Determination of 4-nonylphenol and 4-tert-octylphenol compounds in various types of wastewater and their removal rates in different treatment processes in nine wastewater treatment plants of Iran
2018-05-25BijanBinaFarzanehMohammadiMohammadMehdiAminHamidRezaPourzamaniZeynabYavari
Bijan Bina ,Farzaneh Mohammadi*,Mohammad Mehdi Amin ,Hamid Reza Pourzamani,Zeynab Yavari
1 Environment Research Center,School of Health,Isfahan University of Medical Sciences,Isfahan,Iran
2 Student Research Center,School of Health,Isfahan University of Medical Sciences,Isfahan,Iran
1.Introduction
In recent years,the presence of emerging pollutants,especially in the aquatic environments,has drawn the attention of environmental experts[1].These chemicals are widely used in various industries and include many substances such as endocrine disrupting chemicals(EDCs),pharmaceuticals and personal care products(PPCPs)and persistent organic pollutants(POPs)[2].The sewage and sludge of municipal and industrial wastewater treatment plants are one of the entry routes of emerging contaminants to water resources[3].Many of priority pollutants are from EDCs[2].Alkylphenols(APs)fall in the EDCs group and have estrogenic effects.These materials are used in the production of lubricants,phenolic resins,rubber and plastic,polymers,antioxidants,heat stabilizers and other materials[4].The long chain APEs degrade quickly to the corresponding APs,which leads to permanent entry of these compounds into the environment[5].APs which are the metabolites of APEs,are more stable,toxic,lipophilic compounds and with stronger estrogenic properties than their precursors.4-NP and 4-t-OP are the most important and widely used Alkylphenolic compounds[2].According to the research conducted on the APs,they are highly toxic to many aquatic organisms,humans,animals and the environment.These effects may include cancerous tumors,reproductive disruptor,immune system disorders and obesity[6].As mentioned above,one of the most important ways to transfer 4-NP and 4-t-OP to the environment is through wastewater[7].Thus,in recent years,many studies have reported monitoring of APs in waste waters and water resources and the fate of ones in wastewater treatment plants[2,4,8–15].However,most of the sestu dies have been focused on surface water resources.In addition,the sere searches have been doneon wastewater treatment plants without considering the factors like the type of sewage such as urban wastewater treatment plants(U-WWTPs),rural wastewater treatment plants(R-WWTPs),livestock wastewater treatment plants(L-WWTPs),commercial wastewater treatment plants(C-WWTPs)and hospital wastewater treatment plants(H-WWTPs)[16].
4-NP and 4-t-OP due to high Kow1(4-NP lg Kow=4.48,4-t-OP lg Kow=5.3),have a high tendency to be adsorbed to surfaces such as sludge,sediments and soils.Also persistence of APEs and APs in wastewater sludgeisan important problem becauseamong other sludgehandling techniques,land application is the most desirable due to its low cost and beneficial recycle of nutrients.But wastewater sludge does not only contain nutrients,it also have contaminants such as APs compounds;they should monitored appropriately to judge about their capability in land application.It allows avoiding these compounds entering the food chain and accumulate in the fatty tissues of living organisms.Concerns about the presence of these contaminants in the wastewater,sewage and sludge have encouraged the researchers to monitor APs Specially 4-NP and 4-t-OP in the water and wastewater resources[8].
Despite the development of several methods for measuring organic compounds with small amounts,the study on methods for determining the values of 4-NP and 4-t-OP in a variety of samples is still ongoing.
These compounds are determined mostly by techniques such as gas chromatography(GC)coupled with mass spectro metric detection(MS),liquid chromatography(LC)coupled with MS and high-performance liquid chromatography(HPLC).The GC–MS method achieves significantly higher separation,sharper peak shapes and better resolution.However,the derivatization phase prior to injection to GC must be done for polar compounds that have low volatility.Chemical derivatization of the analytes is often used to improve volatility,increase thermal stability or to enhance the detection sensitivity in GC.Methods such as solid phase micro extraction(SPME)and liquid phase micro extraction(LPME)have been applied in many studies.The SPME method is time consuming and expensive.The LPME method has also some Difficulties such as operational problems,instability of the micro-drop and bubble formation formed by the gas produced during the reaction[17].Such disadvantages have led to news ample preparation techniques,aiming for faster and simpler methods that consume less solvent with higher recovery and compatibility with GC techniques.Therefore,in 2006,the dispersive liquid–liquid micro extraction(DLLME)technique was introduced.In this method,a combination of extraction and dispersive solvent is injected quickly to the liquid sample,which will produce a cloudy solution.This solution is centrifuged,and finally,the contaminant is dissolved in the extraction solvent and will accumulate at the bottom of the centrifuge tube as a tiny drop[18].The DLLME method has been used in recent years to extract various pollutants,such as steroid hormones[19],Bisphenol A[20],a variety of pharmaceuticals[21],NP and OP[17].
In this paper,a comprehensive study was conducted of the occurrence and distribution of 4-NP and 4-t-OP discharged from important sources such as in urban,rural,commercial,livestock and hospital wastewater.Therefore,we investigated APs in rawsewage,effluent and sludge to study their occurrence and fate in various WWTPs.A total of 4 U-WWTPs,2 R-WWTPs,a L-WWTP,a H-WWTP and a C-WWTP were selected for observations.
The treatment processes in the studied WWTPs include activated sludge(AS),moving bed bio film reactor(MBBR),aerated lagoon(AL)and activated sludge along with wetland(AS+WL).Accordingly,the ability of various processes for the removal of 4-NP and 4-t-OP and the adsorption rates of pollutants into the sludge was evaluated.
2.Materials and Methods
2.1.Standards and reagents
Standard solutions of 4-NP(CAS:84852-15-3),4-t-OP(97%,CAS:140-66-9),internal standard of n-OP(99%,CAS:1806-26-4,liner compound)and the derivatization agent(BSTFA+TMCS,99:1,CAS:25561-30-2)were purchased from Sigma-Aldrich Company.GC grade methanol,chloroform and n-hexane were also obtained from Merck Company.Standard and internal standard Solutions of 4-NP,4-t-OP and n-OPat concentrations of 1000 mg·L−1were prepared in methanol and kept in the dark at−18 °C.Working solution was prepared freshly by suiable dilution of the mixed stock solution with distilled water.
2.2.Sampling
The in fluent and effluent samples were collected by 24 h composite samples approach from 4 U-WWTPs(Activated sludge and aerated lagoon),2 R-WWTPs(Aerated lagoon),a L-WWTP(Activated sludge with wetland),a C-WWTP(MBBR)and a H-WWTP(Activated sludge).Samples collection is done in triplicate in each WWTP and its average reported.The information of the studied treatment plants are given in Table 1.The samples were kept in the 500 ml dark glass bottles at 4°C.All the samples were analyzed within one week.
In the activated sludge process,sludge samples were collected and analyzed from the thickener tank.Thickened Sludge is mixture of primary and secondary Sludge.In the MBBR process,due to lack of primary sedimentation tank,the samples were taken from the waste sludge of the sedimentation tank.By determining the flow and the percentage of solids in the sludge,the daily sludge solids were calculated.Then,the amounts of pollutants adsorbed into the sludge were obtained.
The sludge samples were transported to the laboratory as soon as possible and were kept in the dark at a temperature of−18 °C until analysis.Prior to performing analysis the samples were dried in lab at room temperature during about 3 days.The dried sludge was crushed and passed through a 1 mm sieve.
2.3.Extraction
In the extraction procedure,5 ml of sample was poured into a 10 ml glass conical tube.5 μl of the internal standard(n-OP)was added to the sample.Then,500 μl methanol as dispersive solvent were mixed with 100μl chloroform as extraction solvent,and the 600μl resulting mixture was injected rapidly into the sample by using a 1000 μl syringe.The resulting cloudy solution was centrifuged for 5 min at 5000 r·min−1.The fine droplets of extraction solvent that contain analytes were sedimented at the bottom of the centrifuge tube.The sedimented phase[(30 ± 2) μl]was withdrawn and calculated by a 50 μl syringe.The extracts from DLLME were evaporated to dryness in a gentle passage of nitrogen gas at room temperature.

Table 1 Information of WWTPs surveyed in this study
Subsequently the sonication-assisted extraction method for sludge was selected,1 g of dried sludges amples were placed in 15 mlglass centrifuge tube.Then 10 ml methanol were added in to tube and the mixture was placed in an ultrasonic bath and sonicated for 10 min.The vials were then centrifuged at 5000 r·min−1for 5 min.The sludge was then separated from methanol by a 0.45 μm filter.With nitrogen gas passage,the resulting methanol was dried at laboratory temperature and analyzed.
2.4.Derivatization
Due to the polar and semi-volatile nature of Alkylphenolic compounds,the derivatization process seems to be essential.Derivatization turns these compounds into derivatives with more volatility and higher thermal stability.In these circumstances,these derivatives would gain more favorable conditions for analysis in the GC–MS device.By the experiments conducted in this study,the N,O-bis(trimethylsilyl)tri fluoroacetamide(BSTFA)+trimethylchlorosilane(TMCS)(1:99)was selected as the derivatization agent.
The derivatization reagent 10.0 μl and n-hexane 10 μl were added to the test vial containing extracts from DLLME which generates sharper peaksand higher sensitivity.The sample wasvortexed for 1 min.Finally,3 μl sample was injected to the GC–MS for analysis.
2.5.Instruments
The analyses were done using GC–MS instrument(Agilent Technologies,USA),which includes gas chromatography(7890A series)and mass spectrometry(5975C series).The column used in this device was HP-5MS column(30 m × 0.25 mm,0.25 μm),and the carrier gas was helium at a constant rate of 1 ml·min−1.Then,3 μl of the derived sample were injected into the GC–MS system with a 1:10 split at 280°C.The final program used in this study for analysis in the GC–MS system was as follows:
The initially oven temperature was 60°C and held on it for 1 min;then temperature reached to 170°C with temperature ramp of 10 °C·min−1;continue to reach 300 °C with a temperature ramp of 15 °C·min−1and kept on this temperature for about 2 min.
For all compounds the selective ion monitoring(SIM)mode was used.The mass-to-charge(m/z)ratio values for derivatized 4-NP,4-t-OPand n-OP were(207,179 and 193),(207)and(179 and 278),respectively.The 4-NP is indeed a combination of different isomers that are generated due to diversity in the branches of the nonyl chain section.These isomers are seen as a cluster of peaks in the chromatogram.Therefore,the total area of these peaks is used to determine the amount of 4-NP.Due to the unique structure,n-OP and 4-t-OP appear as single peaks.For each compound,the target ions and their exit times during analysis in the GC–MS device were specified.Fig.1,shows the final chromatogram obtained with the selected program of Alkylphenolic compounds.
2.6.Determining the limit of detection(LOD)and the limit of quantitation(LOQ)
The calibration curves were drawn for each of the 4-NP and 4-t-OP compounds in the intervals of 1 ng·L−1−1 mg·L−1using 10 standard solutions.The correlation coefficient(R2)was obtained more than 99%for the calibration curves.
The limit of detection(LOD)and the limit of quantitation(LOQ)were calculated using the response standard deviation and calibration curve slope,which are given in the following equations[23].The values of LOD,LOQ and recoveries percentage in the analysis can be observed in Table 2.


Fig.1.The chromatogram of 4-NP and 4-t-OP compounds and internal standard of n-OP after extraction by DLLME method and derivatization.

s-response standard deviation
S-calibration curve slope
2.7.Determining the adsorption coefficients of APs by sludge
Adsorption to biomass is a key mechanism which results in the removal of APs from wastewater.One of the most important indicators used to evaluate the adsorption behaviors of different compounds is the distribution coefficient or specific adsorption coefficient(kd,kg−1),which is calculated by Eq.(3)[22].

This parameter can be normalized with organic matter of the adsorbent by Eq.(4).The resulting value is the organic carbon–water partition coefficient(kOC,kg−1),which reflects the fact that the organic carbon content is the main adsorption area for neutral organic materials[22,23].


Table 2 LOD,LOQ and recoveries in 4-NP and 4-t-OP analysis
Cs:the amount of pollutants in sludge μg·kg−1,Cw:the amount of pollutants in liquid,μg·L−1,OC:organic carbon content in sludge,%.
One of the objectives of this study was to investigate the adsorption coefficients of 4-NP and 4-t-OP by sludge in various WWTPs.ifciency,and mass gain and milk production by livestock.Therefore,the non-ionic surfactants are used as permitted food additives in livestocks.In addition,in livestock farms where milking is done,the need to use detergents rises sharply.As a result,these can be the reasons for high concentration of APs in livestock wastewater[27,28].
The concentrations of 4-NP and 4-t-OP compounds in urban and hospital waste waters have been higher than rural sewage.This indicates that detergents and surfactants containing Alkyl Phenols have been used more in urban and hospital areas,which has caused the higher concentration of APs in these waste waters.
The concentrations of NP and OP in urban waste waters were measured in two treatment plants in Tokyo,Japan,by Isobe and Takada.
3.Results and Discussion
3.1.Concentrations of 4-NP and 4-t-OP in the in fluent wastewater
The concentrations of 4-NPand 4-t-OPin the raw sewage are summarized in Table 3.The target analytes were detected in the all wastewater samples.In the in fluent wastewater of the WWTPs,the concentration of 4-NPin livestock wastewater(17.02μg·L−1)and commercial wastewater(8.23 μg·L−1)showed the highest values compared to urban wastewater(3.78–5.57 μg·L−1),rural wastewater(1.25–2.56 μg·L−1)and hospital wastewater(4.02 μg·L−1).
In case of 4-t-OP,in which the endocrine disrupting effect is almost 25%more than of the 4-NP[24],the commercial wastewater(718.12 ng·L−1)and livestock wastewater(686.41 ng·L−1)showed higher concentration compared to urban wastewater(86.1–183.34 μg·L−1),rural wastewater(51.45–35.0 μg·L−1),and hospital wastewater(139.35 μg·L−1).
The main use of APEs,which are the parents of APs,is in the production of surfactants and detergents which included about 90%of the total use of these materials in various industries[25].In the commercial centers,the sewage source is sanitary type and APs are used in large quantities as detergents and surfactants;thus,high values of APs are observed in commercial wastewater[26].Non-ionic surfactants have positive effects on degrading enzymes,increasing food consumption ef-The concentrations of NP and OP were determined about 1 μg·L−1and 40–190 ng·L−1,respectively[12].Gao et al.determined the amounts of NP,NP1E and NP2EO in sewage samples.The scope of changes of the mentioned compounds were obtained respectively as 3.9–7.0μg·L−1,4.0–4.8μg·L−1and 5.2–7.2μg·L−1[13].Lian et al.determined the concentration of NP(max:2 μg·L−1,min:0.91 μg·L−1)in four domestic wastewater treatment plants in Beijing[15].Bergé et al.reviewed the literatures about monitoring NP in different waste waters up to 2012.The values of NPin USA were reported in hospital wastewater(<LOQ-2.50 μg·L−1),man-made wastewater(0.25–193 μg·L−1,Median:16.7 μg·L−1),domestic wastewater(0.24–170 μg·L−1,Median:5.09 μg·L−1)and industrial wastewater(0.25–400 μg·L−1,Median:10 μg·L−1)[14].In studies by Petrovic et al.the range of NP and OP in four wastewater treatment plants in Spain were respectively determined as 1–80 μg·L−1and 0.01–5 μg·L−1[11].
Several studies have been done on monitoring of Alkyl Phenols in surface waters,sediments and sewages[4,8,13,24,29–31].But there is not enough research on waste waters with different sources that have been investigated in this study.For this reason,the amounts of 4-NP and 4-t-OP compounds were not mentioned for livestock,commercial and rural waste waters in literature review.
3.2.Concentrations of 4-NP and 4-t-OP in effluent wastewater
The4-NPand 4-t-OPconcentrationsin the effluent wastewater from U-WWTPs(0.605–2.12 μg·L−1,12.47–54.81 ng·L−1),R-WWTPs(0.42–0.932 μg·L−1,11.24–14.63 ng·L−1),L-WWTP (1.163 μg·L−1,5.35 ng·L−1),H-WWTP(0.807 μg·L−1,22.21 ng·L−1)and C-WWTP(0.671μg·L−1,40.24 ng·L−1)can be seen in Table3.Very small concentrations of alkylphenol compounds have been observed in effluent wastewater of treatment plants.Given the wide range of concentrations of these pollutants in in fluent wastewater,the process of sewage treatment has had a significant impact on removal rate of APs from sewages,which will be examined below.
The concentrations of NP and OP in effluent wastewater from two domestic treatment plants in Tokyo,Japan,were measured Isobe and Takada.The concentrations of NP and OP were determined about 0.1 μg·L−1and 10 ng·L−1,respectively[12].Gao et al.measured the NP concentration in the effluent wastewater of treatment plants in the range of 0.39–2.8 μg·L−1[13].Lian et al.determined the amount of NP in effluent wastewater of four domestic wastewater treatment plantsin Beijing as about 0.09–0.5 μg·L−1[15].In studies by Petrovic et al.,the concentrations of NP and OP effluent wastewater of four wastewater treatment plants in Spain were respectively obtained as 1 μg·L−1and 10 ng·L−1[11].Höhne et al.measured 4-NP and 4-t-OP compounds in the effluent wastewater samples at two treatment plants in Germany,respectively,less than 14.4 μg·L−1and 392 ng·L−1[9].Ros et al.measured 4-NP and 4-t-OP compounds in the effluent wastewater of treatment plant less than 81 ng·L−1and <LOD[31].

Table 3 Results from analyses conducted on 4-NP and 4-t-OP compounds
3.3.4-NP and 4-t-OP removal efficiency in different treatment processes
The processes of target WWTPsare activated sludge(AS),aerated lagoon(AL),MBBR and activated sludge along with wetland(AS+WL).The in fluent and effluent concentrations,treatment processes and removal rates of 4-NP are shown in Table 3 and Fig.2.Accordingly,for 4-NP the AS+WL process showed maximum removal rate(93.17%),followed by the MBBR(91.85%),AS(79.93%–83.99%)and AL(61.94%–66.40%).
The in fluent and effluent concentrations,treatment processes and removal rates of 4-t-OP are presented in Table 3 and Fig.3.Generally,the removal rate of 4-t-OP has been higher than 4-NP in all treatment plants.As in the wastewater samples,the removal rates of 4-t-OP in the AS+WL(99.22%)were higher than those in the MBBR(94.40%),AS(84.06%–91.42%)and AL(67.89%–71.56%).
USEPA reported that 4-NP and 4-t-OP removal rates in 17 conventional AS WWTPs were about 76%and 79%,respectively.Also,their average removal rates were mentioned in processes with nitrification(78%and 87%)and denitrification(82%and 91%)[32].According to the USEPA report,with increasing nitrification and denitrification rate,the APsremoval efficiency hasincreased.In the MBBR method,nitrification occurs partly due to high sludge retention time(SRT)in the attached bio film.Anoxic conditions have prevailed in the lower layers of the bio film,and thus,denitrification would occur.Therefore,the MBBR method is expected to showa higher removalrate in compared with activated sludge process,which is consistent with the experiment results.
When ASand AS+WL removal ratesare taken into consideration,it could be understood that WL process is the most effective process that would be able to increase the removal rates of 4-NP and 4-t-OP by about 15%.Alkylphenol pollutants such as 4-NP and 4-t-OP have a high kow,and thus,have a great tendency to be adsorbed to surfaces such as sludge,sediments and soil[8].Therefore,it seems that by passing through the roots of plants in the wetland method;these compounds have been adsorbed by the plants roots.Hence,the factor increasing the AS+WL efficiency is the high tendency to adsorption of these compounds.
In AL process,due to low MLSS in the aeration tank,the rates of 4-NP and 4-t-OP absorbed into the sludge have been also lower.In addition,lower sludge retention time(SRT,day)and non-occurrence of nitrification and denitrification have caused the process to show lower efficiency than other processes for the removal of APs.
3.4.Absorption and biodegradation rates of 4-NP and 4-t-OP in activated sludge and MBBR processes
The units in activated sludge process and moving bed bio film reactor are depicted in Fig.4.The mass balance of the sesystems includes the influent wastewater,effluent wastewater and thickening sludge.Mass balance equations are given in Eqs.(5)and(6).

Qin=in fluent wastewater,m3·d−1,Cin=in fluent concentration,μg·L−1,Qout=effluent wastewater,m3·d−1,Cout=effluent concentration,μg·L−1,˙mThikened Sludge=Mass flow of absorbed pollutants in the thickened sludge,mg·d−1,˙mbiodegradation=Mass flow of biodegraded pollutants,mg·d−1,Cs=absorbed pollutants in the thickened sludge,mg·kg−1,Qs=Mass flow of thickened sludge,kg·d−1.
The Eq.(5)concept is that the in fluent pollutants(1)will have 3 different fates.The first one is biodegradation(2)and mineralization by bacterial activities.The next one is adsorption(3)to the sludge that eventually will be disposed by gravity thickener sludge.The last one is the unchanged pollutants that observed in the effluent wastewater(4).Given that the concentration of pollutants has been measured in the in fluent and effluent wastewater,the 1st and 4th parts of the balancing are known.The rate of pollutants adsorption into the sludge is also measured(Cs),which can be seen in Table 3.The mass flow rate of sludge(Qs)in the thickeners is given in Table 1.Therefore,the third part of the equation is also known.Hence,a part of the pollutant biodegraded can easily be calculated.The results of these calculations can be seen in the diagrams of Fig.5.
According to Fig.5,biodegradation has been considered to be the principle route involved in the removal of APs during all technologies.The biodegradation rates of 4-NPand 4-t-OPin the activated sludge process in the studied treatment plants were respectively obtained more than 50%and 80%.In addition,the adsorption rates into the sludge in the activated sludge process for 4-NP and 4-t-OP were calculated as 22%–32%and 1.8%–3.3%,respectively.

Fig.2.The removal rate of 4-NP in different processes.
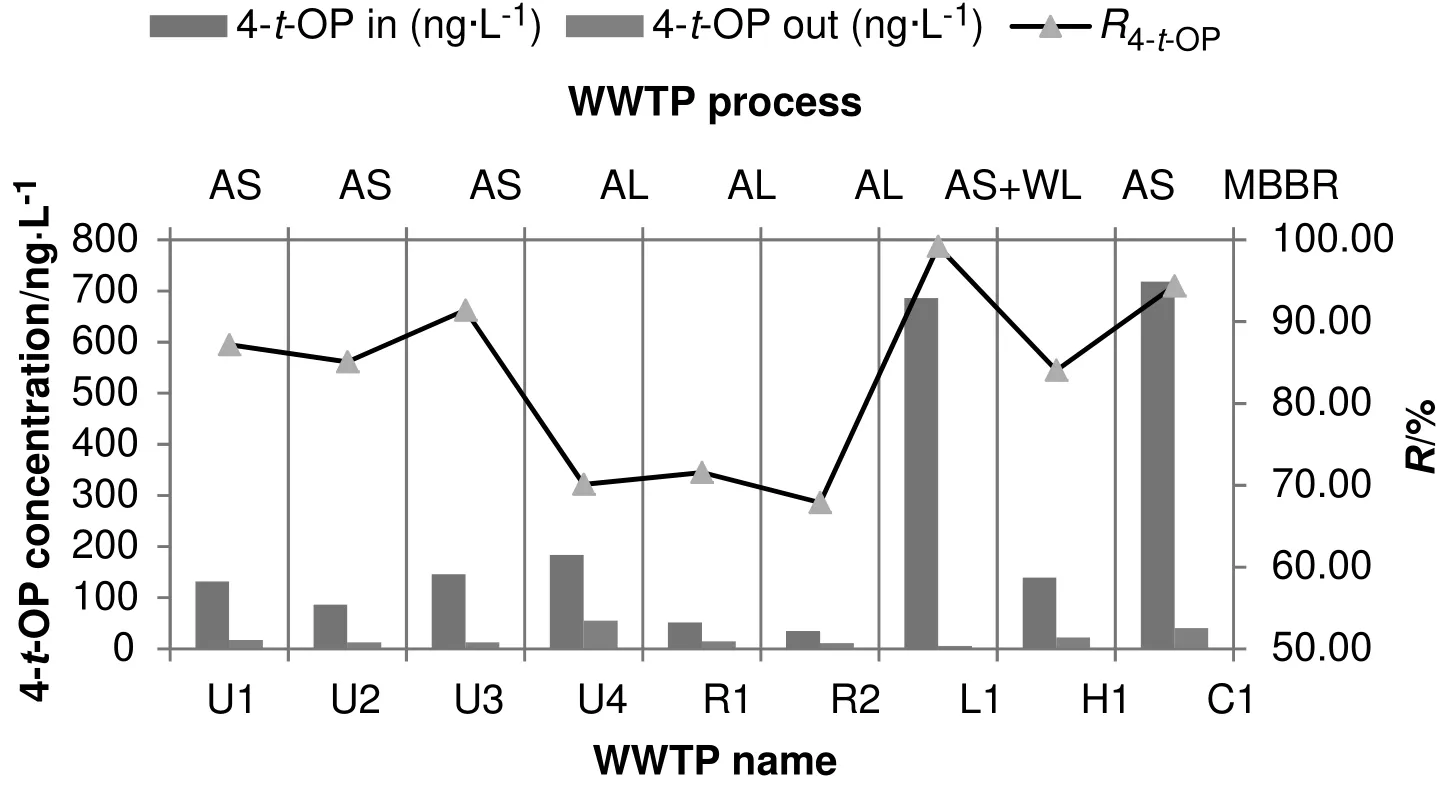
Fig.3.The removal rate of 4-t-OP in different processes.
In the MBBR process,due to high sludge retention time(SRT),there has been more time for biodegradation of the adsorbed pollutants,so its adsorption rate has reduced and its biodegradation rate has increased.The biodegradation rates of 4-NP and 4-t-OP were determined as 75%and 93.5%,respectively.Also,the rates of adsorption into the sludge in this process for 4-NP and 4-t-OP were calculated as 16.8%and 0.9%,respectively.
Bolz et al.examined the phenolic estrogen-like pollutants.In this study,the adsorption rates of 4-NP and 4-t-OP into the sludge were calculated as about 35%and 14%,respectively[33].Isobe et al.measured the NP adsorption rate into the sludgeat about 20%[34].Lian et al.measured the NP adsorption rate into the sludge in four treatment plants in Beijing as about 5%to over 70%[15].

Fig.4.Flowdiagram of A-activated sludge and B-MBBR system for mass balance.

Fig.5.The percentage of adsorption and biodegradation for(A)4-NP and(B)4-t-OP.
3.5.Calculating the adsorption coefficients
As mentioned above,kdand kOCcoefficients were calculated by Eqs.(3)and(4)in WWTPs with AS and MBBR processes,which can be observed in Table 3.The lg kdfor 4-NP and 4-t-OP was calculated respectively in the range 3.55–4.03 and 2.67–2.95.The lg kOCwas also determined for 4-NP and 4-t-OP in the range 4.15–4.63 and 3.69–4.07,respectively.Bergé et al.calculated the value of lg kdcoefficient for NP ranging from 2.70 to 3.82,and the lg kOCcoefficient as 5.39[14].In a review of studies conducted until then,Ying et al.reported the rate of lg kOCfor NP and OP,respectively,as about 4.7 to 5.6 and 3.54 to 4.25.They also announced the value of lg kdfor OP in the range from 0.77 to 2.85[35].
4.Conclusions
In this study,two xenoestrogens compounds 4-NP and 4-t-OP were evaluated in the urban,rural,livestock,commercial and hospital WWTPs.L-WWTP and C-WWTP had the highest concentrations of 4-NP and 4-t-OP in in fluents,respectively.The processes of the examined WWTPs in this study include activated sludge,aerated lagoons,MBBR and activated sludge along with wetland.The AS+WL process had the highest removal rate,while the MBBR process has been the most efficient after it.The biodegradation and adsorption rates of 4-NP and 4-t-OP were calculated.APs were mostly removed through biodegradation and adsorption to sludge is another effective process for APs removal.The lg kdand lg kOCcoefficients of the sludge for 4-NP and 4-t-OP were also determined.
Acknowledgments
This article is theres ult of Ph Dthes is approved in the Isfahan University of Medical Sciences(IUMS).The authors wish to acknowledge Vice Chancellery of Research of IUMS for the financial support,Research Project,#394773.
[1]D.Deyerling,J.Wang,Y.Bi,C.Peng,G.P fister,B.Henkelmann,K.Schramm,Depth pro file of persistent and emerging organic pollutants upstream of the Three Gorges Dam gathered in 2012/2013,Environ.Sci.Pollut.Res.23(2016)5782–5794.
[2]D.Liu,J.Liu,M.Guo,H.Xu,S.Zhang,L.Shi,C.Yao,Occurrence,distribution,and risk assessment of alkylphenols,bisphenol A,and tetrabromobisphenol A in surface water,suspended particulate matter,and sediment in Taihu Lake and its tributaries,Mar.Pollut.Bull.112(2016)142–150.
[3]M.Gavrilescu,K.Demnerová,J.Aamand,S.Agathos,F.Fava,Emerging pollutants in the environment:Present and future challenges in biomonitoring,ecological risks and bioremediation,NewBiotechnol.32(2015)147–156.
[4]J.Cavalheiro,M.Monperrus,D.Amouroux,H.Preud'Homme,A.Prieto,O.Zuloaga,In-port derivatization coupled to different extraction techniques for the determination of alkylphenols in environmental water samples,J.Chromatogr.A 1340(2014)1–7.
[5]W.Giger,F.L.P.Gabriel,N.Jonkers,F.E.Wettstein,H.-P.E.Kohler,Environmental fate of phenolic endocrine disruptors:Field and laboratory studies,Philos.Trans.R.Soc.A Math.Phys.Eng.Sci.367(2009)3941–3963.
[6]S.Massart,B.Redivo,E.Flamion,S.N.Mandiki,E.Falisse,S.Milla,P.Kestemont,The trenbolone acetate affects the immune system in rainbowtrout,Oncorhynchus mykiss,Aquat.Toxicol.163(2015)109–120.
[7]W.Zheng,S.R.Yates,S.A.Bradford,Analysis of steroid hormones in a typical dairy waste disposal system,Environ.Sci.Technol.42(2008)530–535.
[8]S.Ömero,F.Kara,F.D.Sanin,S.Ömeroʇlu,F.K.Murdoch,F.D.Sanin,S.Ömeroğlu,F.Kara Murdoch,F.Dilek Sanin,Investigation of nonylphenol and nonylphenol ethoxylates in sewage sludge samples from a metropolitan wastewater treatment plant in Turkey,Talanta 131(2015)650–655.
[9]C.Höhne,W.Püttmann,Occurrence and temporal variations of the xenoestrogens bisphenol A,4-tert-octylphenol,and tech.4-nonylphenol in two German wastewater treatment plants,Environ.Sci.Pollut.Res.15(2008)405–416.
[10]N.Månsson,L.Sörme,C.Wahlberg,B.Bergbäck,Sources of alkylphenols and alkylphenol ethoxylates in wastewater—A substance flowanalysis in Stockholm,Sweden,water,air,Soil Pollut.Focus.8(2008)445–456.
[11]M.Petrovic,M.Solé,M.J.López de Alda,D.Barceló,Endocrine disruptors in sewage treatment plants,receiving river waters,and sediments:Integration of chemical analysis and biological effects on feral carp,Environ.Toxicol.Chem.21(2002)2146–2156.
[12]T.Isobe,H.Takada,Determination of degradation products of alkylphenol polyethoxylates in municipal wastewaters and rivers in Tokyo,Japan,Environ.Toxicol.Chem.23(2004)599–605.
[13]D.Gao,Z.Li,J.Guan,H.Liang,Seasonal variations in the concentration and removal of nonylphenol ethoxylates from the wastewater of a sewage treatment plant,J.Environ.Sci.54(2017)217–223.
[14]A.Bergé,M.Cladière,J.Gasperi,A.Coursimault,B.Tassin,R.Moilleron,Meta-analysis of environmental contamination by alkylphenols,Environ.Sci.Pollut.Res.19(2012)3798–3819.
[15]J.Lian,J.X.Liu,Y.S.Wei,Fate of nonylphenol polyethoxylates and their metabolites in four Beijing wastewater treatment plants,Sci.Total Environ.407(2009)4261–4268.
[16]W.J.Sim,J.W.Lee,S.K.Shin,K.B.Song,J.E.Oh,Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants,Chemosphere 82(2011)1448–1453.
[17]S.Luo,L.Fang,X.Wang,H.Liu,G.Ouyang,C.Lan,T.Luan,Determination of octylphenol and nonylphenol in aqueous sample using simultaneous derivatization and dispersive liquid–liquid microextraction followed by gas chromatography –Mass spectrometry,J.Chromatogr.A 1217(2010)6762–6768.
[18]M.Rezaee,Y.Assadi,M.-R.Milani Hosseini,E.Aghaee,F.Ahmadi,S.Berijani,Determination of organic compounds in water using dispersive liquid–liquid microextraction,J.Chromatogr.A 1116(2006)1–9.
[19]A.González,J.Avivar,V.Cerdà,Estrogens determination in wastewater samples by automatic in-syringe dispersive liquid–liquid microextraction prior silylation and gas chromatography,J.Chromatogr.A 1413(2015)1–8.
[20]S.C.Cunha,A.Pena,J.O.Fernandes,Dispersive liquid–liquid microextraction followed by microwave-assisted silylation and gas chromatography-mass spectrometry analysis for simultaneous trace quantification of bisphenol A and 13 ultraviolet filters in wastewaters,J.Chromatogr.A 1414(2015)10–21.
[21]M.M.Parrilla Vázquez,P.Parrilla Vázquez,M.Martínez Galera,M.D.Gil García,A.Uclés,Ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction coupled with liquid chromatography-quadrupole-linear ion trap-mass spectrometry for simultaneous analysis of pharmaceuticals in waste waters,J.Chromatogr.A 1291(2013)19–26.
[22]X.Chen,J.Hu,Adsorption of natural estrogens and their conjugates by activated sludge,Water Air Soil Pollut.206(2010)251–261.
[23]S.Roberts,C.Higgins,J.McCray,Sorption of emerging organic wastewater contaminants to four soils,Water(Switzerland)6(2014)1028–1042.
[24]A.A.Oketola,T.K.Fagbemigun,Determination of nonylphenol,octylp henol and bisphenol-A in water and sediments of two major rivers in Lagos,Nigeria,J.Environ.Prot.(Irvine.Calif.)4(2013)38–45.
[25]BKH Consulting Engineers,Chemical Study on Alkylphenols,The Hague,the Netherlands,2001.
[26]M.V.F.Andrade,I.K.Sakamoto,J.J.Corbi,E.L.Silva,M.B.A.Varesche,Effects of hydraulic retention time,co-substrate and nitrogen source on laundry wastewater anionic surfactant degradation in fluidized bed reactors,Bioresour.Technol.224(2017)246–254.
[27]Y.Chen,H.Zhang,H.Wang,K.Yang,Effects of dietary addition of non-ionic surfactants on ruminal metabolism and nutrient digestion of Chinese merino sheep,Asian J.Anim.Vet.Adv.6(2011)688–696.
[28]C.H.Kim,J.N.Kim,J.K.Ha,S.G.Yun,S.S.Lee,Effects of dietary addition of surfactant tween 80 on ruminal fermentation and nutrient digestibility of Hanwoo steers,Asian Australas.J.Anim.Sci.17(2004)337–342.
[29]Y.Gu,J.Yu,X.Hu,D.Yin,Characteristics of the alkylphenol and bisphenol A distributions in marine organisms and implications for human health:A case study of the East China Sea,Sci.Total Environ.539(2016)460–469.
[30]M.Pernica,P.Poloucká,M.Seifertová,Z.Šimek,Determination of alkylphenols in water samples using liquid chromatography-tandem mass spectrometry after precolumn derivatization with dansyl chloride,J.Chromatogr.A 1417(2015)49–56.
[31]O.Ros,A.Vallejo,L.Blanco-Zubiaguirre,M.Olivares,A.Delgado,N.Etxebarria,A.Prieto,Microextraction with polyethersulfone for bisphenol-A,alkylphenols and hormones determination in water samples by means of gas chromatographymass spectrometry and liquid chromatography-tandem mass spectrometry analysis,Talanta 134(2015)247–255.
[32]USEPA,Treating Contaminants of Emerging Concern.A Literature ReviewDatabase,2010 100.
[33]U.Bolz,H.Hagenmaier,W.Körner,Input/output balance of phenolic xenoestrogens in sewage treatment plants(STPs),11th Annu.Meet.SETAC Eur.,Poster at 11th Annual Meeting of SETAC Europe,Madrid 2001,p.72076.
[34]T.Isobe,H.Nishiyama,A.Nakashima,H.Takada,Distribution and behavior of nonylphenol,octylphenol,and nonylphenol monoethoxylate in Tokyo metropolitan area:Their association with aquatic particles and sedimentary distributions,Environ.Sci.Technol.35(2001)1041–1049.
[35]G.-G.Ying,B.Williams,R.Kookana,Environmental fate of alkylphenols and alkylphenol ethoxylates— A review,Environ.Int.28(2002)215–226.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
