Enhanced electrokinetic remediation of cadmium-contaminated natural clay using organophosphonates in comparison with EDTA☆
2018-06-29YingyingGuAlbertYeungHongjiangLi
Yingying Gu *,Albert T.Yeung ,Hongjiang Li
1 Department of Environmental and Safety Engineering,China University of Petroleum(East China),Qingdao 266580,China
2 Department of Engineering,The University of Hong Kong,Pokfulam Road,Hong Kong,China
3 Qingdao Water Group Co.Ltd.,Qingdao 266002,China
4 College of Mining Civil Engineering,Taiyuan University of Technology,Taiyuan 030024,China
1.Introduction
For decades,anthropic activities including mining and smelting,stacking of solid wastes,and irrigation of wastewater have resulted in the world-wide problem of metal contamination of soils[1-3].Unlike organic contaminants,most metals in soil do not undergo microbial or chemical degradation and therefore the total concentration and toxicity of them accumulate after contamination.
Several technologies have been developed to extract metal contaminants from soil including soil flushing[4,5],soil washing[6,7],bioremediation[8-11]and agromining[12].However,most of these technologies are not efficient in extracting metals from fine-grained soil of low hydraulic permeability[13].Electrokinetic extraction has been proven to be a promisingin-situtechnology for the remediation of fine-grained contaminated soils by many laboratory and field experiments[14,15].
Metal contaminants may exist in soil in mobile states(as dissolved species or sorbed species on colloidal particulates suspended in soil solution)or immobile states(as sorbed species on soil particles or solid precipitates)[14],while only metals that exist in mobile states can be extracted from soil by electrokinetic technology.Therefore,in many cases,enhancement techniques are required to solubilize metal contaminants and keep them in the mobile states.These enhancement technologies are essential especially when the contaminated soil has a high buffer capacity which prevents the acidification of soil and dissolution of metals from soil particle surfaces into the soil solution.
The use of enhancement agents is one of the possible approaches to enhance metal extraction from soil by changing the chemical characteristics of metals and transforming them to their mobile phases[15-17].Chelating agents such as EDTA,nitrilotriacetic acid(NTA),(diethylenetriamine)pentaacetic acid(DTPA),and hydroxycarboxylates,such as citric acid,have been widely applied as the enhancement agents in electrokinetic remediation due to their strong chelating ability and availability[18-25].However,other chelating agents such as organophosphonates which also have strong chelating ability and the potential to enhance electrokinetic remediation of metal contaminated soils are seldom studied[15].
Organophosphonates containing one or more C-PO(OH)2or C-PO(OR)2groups are highly water-soluble anthropogenic chelating agents now widely used as corrosion inhibitors,scale inhibitors,and laundry detergents.The most commonly used NTMP and EDTMP are structure analogues to the well-known aminopolycarboxylates of NTA and EDTA,respectively.They also have strong affinity for metal ions and can form soluble,anionic complexes with metals in solution in a wide pH range[15].
The effects of organophosphonates on remobilizing metals sorbed on soil particle surfaces have been studied in previous researches[26-28].Although it has been proven that organophosphonates can promote the electroosmotic flow and desorption ofmetalcontaminants in soils,their potential application in electrokinetic technology as the enhancement agents has not been extensively studied.
In this study,two common organophosphonates,i.e.,NTMP and EDTMP,and EDTA were selected as the enhancement agents to extract cadmium from a naturals oil of high buffer capacity.Batch desorption experiments were firstly carried out to study the influence of soil pH and concentration of enhancement agents on cadmium desorption as a wide pH range from 1 to 12 can be developed across the soil specimen[29].Three bench-scale electrokinetic extraction experiments were then conducted to study the feasibility of using the organophosphonates to enhance cadmium removal from the soil.
2.Materials and Methods
2.1.Soil
The soil used in this study was collected at depths of 0.5-1.0 m in Nanhui District,Shanghai,China which is a slightly alkaline clayey soilof high acid buffer capacity[30].The physicochemical properties of the soil are tabulated in Table 1.

Table 1 Physicochemical properties of the soil
2.2.Organophosphonates and EDTA
The organophosphonates,i.e.,NTMP and EDTMP,and EDTA were obtained from Sigma-Aldrich Chemical Company of St.Louis,Missouri,U.S.A.The structures of these enhancement agents are presented in Fig.1.
2.3.Batch desorption experiments
Cadmium desorption experiments in the presence of NTMP,EDTMP,and EDTA were conducted following the method described by Torrenset al.[31].Cadmium contaminated soil suspensions of 5 mmol·Cd·kg-1soil were firstly prepared by adding 1 g of soil and 10 ml of 0.5 mmol Cd(NO3)2solution to 50-ml centrifuge tubes.The soil suspensions were shaken using a wrist acting shaker for 24 h to allow cadmium to adequately sorb on soil particle surfaces before centrifuged at 4000 r∙min-1for 15 min.The centrifuge tubes were then weighted before and after the supernatants were decanted to determine the volume of cadmium solution retained in the soil.Cadmium concentration in the supernatant was measured using a Perkin Elmer Analyst300 flame atomic absorption spectrometer.Afterwards,10 ml of 0.01 or 0.1 mol·L-1of organophosphonates or EDTA was added to each centrifuge tube as the purging solution.A control test was also conducted by adding 10 ml of deionized(DI)water.The pHs of the soil suspensions were adjusted to 2-11 by adding 0.1 or 1 mol·L-1HNO3or NaOH.The soil suspensions were shaken for 24 h and then centrifuged for cadmium measurement.Each test was performed in duplicate.Detailed calculation of the desorption proportion of cadmium from the soil was given in our previous study[32].
2.4.Electrokinetic experimental setup
The two dimensional(2-d)electrokinetic extraction apparatus used in this study was composed of a specimen cell, fluid and gas volume measurement devices,a power supply,an electrical circuit,and a data logger.Six perforated stainless steel hollow tubes(Φ 18.0 mm)were used as the anodes or cathodes to apply a non-uniform electric field across the soil specimen to better simulate field electrokinetic extraction process.More details of the electrokinetic experimental apparatus were given by Guet al.[31].
2.5.Bench-scale electrokinetic experiments
In this study,three electrokinetic experiments were performed for approximately 5 days to investigate the feasibility of electrokinetic remediation of cadmium-contaminated soil of high buffer capacity enhanced by NTMP and EDTMP in comparison with EDTA.The initial conditions of specimen and operational parameters of electrokinetic extraction experiments are tabulated in Table 2.All the experiments were carried out at room temperature(25°C).

Fig.1.Structure of NTMP,EDTMP&EDTA.

Table 2 Operational parameters of electrokinetic extraction experiments
2.6.Monitoring and analysis of the samples
The electrical current passing through the specimen,electroosmotic flow rates at the anode and the cathode,and voltage distributions at different locations of the specimen were monitored as a function of treatment time during the experiments.At the end of the electrokinetic extraction experiments,soil samples at different positions of the specimen cell(Fig.2)were collected for analyses of water content,soil pH,and cadmium concentration.Methods of determining water content and soil pH were given by Guet al.[32].The measurement of electric conductivity of soil pore fluid followed the method suggested by Page[33].Cadmium concentrations were determined using a flame atomic absorption spectrometer(Perkin Elmer Analyst 300).
3.Results and Discussion
3.1.Batch desorption experiments
Soil pH was reported to greatly influence the sorption/desorption characteristics of the soil particle surfaces,thus affecting the extraction efficiency of electrokinetic remediation[34,35].As shown in Fig.3,at pH 2,almost all the cadmium initially spiked into the soil could be dissolved to the soil solution by DI water.The proportion of cadmium desorption decreased sharply from approximately 93%to 11%when soil pH increased from 3 to 6.At pH≥7,less than 2%of the cadmium could be desorbed from soil by DI water.
With the enhancement of 0.1 mol·L-1EDTA,NTMP,or EDTMP,however,more than 75%of the cadmium initially spiked into the soil could be desorbed in the wide pH range of 1-11.These enhancement agents are very effective in keeping the cadmium in dissolved phase due to the formation of high soluble chelates of Cd-EDTA or Cdorganophosphonates.The capacity of the three enhancement agents for dissolving cadmium from soil was in the order EDTA>EDTMP>NTMP.Nearly complete dissolution of cadmium was found over the entire pH range using 0.1 mol·L-1EDTA.For 0.1 mol·L-1organophosphonates,however,the proportions of cadmium desorption were relatively low in acidic environments.Approximately 75.6%-82.4%and 85.1%-86.9%of cadmium were desorbed from soil particle surfaces by 0.1 mol·L-1NTMP and EDTMP,respectively,at pH 2-5.Cadmium desorption proportions started to increase with soil solution alkalinity and reached 95.2%-100%in the pH range from 6 to 11.
The desorption efficiency of cadmium from soil by chelating agents is largely dependent on the chelating ability of enhancement agents.The log stability constant of Cd-EDTA is 16.4[36],higher than that of Cd-NTMP(lgKCd-NTMPis 12.2[37]),indicating that EDTA has a stronger chelating ability for cadmium.For organophosphonates,the stability of metal complexes generally increases with increasing number of organophosphonic groups[28,38],which explains that EDTMP extracted more cadmium from soil than NTMP.However,the stability constant of Cd-EDTMP is not available due to precipitation and polymerization[39].

Fig.2.Sampling positions(the black spots)of soil(all dimensions are in mm).

Fig.3.Cd desorption using organophosphonates in comparison with EDTA and DI water.

Fig.4.Variation of electrical current during electrokinetic experiments.

Table 3 Log conditional stability constants of Cd-phosphonates complexes as a function of pH[41]
When 0.01 mol·L-1organophosphonates were added as the enhancement agents,cadmium desorption decreased significantly in the pH range of 2-5 and then started to increase with alkalinity at pH 5 and reaches 70%-80%in the pH range from 7 to 11.The cadmium desorption proportions from soil were even lower than those using DI water at pH 2-5.This could be explained by the decrease of stability constant of cadmium complexes with organophosphonates with acidity(Table 3)and the formation of ternary surface complexes which increased the sorption of anionic complexes at lower pHs[26].Similar results have been reported in a previous study on sorption of Cu in the presence of Organophosphonates[40].Cu sorption onto goethite increased at low pH due to electrostatic effects and decreased at high pH due to the formation of soluble Cu-organophosphonate complexes.The maximum sorption was observed at pH approximately 6.This phenomenon was not observed in this study when 0.01 mol·L-1EDTA was used as the enhancement agent.The proportion of cadmium desorbed from soil with EDTA was higher than 92%at all pH levels,maybe due to the weak sorption of the Cd-EDTA complexes in lower pH environment.
3.2.Electrokinetic extraction experiments
3.2.1.Electrical current
The variation of electrical current with treatment time in Fig.4 showed that the electrical current through the specimen system in the three Tests all decreased in the first 2-3 days and then increased gradually to approximately 110 mA after 5 days of electrokinetic treatment.The initial electrical currents in Tests EC-1&EC-3 were 59.0 mA&53.8 mA,respectively.They decreased to 52.4 mA&37.6 mA in the first two days and then increased to 113.5 mA&117.1 mA,respectively.In Test EC-2 when using NTMP as the electrolytes,the initial electrical current was 85.0 mA,which was higher than those in Tests EC-1&EC-3.It may be attributed to the higher ionic concentration of NTMP solution used as the electrolytes.It decreased slowly to 82.2 mA in the first 40 h and then increased to 109.7 mA after 123.8 h of treatment.The same phenomenon of decrease in electrical current followed by an increase in electrical current has also been observed in previous studies[42].
The electrical current flowing through the soil specimen is highly related to the concentration of mobile ions in the soil[43].The decrease of electrical currentat the beginning of the experiments was mainly due to the migration of free ions in the soil specimen into electrolytes,decreasing the electrical conductivity of the specimen.As the enhancement agents used in the electrokinetic experiments contained much more ions/ionic species than the tap water used to prepare the soil specimen,the migration of these ions/ionic species towards electrodes of the opposite polarity greatly increased the electrical conductivity of the specimen and electrical current under the constant electrical gradient applied across the soil specimen.
Other variation trends of electrical current during electrokinetic treatment have also been reported.For example,exponential decay of electrical current with treatment time,at least in the initial stage of the electrokinetic experiment,has been reported by many researchers[13,18,44-47].The exponential decay of electrical current is attributed to the equivalent resistor-capacitor circuit properties of the system[13].Besides,an initial increase of electrical current followed by decaying has also been reported during electrokinetic extraction experiments[48].However,exponential decay of electrical current was not observed in all the experiments in this study.
3.2.2.Electroosmotic flow rates
The electroosmotic flow through the soil specimen largely affects the contaminant removal from soil by accelerating or retarding the electromigration of charged contaminants.Both of the electroosmotic in flow rates and out flow rates can be determined at the anode and at the cathode,respectively,during the electrokinetic experiments.It can be observed from Fig.5 that the electroosmotic flow rates are always positive,i.e.,from the anode towards the cathode.The forward electroosmotic flow direction could be explained by the negative zeta potential of soil particle surfaces in the wide pH range of 2-11 according to the Helmholtz-Smoluchowski equation[29].

Fig.5.Variation of electroosmotic flow rates.
The electroosmotic flow rates at the onset of the three electrokinetic experiments were in the range of4.8-9.0 ml·h-1.They decreased to approximately 3.5-5.4 ml·h-1after 5 days of treatment.The magnitude of electroosmotic flow rate was determined by several parameters including electrical gradient,relative permittivity and viscosity of soil solution,and zeta potential of soil particle surfaces[49].These parameters were similar in Tests EC-1 to EC-3,leading to similar electroosmotic flow rates during the experiments.The decrease of electroosmotic flow rates with treatment time could be explained by:(1)the decrease of electrical gradient applied across the soil specimen with the increase of electrical current and increase of electrical gradient across the external electrical resistance;and(2)clogging of soil near the anode observed after the electrokinetic experiments which decreased the porosity of the soil specimen.
The initial electroosmotic flow rates in Tests EC-1 to EC-3 were much higher than those when Hong Kong tap water was used as the electrolytes(4-6 ml·h-1)(results now shown).The reason may lie in that EDTA and the organophosphonates used in this study could significantly decrease of zeta potential(make more negative)in a wide range of pHs[29],thus enhancing forward electroosmotic flow.Similar results were obtained by other researchers[50-53].This phenomenon may retard the removal of negatively charged chelates of Cd-EDTA or Cdorganophosphonates from soil during the electrokinetic remediation process.
3.2.3.Soil pH distribution
The distributions of soil pH in Row 4 before and after the three electrokinetic remediation experiments are shown in Fig.6.The soil pHs did not exhibit significant change near the anode after all the experiments.In Tests EC-1&EC-3 when using EDTA&EDTMP as the electrolytes,the soil pHs increased significantly in Columns 3-5(4-18 cm from the anode)as shown in Fig.2.They increased from the initial soil pH of 7.39&7.44 to 9.65&8.88,respectively,at 18 cm from the anode after the experiments.At the anode,the neutralization of protons by the alkaline anolyte and the high acid buffer capacity of the soil[31]prevented acidification of soil.The OH-ions generated at the cathode due to electrolytic decomposition of water migrated towards the anode and increased the soil pH in most parts of the specimen.The final soil pH near the cathode was lower in Test EC-3 than that in Test EC-1 due to the lower pH of EDTMP than EDTA added at the cathode.In Test EC-2,the soil pH changed insignificantly throughout the specimen.It demonstrated that the acetic acid in the catholyte as the buffer solution was efficient in controlling soil pH during the electrokinetic extraction.

Fig.6.Soil pH distributions in Tests EC-1 to EC-3.
According to the results of batch desorption experiments,the cadmium spiked into the specimen could hardly exist in dissolved phase at pH≥7 without enhancement as presented in Fig.3.Therefore,electrokinetic extraction of cadmium from the natural clay of high acid/base buffer capacity may not be feasible unless an enhancement agent is applied to desorb cadmium from soil particle surfaces and keep them in the dissolved phase.In the presence of EDTA and organophosphonates,a large proportion of cadmium could be desorbed from soil through complexation and variation of soil pH in the range of 7-10 has insignificant influence on cadmiumdesorption from soil particle surfaces.
3.2.4.Cadmium distributions
The cadmium initially spiked in the soil was uniformly distributed in the specimen before the electrokinetic experiments.In Test EC-1 with the enhancement of 0.1 mol·L-1EDTA,a constant electrical potential of 19.8 V was applied through electrodes in Rows 2&6 for 130.5 h.The initial cadmium concentration in the soil was approximately 294 mg·kg-1.The injection of EDTA significantly enhanced the mobilization the cadmium in the soil specimen.The cadmium content in more than 60%of the soil specimen significantly decreased as displayed in Fig.7(a)&(b).The final cadmium concentrations in Columns 3&4(10&14 cm from the anode)were approximately 200 mg·kg-1and those in Column 5(18 cm from the anode)decreased to 138-185 mg·kg-1.On the other hand,the final cadmium concentrations in Column 2 increased to 291-412 mg·kg-1and those in Rows 2&6 in Colum 1 were nearly twice that of the initial concentration.It indicated that cadmium was desorbed from soil through the formation of highly soluble negatively charged Cd-EDTA complexes and transported towards the anode and accumulated in the vicinity of the anode.The results are in agreement with many previous researches[19,54,55].
In Tests EC-2&EC-3,0.1 mol·L-1NTMP&EDTMP were used as the electrolytes to enhance cadmium extraction from the soil specimen,respectively.As shown in Fig.7(c)-(f),the injection of the two organophosphates into the specimen also greatly changed the distribution of cadmium after electrokinetic treatment.The final cadmium concentrations after electrokinetics extraction enhanced by 0.1 mol∙L-1NTMP&EDTMP were similar in most parts of soil specimen(they decreased from the initial concentrations of 285&282 mg·kg-1to approximately 200 mg·kg-1with an average cadmium removal efficiency of approximately 30%)except those in the vicinity of the anode.The removal efficiencies of cadmium in the vicinity of the cathode enhanced by 0.1 mol·L-1NTMP&EDTMP were lower than those enhanced by 0.1 mol·L-1EDTA.This might be attributed to their relatively lower complexing ability with cadmium compared with EDTA and the low concentrations of ligands in the soil pore fluid which might not be efficient in desorbing cadmium from soil during the short treatment time.
Although NTMP&EDTMP were not as efficient in mobilizing cadmium in soil as EDTA,accumulation of cadmium near the anode was not observed after the electrokinetic extraction in Tests EC-2&EC-3.Cadmium concentrations in the anolytes were determined to be approximately 17.7%and 18.6%of the total concentration spiked into the specimens,respectively.The results indicated that the complexes of Cd-NTMP or Cd-EDTMP could migrate from the soil specimen into the anolyte under the electrical field.The average cadmium removal efficiencies from the soil samples collected after 5 days of electrokinetic extraction enhanced by 0.1 mol·L-1NTMP(22.8%)and 0.1 mol·L-1EDTMP(22.4%)were higher than that by 0.1 mol·L-1EDTA(15.1%).
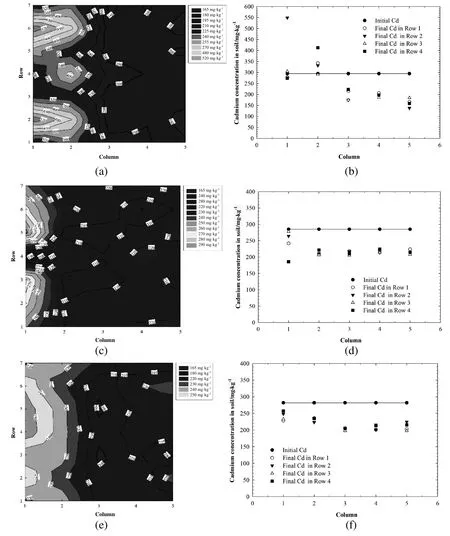
Fig.7.Cd distributions(a)2-d distribution in TestEC-1;(b)1-d distribution in TestEC-1;(c)2-d distribution in TestEC-2;(d)1-d distribution in Test EC-2;(e)2-d distribution in Test EC-3;(f)1-d distribution in Test EC-3.
It should be noted that although the organophosphonates and EDTA used in this study could form water-soluble anionic complexes and enhance desorption and migration of Cd in the soil,EDTA with amino carboxylic acids has stronger complexing ability than NTMP&EDTMP with C-PO(OH)2 ligand as EDTA could be attached to a metal ion up to six sites.Therefore,it could be expected that the mobilization of Cd during electrokinetic extraction experiments enhanced by EDTA was much more efficient than that by NTMP&EDTMP.However,the accumulation of Cd nearthe anode retarded its removal from soil when using EDTA as the enhancement agent.The accumulation of metals in the soil during electrokinetic remediation enhanced by EDTA or NTA has also been observed by many other researchers[18,55].It was explained by the retardation of metal transportation due to forward electroosmotic flow towards the cathode and the opposite electromigration of the anionic complexes of metal-EDTA[56]or the adsorption of metal-EDTA at lower pH near the anode region[54].However,as the electroosmotic flow rates in Tests EC-1 to EC-3 were in the same order and final soil pH were in the narrow range of 7-10,the situations were similar when using the organophosphonates as the enhancement agents.On the other hand,we have conducted several electrokinetic extraction experiments with different concentrations of NTMP,pHs of electrolytes and voltage gradients,the accumulation of Cd in the vicinity of the anode was neither observed(results not shown).The reasons for the different distribution of Cd enhanced by NTMP&EDTMP and EDTA still require further investigation.
It has been reported that metals in exchangeable,carbonate,and Fe/Mn oxides fractions correlate with the metal bioavailability in the order of exchangeable fraction>carbonates fraction>Fe/Mn oxides fraction[57].According to our previous results[29],the fractions of Cd after desorption by the organophosphonates(NTMP&EDTMP)depended largely on soil pH.The exchangeable,carbonate,and Fe/Mn oxide fractions of cadmium could be desorbed from soil particles after extraction by NTMP&EDTMP.However,the proportions of exchangeable and carbonate fractions of cadmium decreased while those of Fe/Mn oxide and organic fractions increased after extraction by NTMP&EDTMP at pHs 7&10 as the exchangeable and carbonate fractions were desorbed most promptly.In the electrokinetic experiments EC-2&EC-3,as the soil pH was in the range of 7.3-8.9(Fig.6),it can be deduced that the bioavailability will decrease significantly after the enhanced extraction of cadmium using the organophosphates.
4.Conclusions
Several conclusions can be drawn from the results of the desorption edge experiments and bench-scale electrokinetic experiments enhanced by organophosphonates,i.e.,NTMP&EDTMP,in comparison with EDTA:
(a)More than 75%of the sorbed cadmium could be dissolved into solution using 0.1 mol·L-1organophosphonates or EDTAin the pH range of 1-11.Cadmium desorption by 0.01 mol·L-1organophosphonates decreased significantly in the pH range of 2-5 due to the decrease of stability constant of Cdorganophosphonates and the formation of ternary surface complexes at lower pHs.
(b)The cadmium in the soil migrated towards the anode with the enhancement of 0.1 mol·L-1NTMP,EDTMP,and EDTA.The average removal efficiencies of cadmium from the soil after approximately 5 days of electrokinetic extraction under a constant voltage gradient of approximately 1.0 V·cm-1enhanced by 0.1 mol·L-1NTMP(22.8%)and EDTMP(22.4%)were 7.3%-7.5%higher than that by 0.1 mol·L-1EDTA(15.1%).
(c)Organophosphonates such as NTMP and EDTMP were proven to be efficient enhancement agents for electrokinetic remediation of metal-contaminated soils.They can be used in replacement of the expensive EDTA although their field applications still need further investigation.
[1]V.Ettler,Soil contamination near non-ferrous metal smelters:A review,Appl.Geochem.64(2016)56-74.
[2]H.K.Kim,T.I.Jang,S.M.Kim,S.W.Park,Impact of domestic wastewater irrigation on heavy metal contamination in soil and vegetables,Environ.Earth Sci.73(2015)2377-2383.
[3]R.A.Wuana,F.E.Okieimen,Heavy metals in contaminated soils:a review of sources,chemistry,risks and best available strategies for remediation,ISRNEcol.2011(2011)1-20.
[4]D.Y.S.Yan,I.M.C.Lo,Pyrophosphate coupling with chelant-enhanced soil flushing of field contaminated soils for heavy metal extraction,J.Hazard.Mater.199(2012)51-57.
[5]K.R.Reddy,A.Z.Al-Hamdan,Enhanced sequential flushing process for removal of mixed contaminants from soils,Water Air Soil Pollut.224(12)(2013)1709-1721.
[6]N.R.Hartley,D.C.W.Tsang,W.E.Old,P.A.Weber,Soil washing enhanced by humic substances and biodegradable chelating agents,Soil Sediment Contam.23(6)(2014)599-613.
[7]M.Ye,M.Sun,Z.Liu,N.Ni,Y.Chen,C.Gu,F.O.Kengara,H.Li,X.Jiang,Evaluation of enhanced soil washing process and phytoremediation with maize oil,carboxymethyl-beta-cyclodextrin,and vetiver grass for the recovery of organochlorine pesticides and heavy metals from a pesticide factory site,J.Environ.Manag.141(2014)161-168.
[8]S.Ehsan,S.Ali,S.Noureen,M.Rizwan,Citric acid assisted phytoremediation of cadmium byBrassica napusL,Ecotoxicol.Environ.Saf.106(2014)164-172.
[9]A.Van Der Ent,A.J.M.Baker,R.D.Reeves,R.L.Chaney,C.W.N.Anderson,J.A.Meech,P.D.Erskine,M.O.Simonnot,J.Vaughan,J.L.Morel,G.Echevarria,B.Fogliani,Q.Rongliang,D.R.Mulligan,Agromining:farming for metals in the future?Environ.Sci.Technol.49(2005)4773-4780.
[10]O.M.Ontanon,P.S.Gonzalez,L.F.Ambrosio,C.E.Paisio,E.Agostini,Rhizoremediation of phenol and chromium by the synergistic combination of a native bacterial strain andBrassica napushairy roots,Int.Biodeterior.Biodegrad.88(2014)192-198.
[11]M.A.Polti,J.D.Aparicio,C.Benimeli,M.J.Amoroso,Simultaneous bioremediation of Cr(VI)and lindane in soilby actinobacteria,Int.Biodeterior.Biodegrad.88(2014)48-55.
[12]J.N.Meegoda,R.Perera,Ultrasound to decontaminate heavy metals in dredged sediments,J.Hazard.Mater.85(1-2)(2011)73-89.
[13]A.T.Yeung,Contaminant extract ability by electrokinetics,Environ.Eng.Sci.23(1)(2006)202-224.
[14]A.T.Yeung,Y.-Y.Gu,A review on techniques to enhance electrochemical remediation of contaminated soils,J.Hazard.Mater.195(2011)11-29.
[15]A.T.Yeung,Y.-Y.Gu,“Use of Chelating Agents in Electrochemical Remediation of Contaminated Soil”,in:Chelating Agents for Land Decontamination Technologies,ASCE Press,Virginia,2012 212-280.
[16]J.S.H.Wong,R.E.Hicks,R.F.Probstein,EDTA-enhanced electroremediation of metal contaminated soils,J.Hazard.Mater.55(1-3)(1997)61-79.
[17]K.R.Reddy,C.Chaparro,R.E.Saichek,Removal of mercury from clayey soils using electrokinetics,J.Environ.Sci.Health A38(2)(2003)307-338.
[18]K.R.Reddy,P.R.Ala,Electrokinetic remediation of metal-contaminated field soil,Sep.Sci.Technol.40(8)(2005)1701-1720.
[19]A.T.Yeung,C.Hsu,Electrokinetic remediation of cadmium-contaminated clay,J.Environ.Eng.131(2)(2005)298-304.
[20]D.M.Zhou,C.F.Deng,A.N.Alshawabkeh,L.Cang,Effects of catholyte conditioning on electrokinetic extraction of copper from mine tailings,Environ.Int.31(6)(2005)885-890.
[21]E.Gidarakos,A.Giannis,Chelate agents enhanced electrokinetic remediation for removal cadmium and zinc by conditioning catholyte pH,Water Air Soil Pollut.172(1-4)(2006)295-312.
[22]T.Kimura,K.I.Takase,S.Tanaka,Concentration of copper and a copper-EDTA complex at the pH junction formed in soil by an electrokinetic remediation process,J.Hazard.Mater.143(3)(2007)668-672.
[23]M.Masi,R.Iannelli,G.Losito,Ligand-enhanced electrokinetic remediation of metal contaminated marine sediments with high acid buffering capacity,Environ.Sci.Pollut.Res.23(11)(2015)10566-10576.
[24]R.B.Fu,D.D.Wen,X.Q.Xia,W.Zhang,Y.-Y.Gu,Electrokinetic remediation of chromium(Cr)-contaminated soil with citric acid(CA)and polyaspartic acid(PASP)as electrolytes,Chem.Eng.J.316(2017)601-608.
[25]F.S.Meng,H.Xue,Y.Y.Wang,B.H.Zheng,J.L.Wang,Citric-acid preacidification enhanced electrokinetic remediation for removal of chromium from chromiumresidue-contaminated soil,Environ.Technol.39(3)(2018)356-362.
[26]F.Bordas,A.C.M.Bourg,Effect of complexing agents(EDTA and ATMP)on the remobilization of heavy metals from a polluted river sediment,Aquat.Geochem.4(2)(1998)201-214.
[27]P.K.A.Hong,W.Jiang,Factors in the selection of chelating agents for extraction of lead from contaminated soil:effectiveness,selectivity,and recoverability,Biogeochemistry of Chelating Agents,Oxford University Press,Washington,DS 2005,pp.421-432.
[28]Y.-Y.Gu,A.T.Yeung,D.C.W.Tsang,R.B.Fu,Applications of citric acid industrial wastewater and phosphonates for soil remediation:effects on temporal change of cadmium distribution,Soil Sediment Contam.22(2013)876-889.
[29]Y.-Y.Gu,A.T.Yeung,A.Koenig,H.J.Li,Effects of chelating agents on zeta potential of cadmium-contaminated natural clay,Sep.Sci.Technol.44(2009)2203-2222.
[30]Y.-Y.Gu,A.T.Yeung,Use of citric acid industrial wastewater to enhance electrochemical remediation of cadmium-contaminated natural clay,Geotechnical Special Publication No.225,ASCE Press 2012,pp.3995-4004.
[31]J.L.Torrens,D.C.Herman,R.M.Miller-Maier,Biosurfactant(Rhamnolipid)sorption and the impact on rhamnolipid-facilitated removal of cadmium from various soils under saturated flow conditions,Environ.Sci.Technol.32(6)(1998)776-781.
[32]Y.-Y.Gu,R.B.Fu,H.J.Li,H.An,A new two-dimensional experimental apparatus for electrochemical remediation processes,Chin.J.Chem.Eng.23(2015)1389-1397.
[33]A.L.Page,Methods of Soil Analysis,Part 2.Chemical and Microbiological Properties,Am Soc of Agron,Inc,Madison,1982.
[34]Y.-Y.Gu,A.T.Yeung,Desorption of cadmium from a natural Shanghai clay using citric acid industrial wastewater,J.Hazard.Mater.191(2011)144-149.
[35]R.Iannelli,M.Masi,A.Ceccarini,M.B.Ostuni,R.Lageman,A.Muntoni,D.Spiga,A.Polettini,A.Marini,R.Pomi,Electrokinetic remediation of metal-polluted marine sediments:experimental investigation for plant design,Electrochim.Acta181(2015)146-159.
[36]K.Wolf,P.A.Gilbert,EDTA-ethylenediaminetetraacetic acid,The Handbook of Environmental Chemistry,vol.3(F),Springer-Verlag,Berlin,1992,pp.243-259.
[37]A.E.Martell,R.M.Smith,R.J.Motekaitis,NIST critically selected stability constants of metal complexes,Version 8.0,National Institute of Standards and Technology,Gaithersburg,Maryland,2004.
[38]B.Nowack,Environmental chemistry of phosphonates,Water Res.37(11)(2003)2533-2546.
[39]K.Popov,A.Kolosov,V.G.Yachmenev,N.Shabanova,A.Artemyeva,A.Frid,B.Kogut,S.Vesnovskii,V.Sukharenko,A laboratory-scale study of applied voltage and chelating agent on the electrokinetic separation of phenol from soil,Sep.Sci.Technol.36(13)(2001)2971-2982.
[40]B.Nowack,A.T.Stone,The influence of metal ions on the adsorption of phosphonates onto goethite,Environ.Sci.Technol.33(20)(1999)3627-3633.
[41]W.E.Gledhill,T.C.J.Feijtel,Environmental properties and safety assessment of organic phosphonates used for detergent and water treatment applications,The Handbook of Environmental Chemistry,vol.3(F),Springer-Verlag,Berlin,1992,pp.261-285.
[42]D.M.Zhou,C.F.Deng,L.Cang,Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents,Chemosphere56(3)(2004)265-273.
[43]Y.B.Acar,A.N.Alshawabkeh,Principles of electrokinetic remediation,Environ.Sci.Technol.27(3)(1993)2638-2647.
[44]C.J.Bruell,B.A.Segall,M.T.Walsh,Electroosmotic removal of gasoline hydrocarbons and TCE from clay,J.Environ.Eng.ASCE118(1)(1992)68-83.
[45]R.F.Probstein,R.E.Hicks,Removal of contaminants from soils by electric- fields,Science260(5107)(1993)498-503.
[46]G.R.Eykholt,D.E.Daniel,Impact of system chemistry on electroosmosis in contaminated soil,J.Geotech.Eng.ASCE120(5)(1994)797-815.
[47]A.T.Yeung,T.B.Scott,S.Gopinath,R.M.Menon,C.Hsu,Design,fabrication,and assembly of an apparatus for electrokinetic remediation studies,Geotech.Test.J.20(2)(1997)199-210.
[48]K.R.Reddy,S.Chinthamreddy,Effects of initial form of chromium on electrokinetic remediation in clays,Adv.Environ.Res.7(2)(2003)353-365.
[49]S.Y.Shin,S.M.Park,K.Baek,Soil moisture could enhance electrokinetic remediation of arsenic-contaminated soil,Environ.Sci.Pollut.Res.24(10)(2017)9820-9825.
[50]K.I.Popov,N.A.Shabanova,A.A.Artemeva,E.M.Urinovich,Y.V.Tulaeva,Influence of chelating agents on the electrokinetic potential of the clay fraction of soddy podzolic soils,Colloid J.59(2)(1997)212-214.
[51]K.Popov,V.Yachmenev,A.Kolosov,N.Shabanova,Effect of soil electroosmotic flow enhancement by chelating reagents,Colloids Surf.A Physicochem.Eng.Asp.160(2)(1999)135-140.
[52]A.Y.Kolosov,K.I.Popov,N.A.Shabanova,A.A.Artem'eva,B.M.Kogut,Electrokinetic removal of hydrophobic organic compounds from soil,Russ.J.Appl.Chem.74(4)(2001)631-635.
[53]K.Popov,H.Ronkkomaki,L.H.J.Lajunen,Critical evaluation of stability constants of phosphonic acids(IUPAC technical report),Pure Appl.Chem.73(10)(2001)1641-1677.
[54]Y.S.Ng,B.Sen Gupta,M.A.Hashim,Remediation of Pb/Cr co-contaminated soil using electrokinetic process and approaching electrode technique,Environ.Sci.Pollut.Res.23(1)(2016)546-555.
[55]Y.Song,M.T.Ammami,A.Benamar,S.Mezazigh,H.Q.Wang,Effect of EDTA,EDDS,NTA and citric acid on electrokinetic remediation of As,Cd,Cr,Cu,Ni,Pb and Zn contaminated dredged marine sediment,Environ.Sci.Pollut.Res.23(11)(2015)10577-10586.
[56]G.C.C.Yang,S.L.Lin,Removal of lead from a silt loam soil by electrokinetic remediation,J.Hazard.Mater.58(1-3)(1998)285-299.
[57]M.Pociecha,D.Lestan,EDTA leaching of Cu contaminated soil using electrochemical treatment of the washing solution,J.Hazard.Mater.165(2009)533-539.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
