兼具抗癌和药物递送作用的硒掺杂羟基磷灰石微球
2018-08-01王艳华巫剑雄何文聪
王艳华 郝 颃 巫剑雄 姚 媛 覃 娜 何文聪
(1三峡大学医学院,宜昌 443002)
(2华中科技大学先进生物材料与组织工程研究中心,武汉 430074)
(3三峡大学第一临床医学院,宜昌 443002)
0 Introduction
The therapy of osteosarcoma is still far from satisfactory due to the high tumor relapse postoperatively.Hence,it is imperative to develop new therapeutic strategies.Hydroxyapatite(Ca10(PO4)6(OH)2,HA)is the main component of human bones and teeth[1],and has been widely applied in various biomedical fields such as bone repair and tissue engineering[2-3],anticancer therapy[4-5],and drug delivery[6-8].In recent decades,HA has been tremendously studied as bone matrix material and drug carriers,targeting the rapid bone repair,high bactericidal and antitumor effect[9-11].Benedetti used the hydroxyapatite nanocrystals to adsorb cisplatin,and got increased antitumor efficacy for HeLa,MCF-7 and HS-5 cell lines[12].In our previous study,the HASe nanocarrier,with high loading capacity and biologic activity,was applied to deliver lysozyme to the infectious sites[13].Except for HA-nanocarrier,HA-micron scale particles,because of the large specific surface area and high loading capacity,are also good candidates to deliver doxorubicin hydrochloride(DOX)to kill the cancerous cells[14].Kamitakahara prepared micrometer-sized composites of magnetic HA nanoparticles via hydrothermal process,and found these particles generate sufficient heat energy under an alternating magnetic field,thus killing bone tumor cells[10].Regardless of morphology and size,HA is of great potential served as carriers for drug delivery in the therapy of tumors[15-18].
Selenium (Se)is a trace mineral element with various biological effects,especially in the aspect of bone remodel and growth.Se also has strong anticancer activity on various types of carcinoma.Dual-functional selenium substituted hydroxyapatite nanoparticles(HASe)were synthesized in our lab[19].Good results were obtained in the treatment of osteosarcoma-related bone defect[20].Besides,it was reported that the doped selenium improves the loading efficacy of HA lattice for lysozyme,as well as changes the release fashion[13].For broadening the range of biomedical applications of HASe particles in our group,the hollow porous HASe microspheres were fabricated to enhance the anticancer effect on osteosarcoma.Many literatures provided various strategies to synthesize this type of microspheres[21-28].One of these methods is the template assisted route.For example,HA nanorod-assembled porous hollow polyhedra can be successfully prepared at room temperature with a self-sacrificing Ca(OH)2template,which shows increased bone regenerative capability for bone defect[23].Ma et al.prepared nanostructured porous hollow ellipsoidal capsules of HA using CaCO3templates[29].Ye et al.prepared HA hollow nanospheres using poloxamer (Pluronic P123)and polysorbate 60(Tween 60)micelles as templates[30].Qi et al.prepared nanosheet-assembled porous hollow microspheres ofHA using DNA molecules as templates by the hydrothermal method[31].Eventually,they all present good biologic effects.However,it remains a challenge to prepare micro-structured porous hollow element doped HA materials by the simple,rapid,and environmentally friendly template route,which presentsgood selectivity and high stability for the loaded drug.
HASe nanocarrier has been developed in our lab[19],and has showed good drug loading and release property,but the fine structure and function of HASe microcarrier has not been studied yet.In this work,the template-hydrothermal route was used to fabricate HASe microspheres by synthetic vaterite CaCO3as the sacrificial template.The as-prepared HASe microspheres were explored for potential application in drug-loading and controlled-release.Curcumin(Cur),a traditional Chinese medicine,was selected as a model drug,owing to the famous broad-spectrum anti-cancer properties on various type of cancers[32-35].The HASe-Cur delivery system shows high loading efficiency,sustainable slow-release,low blood toxicity and high anticancer effect on the growth of bone tumor cells.So,it is feasible to use the HASe microsphere as new microcarrier to deliver anticancer drugs,especially in the oncotherapy of osteosarcoma.
1 Experimental
1.1 Materials
Poly(styrene sulfonic acid)sodium salt(PSS,powder,Mr=70 000,Shanghai Jianglai Biotechnology Corporation,China),Cur (Shanxi Huike Chemical Reagent Corporation,China),sodium selenite(Na2SeO3,Micxy Reagent Company,China),calcium chloride(CaCl2,99%,National Medicine Chemical Reagent Company,China),sodium carbonate (Na2CO3,99.8%,ShanghaiHongguangChemicalReagentFactory,China),disodium hydrogen phosphate (Na2HPO4,99.99%,Wuhan Chemical Reagent Factory,China),sodium hydroxide(NaOH,99%,National Medicine Chemical Reagent Company,China),hydrochloric acid(HCl,ShanghaiSanying ChemicalReagentCo.,Ltd.),ethanol(≥99.7%,Hangzhou Gaojing Fine Chemical Industry Co.,Ltd.),and phosphate buffer saline(pH=7.2±0.2)were obtained commercially,and used as received without further purification.All other reagents of chemical grade or above were used as provided.
1.2 Preparation of CaCO3template
The CaCO3template was synthesized according to the strategies reported by the references[14,26].PSS was used as a regulation template of the reactant aqueous solution at room temperature.Briefly,the CaCl2solution(10 mL,0.2 mol·L-1)was poured into PSS (100 mL,10 mg·mL-1)solution under vigorous magnetic agitation,and then the Na2CO3solution (10 mL,0.2 mol·L-1)was added dropwise into the above mixed solution under vigorous magnetic stirring to obtain a white suspension.After the complete addition,the white suspension was agitated continuously at room temperature for 2 h,and then followed by centrifugation (4 000 r·min-1,15 min),washed three times with distilled water and absolute ethanol respectively,and finally dried at 60℃for 48 h.
1.3 Preparation of HASe microspheres from CaCO3template
The HASe microspheres were prepared by an anion-exchange process using the previously prepared CaCO3as the sacrificial template in a hydrothermal method.Specific operation referred to the reported literature with minor modification[14]. In a typical experiment for HASe10 sample,100 mL of the P and Se mixed solution contained 14.2 mg·mL-1Na2HPO4and 0.1 mg·mL-1Na2SeO3was added into 0.2 g of the CaCO3.The pH value of the resulting suspension was adjusted to 11.0 using 1 mol·L-1NaOH solution.The suspension was transferred into a Teflon-lined stainless steel autoclave,sealed,and heated by a hydrothermal method at 120℃for 45 min under 0.1 MPa of atmosphere pressure.At the end of hydrothermal reaction,the product was collected by centrifugation,washed three times with distilled water and absolute ethanol respectively,and dried at 60℃for 48 h.The powder was stored in vacuum for the further studies.For the fabrication of selenium-free HA and selenium-doped HASe30 microspheres,the clear Na2HPO4solution(14.2 mg·mL-1,100 mL),and 100 mL of the P and Se mixed solution contained 14.2 mg·mL-1Na2HPO4and 0.3 mg·mL-1Na2SeO3,were added into 0.2 g of the CaCO3powders,respectively.
1.4 Characterization
The morphology and size of the as-synthesized samples were characterized by field emission scanning electron microscopy(FE-SEM)(Nova nanoSEM 450,FEI,Netherlands),transmission electron microscopy(TEM)(Tecnai G220,FEI,Netherlands).The samples were ultrasonically dispersed in double distilled water and the size distribution of the samples was characterized by dynamic light scattering (DLS)(Nano-ZS90,Malvern,MA,USA).The crystalline phases of samples were examined with X-ray powder diffraction(XRD)(X′Pert PRO,PANalytical B.V.Co,Netherlands)using a monochromatic Cu Kα radiation(λ=0.154 06 nm,40 mA,40 kV)in the 2θ range of 10°~70°with a step size of 0.02°per second.Fourier transform infrared spectra(FTIR)(NEXUS,Thermo Electron,USA)were collected at room temperature by using the KBr squash technique,working in the wavenumber range of 4 000~400 cm-1with a resolution of 4 cm-1.Thermogravimetric analysis(TGA)was conducted on a thermo-gravimetric analyzer (Diamond,PerkinElmer Instruments,USA)heated to 800℃with a rate of 10℃·min-1under nitrogen atmosphere.
1.5 In vitro materials degradation
For the in vitro degradation experiment,50 mg samples were immersed in 10 mL phosphate buffered saline(PBS,pH 3.5 or 10.5),and constantly stirred in a shaking bath at 37℃.At pre-determinant time intervals,1 mL of the supernatant was withdrawn by centrifugation and replaced by the same volume of fresh medium.The concentration of calcium ions and selenium ions in the supernatant was measured by UV-Vis spectrophotometer(Nanoquant infinite M200 PRO,Tecan)at a wavelength of 612 and 365 nm,respectively,and calculated by using a standard calibration curve obtained under the same conditions.All the tests were carried out in triplicate and the average values were shown in this study.
1.6 Drug loading and in vitro drug release
The drug loading and in vitro drug release experiments were performed using Cur as a drug model.The procedure of Cur loading was performed as follows:50 mg of sample was blended into Cur solution(5 mL,1 mg·mL-1)over 72 h under gentle shaking at 37℃.Then,the Cur-loaded particles were collected by centrifugation,and then dried in vacuum at 60℃for 48 h.The amount of Cur in the supernatant was measured by UV-Vis spectrophotometer at a wavelength of 430 nm and calculated via a calibration curve.The amount of Cur encapsulated in the samples can be calculated by subtracting the amount in the supernatant from the initial dose.Therefore,the drug loading amount(DLA)in the final products was estimated by the following equation:DLA=(wa-ws)/wc×100%,where wais the amount of Cur added to the reaction,wsis the amount of Cur presented in the supernatant,wcis the amount of obtained final dried products.The drug entrapment efficiency(DEE)was calculated by using the equation:DEE=(wa-ws)/wa×100%.
For the in vitro drug release experiment,the Curloaded HA samples(5 mg)were immersed in 15 mL PBS with pH value equal to 7.2±0.2 under magnetic stirring at a constant rate of 150 r·min-1at 37 ℃.The sample(1 mL)was removed at given time intervals for UV-Vis analysis and replaced by the same volume of fresh medium.The adsorption data (OD430nm)were representative as the mean value of three parallel measurements.
1.7 Hemolysis test
For the hemolysis evaluation,the whole blood of forty healthy individuals was collected from the first college of clinical medical science of China Three Gorges University,and confirmed in the normalcy via blood-routine test.For the hemolysis analysis,10 mg of each sample was resolved in 10 mL PBS (pH 7.2),and dispersed by ultrasonic for 20 minutes,then 20 μL of the above sample was added into 1mL human whole blood,thereafter incubated in 37℃ for 15 minutes,and finally the changes of the whole blood was monitored via blood routine test by blood analyzer(Mindray BC-1800,China).And the increase of the hemoglobin quantity in the blood could reflect the strong hemolytic toxicity of the materials.
1.8 Cytotoxicity assay
The human osteosarcoma SOSP-9607 cell and normal macrophages RAW-264.7 cell were used to test the cytotoxic effect of these microspheres.The human osteosarcoma SOSP-9607 celllineswere provided from the 4th military medical university,Xi′an,Shaanxi,China.The macrophages RAW-264.7 cell lineswereprovided from theChineseMedicine Pharmacology Level 3 Laboratory of National Chinese Medicine Administrator atChina Three Gorges University.The cancerous SOSP-9607 cell and normal RAW-264.7 cells were respectively cultured in RPMI 1640 medium and high glucose DMEM medium,supplemented with 10%fetal bovine serum in the 5%(V/V)CO2,saturated humidity,37℃incubator(Sanyo CO2incubator,Japan).MTT test was used to monitor the cytotoxicity of the microspheres.Briefly,1×104cells were implanted into 96 well cell plate,and after 24 h of pasting,1 mg microspheres were added into the medium.At the pre-determined intervals,the MTT test was applied to evaluate the cytotoxicity of the biomaterials.Meanwhile,in order to observe the impact of the microspheres on the cell migration ability,5×103cells were implanted into twelve well cell plate,and 5 mg microspheres were added into the medium,and a line at the bottom of the plate was given by the head of a needle from 10 mL syringe.After cocultured for 48 h,the cell density and migration effect were monitored under a light microscope after dyed by the Wright′s-Gimsa.
1.9 Statistical analysis
The data was exhibited via mean±SD values of six independent experiments.For the comparison between two groups,two-tailed student′s t-test was used to calculate the statistical significance of the experimental results.Comparisons among three groups were tested using analysis of variance.*p<0.05 was considered to indicate significant difference,and**p<0.01 was considered to be greatly significant.
2 Results and discussion
2.1 Characterization of CaCO3template
The morphology ofthe synthesized vaterite CaCO3was observed from the SEM(Fig.1a).It showed a uniform spherical morphology with the average diameter of 1.0 μm.The XRD patterns ofthe synthesized vaterite CaCO3(Fig.1b)revealed that all the diffraction peaks accord well with the vaterite phase (PDF No.15-0020).The results show that the addition of PSS can greatly control the formation of vaterite phase. Literatures have provided good explanation that,PSS,a crystal growth regulator,has the ability of accelerating the transformation of CaCO3particles from calcite to vaterite[36].The functional groups in the synthesized vaterite CaCO3have been characterized by FTIR spectra.As shown in Fig.1c,two absorption bands located at 877 and 745 cm-1are the characteristic peaks of CO32-,which are assigned as carbonate out-of-plane deformation and in-plane deformation,respectively.The absorption band at 745 cm-1is the typical carbonate band deformation in vaterite CaCO3,which is consistent with the observation from XRD results(Fig.1b).The broad absorption bands located at 1 566 and 1 358 cm-1are the characteristic peaks of carbonyl groups (C-O bonds).Lots of sharp absorption bands aggregated in the range of 1 000~1 250 cm-1are attributed to PSS[14],implying that a small amount of PSS is adsorbed on the surface or trapped in the nanopores of the vaterite CaCO3.The wide band located at 3 435 cm-1corresponds to the adsorbed H2O molecules.The results of FTIR indicate that,the functional groups of CaCO3are visible in the synthesized sample, which could provide the carboxylic group serving as interactive site to initiate the transformation of the CaCO3to HA during the hydrothermal process.

Fig.1 Characterization of CaCO3template:(a)SEM image;(b)XRD pattern(c)FTIR spectrum
2.2 Characterization of HASe microspheres
The morphologies of different HASe microspheres were confirmed by FE-SEM (Fig.2).The single dispersed spherical structure with cauliflower surface was apparent in the FE-SEM images of all samples.For HA microsphere,the wall was constructed with flake-like hydroxyapatite crystals,which distributed randomly and seemed to grow out of the center.For HASe10 microsphere,short clavite-like surface was visible.Such structure consisted of HA crystalline which arranges densely.For HASe30 microsphere,many flowerlike bundles were clearly observed.The microspheres with nano-flowers structure clustered together,but they were facilely distinguished from each other.Hydroxyapatite crystals grew from the center of the ball to the periphery,and constructed the fuzz-like surface.Such wispy crystals aligned precisely,which made the sphere present an urchinlike appearance.

Fig.2 Morphology characterization of HA and HASe microspheres

Fig.3 TEM images of HA and HASe microspheres
As shown in Fig.3,the TEM images of HASe microspheres exhibit a translucent center and a black edge,indicating that the synthetic microsphere has hollow structure.The wall of HASe microsphere was constructed by subtle units of hydroxyapatite nanorods with an average length of 150 nm and an average width of 20 nm.There were also small nuances among the three samples.Long nanorods units were shown in the HA group,whereas slightly short nanorods units were obtained in the HASe10 group.Most intriguing,some stubs and small brokens were shown on the wall of the HASe30 group.The formation of the nanorods units has greatly enlarged the surface area of the sphere,and such large interface provides a niche for them tointeractwith drugmolecules,which is facilitated to increase the drug loading capacity.
The size of HASe microspheres were tested by dynamic light scattering (DLS),and the data are summarized in Table 1.The size of all samples was slightly largervia DLS measurementthan SEM observations.In the SEM images,the HASe microspheres had a size distribution from 0.8 to 1.6 μm and the average diameter was about 1.0 μm.However,DLS measurements indicated that the size of the same sample was over 2.0 μm.The deviation stems from the fact that,as the DLS measurements provided the hydration size of microspheres,and all samples are dispersed in H2O,there are inevitably some water molecules adsorbed on the surface of the microspheres,which increases the value of the sizes.Additionally,the size of HASe microspheres was slightly larger than that of the CaCO3template due to the formation ofhydroxyapatite nanorods during the hydrothermal process.So,the surface of HASe microspheres become coarser than that of vaterite CaCO3.
The XRD patterns of the HASe microspheres are presented in Fig.4a.All the peaks can be indexed and matched well with the hexagonal phase of hydroxyapatite(PDF No.09-0432),proving that hydroxyapatite is the main phase of the synthetic HASe microspheres.Hydrothermal fabrication is a successful route to prepare selenium doped HA microsphere with hydroxyapatite phase.As shown in the XRD patterns,the intensity of certain peaks increased with the increase of the dopant dose,which indicates the doped element could increase the crystallinity of the HA microspheres.In addition,certain peaks became broadened after doped.As shown in the inset of Fig.4a,the more selenium was doped into hydroxyapatite,the wider band of this plane is shown in the XRD pattern.The XRD resultsindicated that,thesynthetic HASe microspheres are of the hydroxyapatite phase with better crystallinity,and selenium doping has no evidentinterference in the transformation from CaCO3to HA,which indicates the synthetic route was successful.

Table 1 Size distribution of HA and HASe microspheres
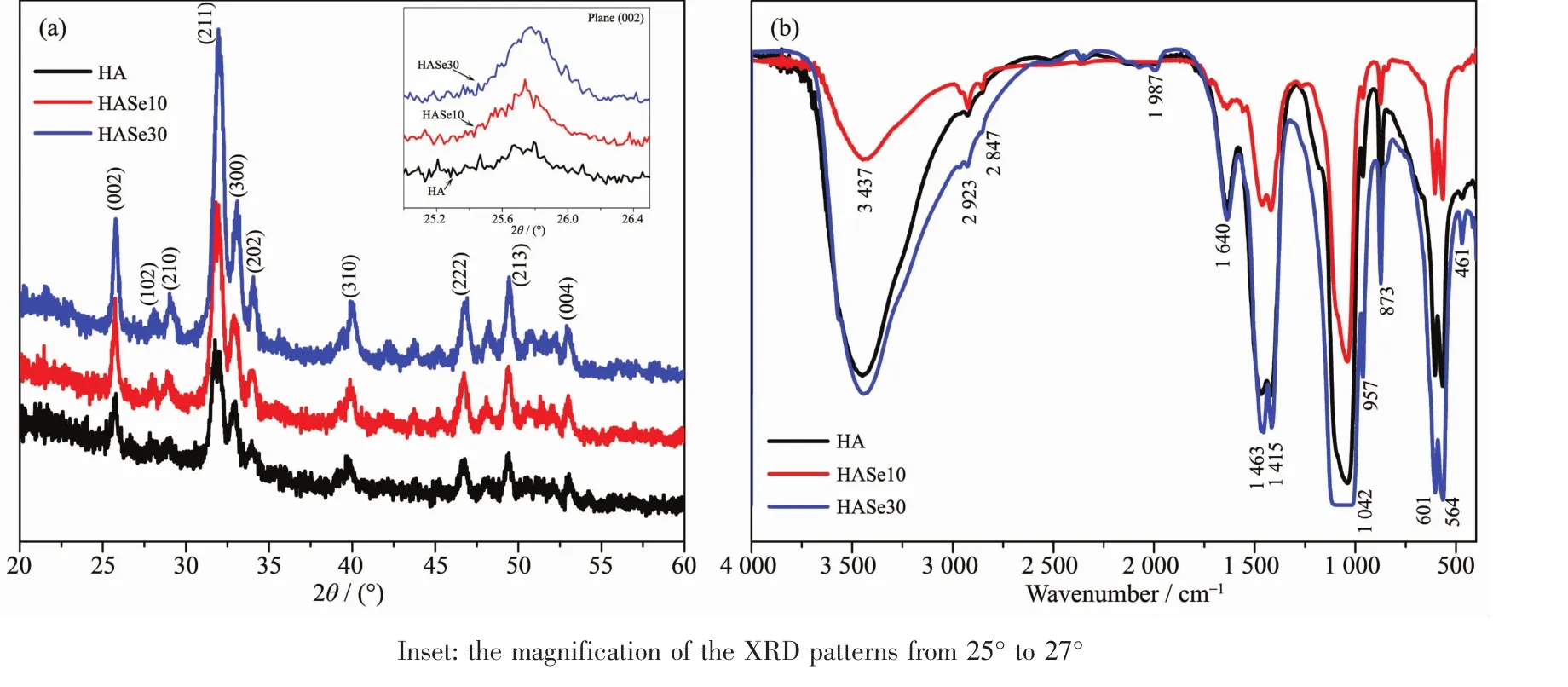
Fig.4 (a)XRD patterns of HA and HASe microspheres;(b)FTIR spectra of HA and HASe microspheres
The functional groups in the HASe microspheres and the pure hydroxyapatite have been characterized by FTIR spectra.As shown in Fig.4b,the intense absorption band located at 1 042 cm-1is ascribed to the stretching vibration (ν3)of the phosphate (PO43-)groups,and the absorption bands located at 564 and 601 cm-1are attributed to the bending vibration(ν4)of the PO43-groups[19]The absorption bands located at 3 437 and 1 640 cm-1are ascribed to the adsorbed water and hydroxyl group,respectively.In addition,the appearance of CO32-absorption peaks at 873 cm-1(ν2),1 415 and 1 463 cm-1(ν3)indicates the incorporation of CO32-groups into hydroxyapatite lattice structure under the ambient atmosphere condition[13].The FTIR results indicated that the synthetic HASe microspheres are the typical carbonated hydroxyapatite.Hydrothermal process could expedite the complete transformation of vaterite CaCO3into hydroxyapatite phase.
The HASe microspheres were subjected to thermal gravimetric analysis.Fig.5 shows the TG/DSC plots of all samples.The weight loss curves of the microspheres could be roughly divided into three steps.The initial weight loss from room temperature to 200℃ is due to the volatilization of physically adsorbed water.When the temperature arose from 200 to 500℃,the weight loss of about 4.86%,3.30%and 3.11%in each curve was observed,which could be associated with the thermal decomposition of PSS,indicating that the polymer contents in HA,HASe10 and HASe30 microspheres are about 4.86%,3.30%and 3.11%,respectively.At 500℃,the organic molecules were removed completely and only the HASe mineral microspheres were remained.There is still visible small weight loss,which might be ascribed to the decomposition of the carbonate groups substituted in the HA structure.According to the remained weight at 800℃,the mineral contents in the microspheres calculated from the TGA curve decreased from 21.12%,13.49%to 11.82%,when the doped Na2SeO3amount increased from 0 mg(HA),10 mg(HASe10)to 30 mg (HASe30),respectively.Such changes further confirm the selenite ions substitute the carbonate groups of the HA structure,and the synthetic HASe microspheres are selenite-substituted-carbonate HA phase. The substitution mechanism involved is complex,and our previous work has provided detailed explanation[19].

Fig.5 Thermal-gravity changes profiles of HA and HASe microspheres
2.3 In vitro degradation experiment

Fig.6 In vitro degradation analysis of HA and HASe microspheres
The in vitro degradation profile of HASe10 microspheres was monitored by immersing them into PBS(pH 3.5 or 10.5),which was incubated at 37℃in a water bath with constant shaking.As shown in Fig.6,the in vitro degradation behavior and selenium release profiles of the HASe10 microspheres exhibit a similar feature.Fig.6a shows that the cumulative degradation percentage ofthe HASe10 microsphereswasa proximately linear relationship over a time period of one week.The degradation curve of the HASe10 microspheres presented two stages.In no more than 76 h (0~76 h),the degradation percentage decreased with the increase of pH value from 3.5 to 10.5.After 76 h till 164 h (76~164 h),the degradation percentage increased with the increase of pH value.During one week,approximately 12.99%and 15.43%of the HASe10 microspheres were degraded in the PBS with a pH of 3.5 and 10.5.The cumulative degradation percentage at pH 10.5 was somewhat higher than that at pH 3.5.Although many evidences have proved the dissolution rate of the HA microspheres was faster at a lower pH value[1,14,16],in this work,the faster degradation was observed at the higher pH value.As the HASe microspheres presented sustainable biphasic degradation pattern,it is inferred that,in the initial phase,the faster dissolution of HASe nanorods of the sphere improves the degradation in the lower pH value,then the pH value of the degradable solution arises close to the neutral which slows down the degradation speed.The selenium release profile(Fig.6b)of the HASe10 microspheres was almost similar with the degradation pattern,which exhibited a pH-responsive effect.It is speculated that,only when the spheres degrade in the buffer solution,the selenite ions could be released from it.
2.4 Drug loading and in vitro drug release
The amounts of Cur loaded on the HA,HASe10 and HASe30 microspheres were(0.00±0.00),(88.72±0.01)and(72.82±0.15)mg·g-1,respectively.The HASe microspheres could be used forcontrolling drug release because they possessed large specific surface areas,hollow porosity and good bioactivity.And our previous study about HASe nanocarriers provided certain useful information:the selenite ions doped into HA structure could cause the decreased crystallinity and increased solubility of the crystal[19].For loading lysozyme,the HASe10 was the sole phase with the highest loading capability.The addition of selenium into HA structure seems to make this specific drug more facilely affine with the crystal,and this is the reason why drug loading efficacy of HA increased especially after elemental doping.Selenite ions itself exhibit antitumor effect on various type of carcinoma,whereas HA is employed as novel drug vehicle owing to large drug loading capacity and stable controllable release property.And the related reports[14]showed that the pure HA microspheres via hydrothermal fabrication perform excellent behavior of loading large amounts of DOX,and there is obvious effectiveness of providing useful amount of the loaded drug to the pathologic region.Thus in this view,the HASe have the potential as controlled-release carriers to load certain drugs.In this work,the UV-Vis absorption spectra of the aqueous solution containing Cur before and after drug loading were employed to evaluate the drug loading content and entrapment efficiency of HASe microspheres.The HASe10 microspheres showed an impressively highest drug entrapment efficiency of(88.72±0.06)%,meanwhile,the drug loading content was(88.72±0.01)mg·g-1,which was a relatively high value for hydroxyapatite based carriers commonly reported in the references[14].It is obvious that the porous and hollow structure of the HASe microspheres plays a key role in providing strong adsorption for Cur moleculesand thusenhancing the drug loading capacity.The excellent drug loading capacity is also attributed to the large specific surface area and porosity.The negatively charged PSS remains in the HASe microspheres,and it could provide additional attractive force for the positively charged Cur due to the electrostatic attraction between PSS and Cur.Moreover,the negative units of OH-and PO43-in the hydroxyapatite structure can provide these carriers with greater affinity towards Cur molecules.The drug molecules can attach onto the HASe microspheres through hydrogen bonding interactions due to the presence ofhydroxylgroups in both the drug molecules and the HASe microspheres.
The cumulative Cur release profiles of the Curloaded HASe microspheres in PBS are summarized in Fig.7.The drug release curve showed that,two Curloaded HASe microspheres (HASe10 and HASe30)had the similar tendency under PBS with pH values of 7.2±0.2.The slow release lasted for about 159 h,and Se doping amount had no significant effect on the release effect.In contrast with pure HA,the two HASe microspheres(HASe10 and HASe30)showed slower release effect.It can be visible that the release rate of Cur decreases with the addition of the dopant.There was no burst release presented in the Curloaded HASe microspheres,instead,they showed a slow and sustainable controlled release from beginning to the endpoint of the release test.In the PBS with pH 7.2 at 0~159 h,approximately 34.6%(1.73/5),29.0%(1.45/5)and 27.6% (1.38/5)of loaded Cur were released from the Cur-loaded HA microspheres.Interestingly,the cumulative release curve of Cur presented an approximately linear relationship with drug release time from 0~159 h.The mechanisms about the in vitro Cur release profiles are complex.For example,Pizzoccaro et al.studied the adsorption and release behavior of benzoxaboroles on hydroxyapatite crystalline,and a rapid release pattern was got in the physiological media,which is caused by the weak binding force between the drugs and the crystals[37].Therefore,the knowledge about the phase transformation of the HA microcarriers,desorption and diffusion of Cur,and electrostatic interaction between the carrier and drug,could provide some insights to decipher the excellent controlled-release property of the Cur-loaded HASe microspheres system.

Fig.7 (a)Drug entrapment efficiency of HA and HASe microspheres;(b)Drug release amount of Cur from HA and HASe microspheres
2.5 Hemolysis assay
The whole blood from healthy individuals was applied to test the cytotoxicity and hemolysis properties of the HASe microspheres.Table 2 summarizes the tested data.From the results,the HASe10 microspheres exhibit less toxic to the blood cells than the other two samples (HA and HASe30),and it could hardly cause hemolysis of the red blood cells(RBC),because the content of the hemoglobin was the lowest among them.On contrary,the HA and HASe30 group increased the amount of the HGB,which implied they could cause weakly hemolytic response.There was no significant difference in WBC and RBC number among three samples,but the value of the PLT in the twoHASesampleswashigherthan pure HA microspheres,one of the reasonable assumptions is that,it is possible to split certain platelet by the HASe microspheres,and the PLT fragment attaches onto the surface ofthe nanorods units ofthe microspheres,therefore the recorded value via the equipment arises.This also indicates the two HASe microspheres show mildly toxic to the PLT.Although the value of PLT was high,it was in the normal range.And other parameters presented no statistical difference. Combined the components of the microspheres with the hemolysis analysis, the synthetic HASe10 microspheres could be an effective carrier for drug delivery.Many reports confirm the excellent biocompatibility of HA,because it is the main inorganic component of human bone and teeth.Selenium itself is a nutritional trace element,requisite for the growth of many cells.HASe nanoparticles were synthesized successfully,and could load lysozyme for controlled release in vitro.In this work,the HASe microspheres similarly show large Cur loading capacity and slowly stable release effect.And it possesses no hemolytic toxicity and cytotoxicity to the normal blood cells.Therefore,it has great potentials in the aspect of biomedical application,especially as an effective microcarrierto load drugsforthe oncotherapy of bone tumors.
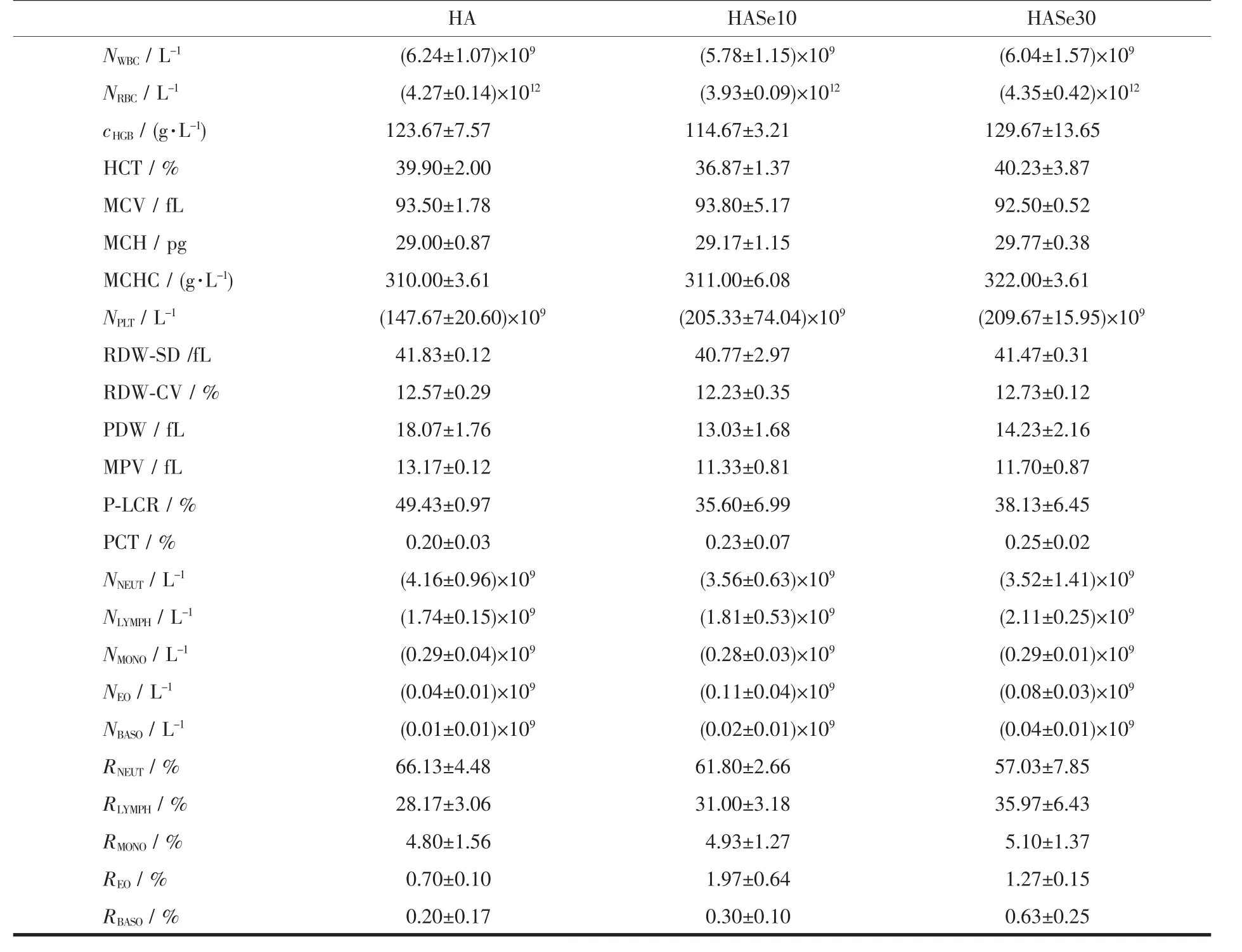
Table 2 Hemolysis properties of the HA and HASe microspheres*
2.6 Cytotoxicity assay
The Fig.8a shows the changes of the cell viability of the cancerous SOSP-9607 cells and the normal RAW-264.7 cells after treated by these microspheres.There was significant difference between the two types ofcellline.Seemingly,the HASe microspheres presented cell type specific effect:They could promote the growth of normal RAW-264.7 cells due to the greatly increased cell viability compared with the control group;simultaneously,they exhibited inhibitory effect on the growth of the cancerous SOSP-9607 cells due to the decreased cell viability compared with the control group.From the above data,it is inferred that,the HASe microspheres have no obvious toxicity to normal cells,but greatly toxic effect to cancerous cells.And selenium doped into the HA structure increases the inhibitory efficacy on the growth of cancerous SOSP-9607 cells.The effectwasalso confirmed in the migration test(Fig.8b):in the control group,many cells had the ability to migrate,and they could cluster together to form cell colony;in the HASe group,it was visible that certain living cells scattered in the bottom of the plate,but many cells died and the cellular compartment became wider.The above effect was slightly similar with the Sr doped hydroxyapatite nanocrystals,which could enhance the osteogenic and inhibit the osteoclastic effect[38].The synthetic HASe microspherescould improve the growth of normal cells,whilst inhibiting the growth of the cancerous cells.Such dual-functional effect could be used to design the multifunctional bone grafts in the therapy of the bone cancer.

Fig.8 Cytotoxicity analysis of the HA and HASe microspheres:(a)cell viability of the cancerous SOSP-9607 and normal RAW-264.7 cells;(b)cell density of the cancerous SOSP-9607 cells
3 Conclusions
The HASe microspheres have been successfully synthesized by a hydrothermal method using the vaterite CaCO3as the sacrificialtemplate.The morphology and size of vaterite CaCO3play a key role in regulating the structure of the HASe microspheres.The HASe microspheres have a size distribution from 0.8 to 1.6 μm,with an average diameter of about 1.0 μm.The wall of HASe microspheres is constructed with subtle units of hydroxyapatite nanorods with an average length of 150 nm and an average width of 20 nm.The synthetic strategy of the microspheres is rapid,highly efficient,energy-saving and environmentally friendly.This work explored the potential application of the HASe microspheres for drug loading and controlled release.The prepared HASe microspheres display a high drug loading capacity and sustainedcontrolled release behavior for Cur.Moreover,the HASe microspheres controlled release system shows no hemolytic and cytotoxic to the blood cells.Cell coculture testifies that the HASe microspheres could inhibit the proliferation of the cancerous SOSP-9607 cells,but promote the growth of normal RAW-264.7 cells.The dual-effect indicates that HASe microspheres are promising for applications in various biomedical fields such as drug delivery system and oncotherapy.From the pointofview,HASe10 microspheres could be a novel micro-carrier for the treatment of bone related diseases such as osteosarcoma.
Acknowledgements:We sincerely thank for the help of Dr.XIANG Hui-Yao at the first college of clinical medical science of China Three Gorges University for the constructive suggestion and data analysis about this study.This work was supported by National Natural Science Foundation of China(Grant No.81602559)and by Youth Science Fund Program of China Three Gorges University(Grant No.1115064).
