scholars proposed to make exosomes a biological carrier to deliver anti-cancer drugs and genes for cancer stem cell-targeted therapy[65,66]. Jc Boseet al[66]found that delivery of anti-miR-21 could inhibit the overexpression of endogenous oncogenic miR-21 in tumor cells. Exosome-mediated anti-miR-21 delivery attenuates doxorubicin resistance in breast cancer cells. This makes the killing-cell efficiency of doxorubicin three times higher than that with doxorubicin alone. They also verified the biodistributionin vivo, tumor specific accumulation of anti-miR-21 loaded TEVGIONs (gold-iron oxide nanoparticles) and its theranostic property. In summary,exosomes are expected to be used as targeted therapeutic vectors for various cancers,including EC in the near future.
OTHER ROLES OF EXOSOME IN ESOPHAGEAL CANCER
A study[13]demonstrated that dysregulated miR-33a, miR-1, miR-326, miR-133a/b,miR-548h, miR-603, miR-141-5p, miR-429, miR-26a and miR-548k contained in exosomes from tumor associated fibroblasts next to EC cells were all involved with stromal remodeling including endocytosis, adhesion, gap and tight junction, focal adhesion, actin cytoskeleton regulation and ubiquitin-mediated proteolysis in tumor microenvironments through targeting different mRNA molecules.
CONCLUSION
Exosomes are another newly discovered vehicle of efficient cell communication in addition to classical intercellular communication (including signaling molecules,membrane binding, channels,etc.). It is an important component of the tumor microenvironment and plays a complex role in the progression and treatment of EC.
To date, the research on exosomes in EC has been successful, but some problems still need to be solved in the clinical diagnosis and treatment of EC. Although studies have shown that the number and molecular contents of exosomes in patients with EC may be helpful for the diagnosis, there are still some questions worth thinking about and require more efforts to solve. Which part of the blood or which content is more sensitive? Is the RNA and protein of the circulating exosomes more sensitive than the corresponding circulating RNA and protein? What should the diagnostic criteria related to exosomes be? Last but not least, the same miRNA plays the opposite role in different cancers. For example, miR-93-5p can promote the formation of EC, lacrimal adenoid cystic carcinoma[67,68]and hepatoma development[69], but it was proved that miR-93-5P may inhibit epithelial ovarian carcinoma tumorigenesis and progression by targeting Ras homolog gene family member C[70]. Therefore, we should consider comorbidities when analyzing the cause of tumor formation. In conclusion, we need to analyze the role and specific mechanisms of exosomes in the formation, monitoring and treatment of EC in a multifaceted way.
杂志排行
World Journal of Clinical Cases的其它文章
- Food additives can act as triggering factors in celiac disease:Current knowledge based on a critical review of the literature
- Optimal use of fielder XT guidewire enhances the success rate of chronic total occlusion percutaneous coronary intervention
- Association between ventricular repolarization variables and cardiac diastolic function: A cross-sectional study of a healthy Chinese population
- Non-invasive home lung impedance monitoring in early post-acute heart failure discharge: Three case reports
- Bilateral adrenocortical adenomas causing adrenocorticotropic hormone-independent Cushing’s syndrome: A case report and review of the literature
- Two case reports and literature review for hepatic epithelioid angiomyolipoma: Pitfall of misdiagnosis
