Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
2018-05-25HongXiaLiuNaixinWangCuiZhaoShulanJiJianRongLi
Hong-Xia Liu,Naixin Wang*,Cui Zhao,Shulan Ji,Jian-Rong Li
Beijing Key Laboratory for Green Catalysis and Separation and Department of Chemistry and Chemical Engineering,College of Environmental and Energy Engineering,Beijing University of Technology,Beijing 100124,China
1.Introduction
The separation of aromatic/aliphatic hydrocarbon mixtures,which has been studied since the 1960s,is of great importance in the chemical industry[1,2].However,because of the very similar physical and chemical properties of them(see Table 1),the separation of aromatic and aliphatic compounds is quite difficult in some cases.Traditional methods for separating aromatic/aliphatic hydrocarbon mixtures include extractive distillation,azeotropic distillation,and liquid–liquid extraction.Compared with these methods,pervaporation technique,as a new and efficient method,has advantages in both economy and environment[2–5].However,the development of pervaporation membranes used for separating aromatic/aliphatic hydrocarbon mixtures is comparatively slow(Fig.1).The main reason is that the separation performance of the pervaporation membrane cannot satisfy industrial requirements.Therefore,the major task at present for the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures is to improve the separation performance of the membrane.
At present,it is generally believed that the separation mechanism of pervaporation is the solution-diffusion.Based on this separation mechanism,pervaporation process can be divided into three consecutive steps:(1)the components are absorbed and dissolved in the feed side of the membrane;(2)the components diffuse through the membrane;and(3)the components are desorbed at the permeation side of the membrane[6].In thus,the separation performance of a membrane depends primarily on the different solution and diffusion ability of aromatic and aliphatic compounds in membrane materials.In order to achieve a good separation performance,the membrane materials are usually suggested to have a stronger affinity towards aromatic compounds,rather than aliphatic partners.
The extensively explored membrane materials for separating aromatic/aliphatic mixtures are organic polymers.They are preferred due to the advantages of good membrane-forming property,abundant species,low cost,and easy fabrication.However,the application of polymers in pervaporation suffers from obstacle in the trade-off between permeability and selectivity[8–10].Furthermore,the polymeric membranes are usually excessively swollen during the pervaporation process,leading to poor stability in long-term running.In order to solve these problems,in recent years,facilitated transport fillers are incorporated into polymers to form mixed matrix membranes(MMMs),in which the interaction between aromatic compounds and membrane materials can be enhanced,thereby improving the separation performances of the resulting membranes.However,new challenges arise in these hybrid membranes,such as how to improve dispersion and compatibility,as well as to efficiently control loading of the particles in polymer.
In this review article,we summarize the latest research advances concerning the membrane materials used for the pervaporationseparation of aromatic/aliphatic mixtures.The structural features and separation performances of explored membrane materials are highlighted and compared.In addition,we also prospect the challenges and opportunities in this research topic.

Table 1 The physical and chemical properties of some aromatic and aliphatic compounds

Fig.1.Publications on the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures.
2.Polymeric Membranes
Polymeric membranes are widely applied in the pervaporation for the dehydration of organic solvents(alcohols,acids,ethers,and ketones)[11],the removal of dilute organic compounds from water(volatile organic compounds,aroma,and biofuels from fermentation broth)[12],the separation of organic liquid mixtures[13],and the de-sulfurization of gasoline[14,15].Among these processes,the separation of aromatic/aliphatic hydrocarbon mixtures is extremely difficult,because of their close physical and chemical properties.The choice of a pervaporation membrane material is dependent on its molecular structure and property.In view of the solution-diffusion mechanism,the membrane materials should have stronger affinity towards aromatic compounds than aliphatic ones.Although both aromatic and aliphatic compounds have weak polarity,the aromatics which have delocalized π electrons,can be polarized under the induction of polar groups,while aliphatic compounds are almost not affected.Therefore,the membrane materials containing polar groups are more conducive to the preferential permeation of aromatics.Meanwhile,according to the principle of like dissolves like,aromatics preferentially permeate through the membranes containing benzene rings on the main chain of polymer,and the diffusion of aliphatic compounds is obstructed.The explored polymeric materials for separating aromatic/aliphatic hydrocarbon mixtures include polyimides,poly(ether amide)s,polyurethanes,poly(methyl methacrylate)s,polyacrylates,polysiloxanes amides,cellulose alkyl esters,and poly(vinyl alcohol)s.
In this section,the pervaporation performances of polymeric membranes fabricated by using different materials are discussed,with related results being summarized in Table 2.
2.1.Polyimides
Polyimides(PIs)have been widely studied as excellent materials for separating aromatic/aliphatic mixtures.They have high chemical and thermal resistance,good mechanical strength,and superior membrane forming properties.As shown in Fig.2,PIs usually contain a benzene ring,which can interact with the aromatics by π-π stacking interaction.In addition,a strong affinity can be formed between the polar imide functional groups of PIs and π electrons of aromatic rings.Therefore,the aromatics can permeate through the PI membrane while the aliphatic compounds are rejected[4,5,16,17].In order to further improve the affinity of PI membranes to aromatics,the structure of molecular chain in PIs can be modified by grafted copolymerization.Ribeiro et al.[18]synthesized poly(siloxane-co-imide)and poly(ether-co-imide)as membrane materials to separate toluene/n-heptane and benzene/n-heptane mixtures.The incorporation of siloxane in the copolymer greatly improved the permeation flux of membranes,while the introduction of ether had no significant effect on pervaporation performance.Their research results also indicated that the chemical structure of PIs could affect the diffusion coefficients of aromatics and aliphatic compounds in the membranes,while the separation factor was a result of the differences in solubility[19].
The poor solubility of PIs in organic solvents is one of the prominent problems,which can be improved by introduction of fluorine groups in PIs.Meanwhile,the free volume of the PI membranes is also increased[20].Therefore,they are found to have potential applications in gas separation[21–23]and pervaporation[24,25].Ye et al.[7]used 2,2-bis(3,4-dicarboxyphenyl)hexa fluoropropane dianhydride(6FDA),diamines including 2,2-bis[4-(4-aminophenoxy)phenyl]hexa fluoropropane(BDAF)and 2,2-bis[4-(4-aminophenoxy)phenyl]propane(BAPP)to prepare fluorine-containing PI membranes.The prepared 6FDA–BDAF and 6FDA–BAPP membranes were used for separating toluene/nheptane mixture.The pervaporation performance of 6FDA–BDAF membrane was superior to that of 6FDA–BAPP membrane,which was due to the strong polarity surface of 6FDA–BDAF membrane and the similar solubility parameters between toluene and 6FDA–BDAF.
Another approach to improve the separation performance of membrane is changing the molecular structure in PIs.Crown ether can be used to modify PIs because of its cavity structure.The permeation flux of PI membranes was thus improved.Yang et al.[26]fabricated a PI membrane(DSDA–DABC/DDBT)from 3,3′,4,4′-diphenylsulfone tetracarboxylic dianhydride(DSDA),trans-4,4′-diaminodibenzo-18-crown-6(DABC)and 2,8(6)-dimethyl-3,7-diaminobenzothiophene-5,5-dioxane(DDBT)using a three-step polymerization method,which was followed by further heat treatment for complete imidization.The membrane contains crown ether groups in the diamine moieties.Because the crown ether group(DABC)plays a similar role to molecular recognition for benzene,the DSDA–DABC/DDBT membrane achieved better pervaporation performance than DSDA/DDBT membrane.
During the pervaporation process,the polymeric membrane materials are prone to excessive swelling,because of the strong affinity withorganic solvent.It will lead to adecline of the separation performance.In order to improve the stability of the pervaporation membrane,crosslinking is usually used during the membrane preparation process.Intra-or inter-molecular bonds are formed to constrict polymer chain mobility and redistribute the number and size of free volume.Bell et al.[27]found that the rubber materials had a lower separation factor in the separation of aromatic/aliphatic hydrocarbon mixtures.However,the cross-linked PIs and per fluoropolymer membranes had higher selectivity and stability.PIs and per fluoropolymers had high cohesion energy,which could reduce the swelling of membranes.Meanwhile,blending different PIs and/or copolymerizing different monomers could obtain large free volume to improve the pervaporation performance of membranes.

Table 2 Pervaporation performances of a series of polymeric membrane materials

Fig.3.Chemical structures of PBI and Matrimid.The dotted line between the polymers represents hydrogen bonding interaction[28].
From the application point of view,some researchers attempt to employ commercialized materials(Matrimid and polybenzimidazole(PBI))to prepare membranes for separating aromatic/aliphatic hydrocarbon mixtures.Kung et al.[28]reported commercialized PBI and PI Matrimid blending membranes to separate toluene/iso-octane mixture.The formation of hydrogen bonding(see Fig.3)between PBI and Matrimid could improve the compatibility between them.Increasing the content of PBI greatly improved the separation factor for the selective transport of toluene,while a corresponding decline in the permeation flux was found.This is mainly due to the strong polarity,tight and rigid structure as well as enhanced anti-swelling property of PBI.For the separation of a 50 wt%toluene/iso-octane mixture,the best performance achieved was a separation factor of about 200,and a permeation flux of about 1350 g·m−2·h−1.
In the real industry,aromatic/aliphatic hydrocarbon mixtures usually contain multi-components.Staudt et al.[17,29–31]used PI copolymer membrane(6FDA–4MPD/DABA)(Fig.4)to separate aromatics from the multi-component mixtures.Three kinds of multi-component mixtures with 5 to 9 components were used as feeds.The separation performances of the non-cross-linked and cross-linked 6FDA-based copolyimide membranes were compared.The results showed that the separation factor of the membrane increased and permeation flux decreased after cross-linking,which was due to the more tightened network of cross-linked membranes.Moreover,the feed temperature has a significant effect on the separation performance of the membrane.The permeation flux of PI membrane increased with the increase of feed temperature[29].Meanwhile,the chemical stability,structural integrity,and anti-swelling property of the cross-linked membranes were better than those of the non-cross-linked membranes[32].In addition,Roychowdhury and Mitra[33]chose a mixture of n-tetradecane and phenanthrene(a PAH present in diesel)as the model diesel composition to study their pervaporation separation performance.The prepared aromatic PI membrane displayed preferential permeation of phenanthrene.Moreover,the high thermal stability,chemical resistance,and good mechanical properties of aromatic PI materials and the simple and low-cost preparation procedure make the membranes have potential possibility of industrial applications.
2.2.Poly(ether amide)s
Poly(ether amide)(PEA)is another membrane material used for separating aromatic/aliphatic mixtures.However,PEA lacks the π electron acceptor,which is usually modified by introducing functional groups to change the sorption and diffusion properties of membranes.Maji et al.[34–36]prepared four different semi fluorinated aromatic PEA copolymeric membranes(PEA I,PEA II,PEA III and PEA IV)through the polymerization reaction of 5-tert-butyl-isophthalic acid(TIPA)and four different semi fluorinated aromatic bis(ether amine)s(Fig.5).These membranes were used to separate benzene/cyclohexane mixture.The effect of copolymer structures on the selective priority of benzene was investigated at different feed temperatures.The PEA IV membrane,which contained cardo phenolphthalein anilide unit in the main chain,exhibited the highest separation factor of 5.9.The PEA III membrane had the highest permeation flux of 748 g·m−2·h−1.The reason is that the cardo fluorene moiety in PEA III can enhance π–π interaction between PEA III and benzene molecules.

Fig.4.The chemical structures of non-cross-linked and cross-linked 6FDA-based copolyimides[29].

Fig.5.The chemical structures of PEAs[34].
The permeation flux of PEA membranes is relatively high because of their affinity with aromatics.However,the excessive swelling leads to a poor stability of the membranes during the pervaporation process.In order to improve the stability of PEA membranes,block copolymers were used to adjust the property of them.Our group used commercial poly(ether-block-amide)(PEBA)containing rigid polyamide(PA)segment and flexible polyether(PE)segment as a membrane-forming material to prepare composite membrane[37].The PEBA separation layer was formed on the tubular ceramic substrate by a thermal cross-linking reaction.The sorption and diffusion behaviors of toluene and n-heptane in PEBA were investigated by the inverse gas chromatography(IGC)technique,which was used to determine the in finite dilute activity coefficient and the in finite dilute diffusion coefficient of toluene and n-heptane in PEBA.The results showed that toluene had a higher solubility and diffusivity in PEBAthan n-heptane.The prepared composite membrane had a separation factor of 4.0 and a permeation flux of 280 g·m−2·h−1at 80 °C when separating 50 wt%toluene/n-heptane mixture.More importantly,the PEBA/ceramic composite membrane exhibited a stable pervaporation performance in a relatively wide feed temperature(40 °C–80 °C)and long-time running of 30 h.For the purpose of investigating the effect of polymer hardness on pervaporation performance of PEBA membranes,Yildirim et al.[38]used different grades of commercial PEBA(PEBA 2533,3533,4033)as membrane materials to separate benzene/cyclohexane mixture.The sorption results showed that all of the PEBA had a higher affinity to benzene than to cyclohexane.The pervaporation results indicated that with the increase of PEBA hardness(hardness:PEBA 4033>3533>2533),the permeation flux decreased and separation factor increased.The degree of swelling(DS)of PEBA membrane was also decreased.The reason was because PEs oft segment has a higher affinity towards benzene than PA hard segment.Therefore,the pervaporation performance of PEBA-based membranes can be optimized via regulating the ratio of rigid segment and flexible segment in the PEBA chains.
2.3.Polyurethanes
The segmented polyurethanes(PUs)as multiblock copolymers which contain two segments are widely used in pervaporation.Wolińska-Grabczyk[39]used the soft–hard segment block co-polymer PUs to synthesize pervaporation membranes for the separation of benzene/cyclohexane mixture.The soft segment of PU is poly(oxytetramethylene)(PTMO)and the hard segment is 2,4-tolylene diisocyanate(TDI)(Fig.6).Two low molecular diols,4,4′-bis(2-hydroxyethoxy)biphenyl(BHBP)and hydroquinone bis(2-hydroxyethyl)ether(HQE)are used to extend the molecular chain of TDI.The pervaporation results showed that the chain extended PU membranes had better permselectivity towards benzene,which mainly resulted from the microphase-separated structure of the segmented PUs.The hard segments in PU membranes suppress the excessive swelling,and the soft segments increase the permeability of the membranes.Ye et al.[40]prepared polyurethaneurea(PUU)and polyurethaneimide(PUI)membranes,which contained the same soft segment of poly(ethylene adipate)diol and different hard segments(TDI–MDA hard block in PUU and MDI–PMDA in PUI).The results showed that for a50 wt%benzene/cyclohexane feed solution,PUU membrane gave a separation factor of 6.29 and a permeation flux of 264 g·m−2·h−1,while the separation factor and permeation flux of PUI membrane were 8.25 and 121 g·m−2·h−1,respectively.Compared with the PUI membrane,PUU membrane easily swelled and had higher permeation flux with a lower separation factor.It was because that the hydrogen density between soft and hard segments could be weakened during the pervaporation process,which further led to the poly(ethylene adipate)diol segment in PUU obtained good mobility.
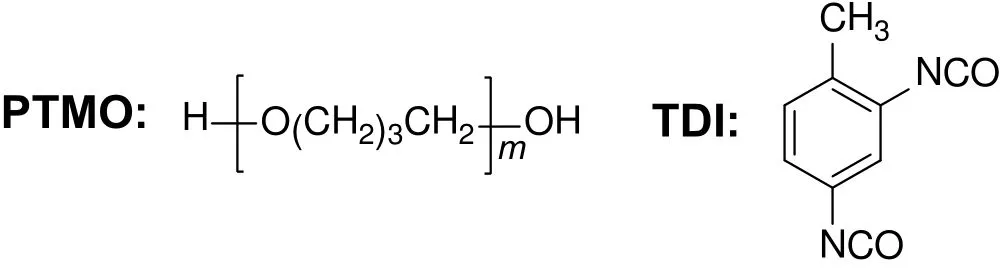
Fig.6.The soft segment and hard segment of PU[39].
2.4.Poly(methyl methacrylate)s
Poly(methyl methacrylate)(PMMA)has recently attracted considerable attention as an organic matrix in membrane preparation because of its exceptional thermal and mechanical stability,good chemical resistance and its compatibility with other membrane materials.Membranes prepared by PMMA mixed with other organic or inorganic materials are widely used in polymer electrolyte fuel cells(PEFCs)[41]and pervaporation.Okeowo et al.[42]reported a series of nonequilibrium nanoblend NBR/Hydrin/PMMA membranes to separate benzene/cyclohexane mixture.The membranes were prepared via the thermal cross-linking method.The used membrane materials were PMMA,acrylonitrile butadiene rubber(NBR),and a tercopolymer of ethylene oxide/epichlorohydrin/allyl glycidyl ether(Hydrin).The chemical structures of NBR,Hydrin and PMMA are shown in Fig.7.NBR and Hydrin have good heat resistance.They are usually used to control the permeant solubility.As a result,they can prevent the excessive swelling of membranes to preserve selectivity.The rigidity and hardness of PMMA improve the mechanical strength of the membranes.Moreover,the solubility parameter of PMMA is more similar to benzene,resulting in increasing the selectivity of the membranes.It was found that the membrane containing 80 wt%NBR,10 wt%Hydrin,and 10 wt%PMMA had a permeation flux of 160 g·m−2·h−1and a separation factor of 7.3 when separating 50 wt%benzene/cyclohexane mixture.With the decrease of NBR content,the permeation flux of the membrane increased,while the separation factor had no significant change.The blend membrane could be operated stably in 48 h.
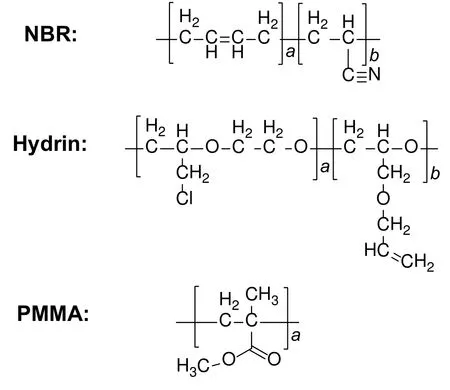
Fig.7.The chemical structures of NBR,Hydrin,and PMMA[42].
Methyl methacrylate(MMA)can also be used to prepare pervaporation membranes for separating aromatic/aliphatic mixtures[43].The composite membranes were prepared by an interfacial reaction with different polyelectrolytes.An oppositely charged ionic reagent served as the ionic surfactant.The pervaporation results showed that benzene had better permeability in the separation of multicomponents aromatic/aliphatic hydrocarbon mixtures.Meanwhile,the active groupssuch assulfoethyl groupsin the membrane had a stronger coordination with the π orbitals of benzene than toluene.Therefore,the cross-linked p-(MMA-co-MASPE)(Fig.8)membranes had a higher permeation flux in the separation of benzene/cyclohexane mixture than that of toluene/n-heptane mixture.

Fig.8.The chemical structure of poly-(methyl methacrylate-co-methacrylic acid-[3-sulfopropyl ester]potassium salt)[p-(MMA-co-MASPE)][43].
2.5.Polydimethylsiloxanes
Polydimethylsiloxane(PDMS)membranes are widely used in the field of alcohol permselective pervaporation[3,44],but they have not been extensively investigated for the separation of aromatic/aliphatic hydrocarbon mixtures.Chen et al.[45]synthesized cross-linked polydimethylsiloxane/polyetherimide(PDMS/PEI) flat-sheet composite membranes to separate benzene/cyclohexane mixture.PEI was prepared as the asymmetric microporous supporting layer by a phase inversion method.PDMS was cross-linked as the separation layer on PEI membrane.PDMS and PEI both have--O--bond,so their interface can cement closely.They found that cross-linking was important for the anti-swellingproperty of membranes.Both theseparation factor and stability performance of the membrane were improved after cross-linking.The average separation factor of PDMS/PEI membranes was 13.2,and the permeation flux was 218 g·m−2·h−1for benzene/cyclohexane mixture.Moreover,the pervaporation performance of the membrane remained stable in 180 h.In order to improve the interfacial stability between PDMSseparation layer and substrate,Zhou et al.[46]modified the polyacrylonitrile(PAN)substrates with poly(methylhydrosiloxane)(PMHS)by plasma treatment.The results showed that the corresponding PDMS separation layer displayed quite lowDS in toluene and n-heptane,due to the enhanced interfacial interaction.
2.6.Polyacrylates
Due to the swelling effect of the aromatic/aliphatic mixtures,the stability of the pervaporation membrane is required to be improved.Block copolymers are usually used to enhance the anti-swelling property of the membrane.An et al.[47]prepared polyacrylonitrileblock-poly(methyl acrylate)(P(AN-b-MA))membranes to separate benzene/cyclohexane mixture.The effect of MA content in P(AN-b-MA)membranes on separation performance was discussed.When the MA content in the membrane increased,the permeation flux of benzene increased with the separation factor decreased.However,the relationship between the swelling behavior and the MA content in the membranes revealed a discontinuity phenomenon.The DS and the benzene flux increased dramatically when the MA content in the membrane exceeded 40 mol%.All these might be explained by the transition of MA segment from a dispersion phase to a continuous phase with increasing MA content in the P(AN-b-MA)block copolymer membranes.
In addition to the membrane material,the structure of the composite membrane also has a significant influence on the swelling resistance.Li et al.[48]developed a novel atmospheric dielectric barrier discharge(DBD)plasma graft- filling technique to prepare “pore- filling”composite membranes.The PEO526OHMA/PAN membrane was prepared by grafting poly(ethylene glycol)methacrylate(PEO526OHMA)in the sublayer pores and onto the surface of the asymmetric PAN ultra filtration membrane.By the double-plasma grafting strategy combining the syn-irradiation grafting with the post-irradiation grafting,the “pore- filling”composite membrane exhibited excellent performance for pervaporation of aromatic/aliphatic hydrocarbon mixtures.When the feed solution was 20 wt%toluene/n-heptane mixture,the separation factor of the membrane was 7.8,while the permeation flux was 1620 g·m−2·h−1.Meanwhile,the “pore- filling”structure effectively suppressed excessive swelling of the membrane during the pervaporation process,thereby improving the stability of the membrane.Iravaninia et al.[49]used molecular surface engineering(MSE)technique to modify the surface of asymmetric PAN membrane with polyoxyethylene methacrylates and monomethyl polyoxyethylene methacrylate as pore- filling agents.The MSE-modified membrane exhibited a high flux of 4610 g·m−2·h−1with a separation factor of 4.985,when separating 10 wt%toluene/n-heptane mixture.Meanwhile,they predicted the unsteady state transport of toluene and n-heptane through the membranes in a pervaporation process via a 2D mathematical model[50].The simulation resultswerein good agreement with the experimental data,and revealed that the developed model could provide a general simulation of mass transport in the pervaporation process.
2.7.Celluloses
Cellulose(Fig.9)has a good durability to organic solvents,so it can be used to control the excessive swelling of membranes during the pervaporation process.However,the permeability of cellulose membranes is lowdue to their weak affinity with aromatic and aliphatic compounds.Therefore,cellulose should be modified with functional groups to improve the affinity with aromatics before used to prepare membranes.Uragami et al.[51]prepared a series of cellulose alkyl ester membranes containing ethyl,butyryl,pentyl,and heptyl groups.These membranes were used to separate benzene/cyclohexane mixture.The permeation flux of cellulose alkyl ester membranes with different substitution groups was improved compared to that of pristine cellulose membranes.This could be due to the increased DS of membranes after incorporation of alkyl ester.In addition,they found that the carbon number in the ester groups had an important effect on the permeation flux and separation factor.When the carbon number in the ester groups increased,the swelling of membrane obviously increased,resulting in an increase of the permeation flux.Meanwhile,for all cellulose alkyl ester membranes with different number of carbon in the alkyl ester groups,the diffusion selectivity was greater than the sorption selectivity,which indicated that the separation of benzene/cyclohexane mixture through the cellulose alkyl ester membranes was mostly governed by the diffusion process.
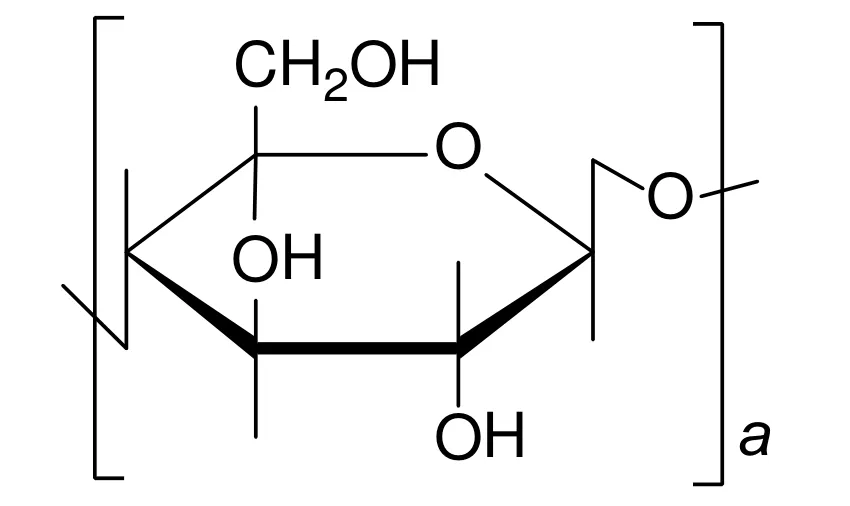
Fig.9.The chemical structure of cellulose.
The separation performance of the cellulose membrane can also be controlled by blending other substances.Bai et al.[52]prepared poly(trimethyleneco-ethylene terephthalate)/cellulose acetate(PTET-60/CA)blend membranes to separate benzene/cyclohexane mixture.When the mass fraction of PTET-60 in PTET-60/CA blends(WPTET-60)was lower than 0.35 or more than 0.5,PTET-60 had good compatibility with CA.When the weight fraction of PTET-60 increased from 0 to 0.35,the DS and permeation flux of the blend membranes increased.A series of blend membranes with different compositions were synthesized via solution blending of sodium alginate(SA)(Fig.10)and sodium carboxymethyl cellulose(CMC)[53].The pervaporation results demonstrated that the permeation flux increased with the increase of CMC concentration.However,when the concentration of CMC increased to 75 wt%,extensive swelling was found and the separation performance declined.For a 19.6 wt%benzene/cyclohexane mixture,the blend membrane containing 25%SA and 75%CMC showed optimum flux and separation factor.The separation factor and permeation flux could achieve 57.90 and 2233.67 g·m−2·h−1,respectively.
3.Hybrid Membranes
Although pure polymers can be used to prepare membranes for the separation of aromatic/aliphatic hydrocarbon mixtures,it is difficult to simultaneously obtain high membrane flux and separation factor.In addition,the excessive swelling of the polymeric membranes in organic solvent will lead to the degradation of separation performance during the long-term running.In recent years,hybrid membranes have attracted a great deal of attention because of their prefect adsorption/separation properties and comprehensive potential applications.Organic/inorganic hybrids are the most common materials,which have been studied by many researchers.Organic polymer materials have good flexibility and toughness,while inorganic materials can improve the anti-swelling property,mechanical strength,chemical resistance and thermal stability of the membrane.In addition,the incorporated particles have a strong interaction with the aromatic hydrocarbons,which can enhance the interaction between the membrane materials and aromatic hydrocarbons,thus to improve the solubility selectivity of the membrane.The incorporation of particlesin polymer could also increase the free volume of the membrane and mass transfer channel,so that the permeability of the membrane can be improved.At present,facilitated transport fillers that have been used include organic macromolecules(cyclodextrin,chitosan and calixarene),inorganic particles(transition metal ions,molecular sieves,carbon nanotubes,graphite,graphene oxide and silicon dioxide),and metal–organic materials(MOFs and MOPs).
In this section,the effect of different facilitated transport fillers on pervaporation performances of hybrid membranes is discussed,with related results being summarized in Table 3.
3.1.Organic macromolecules as the dispersion phase
Organic macromolecules as the dispersion phase for preparing hybrid membranes have the advantage of good membrane-forming properties and compatibility with polymer matrix.They are doped into polymer membranes to improve the affinity of membranes with aromatics.The mass transfer of aromatics is promoted to improve the separation performance of membranes.
3.1.1.Cyclodextrin

Fig.10.The chemical structure of sodium alginate(SA).
Cyclodextrins are a series of cyclic oligosaccharides which can be produced by the hydrolysis of amylose.The α-cyclodextrin(α-CD)or β-cyclodextrin(β-CD)containing hydrophobic cavities are generally composed of six or seven glucose units by an α-1,4-glycosidic linkage(Fig.11).Peng et al.[60]doped commercial poly(vinyl alcohol)(PVA)with β-CD to prepare β-CD/PVA hybrid membranes for the pervaporation separation of benzene/cyclohexane mixture.Glutaraldehyde(GA)was used as the cross-linker to immobilize cyclodextrins in the polymer matrix.Compared to pure PVA membranes,the β-CD/PVA hybrid membranes had an increased benzene permeation flux of 30.9 g·m−2·h−1and a separation factor of 27 for a 50 wt%benzene/cyclohexane mixture.This is due to the fact that the doping of β-CD could increase the solubility selectivity and diffusion selectivity of membranes.They also presented a possible separation mechanism of benzene and cyclohexane molecules: firstly,benzene molecules are absorbed onto the β-CD/PVA hybrid membranes;then benzene molecules are transported through the membranes by jumping from one β-CD to another.However,cyclohexane molecules are strongly adsorbed onβ-CD.Hence,benzene molecules will preferentially permeate through the β-CD/PVA hybrid membranes while cyclohexane molecules are rejected.
Rölling et al.[61]used polyethylene glycol dimethacrylate(PEGDMA)and acrylated cyclodextrin to prepare cross-linked copolymer for separating toluene/cyclohexane mixture.The copolymer was cross-linked by ultraviolet(UV).They found that compared with pure PEG-DMA membranes,the membranes incorporated with α-CD and β-CD showed a higher selectivity to aromatics.However,the selectivity of the membranes based on α-CD and β-CD had no obvious difference,but the permeation flux of the membranes containing α-CD was higher at low aromatic feed concentrations.This might be explained by the fact that the size of aromatic molecule is larger than the cavity of α-CDand is difficult to be trapped.The complex formation constant is lower than that of the β-CD and toluene.

Fig.11.The chemical structures of α-cyclodextrin and β-cyclodextrin.
Dubey et al.[62]compared the separation performances of the poly(vinyl acetal)(PVAc)membranes incorporated with different macromolecules,such as α-CD,β-CD and butyl calixarene(calix).The diameter of the cavity varies in the order:β-CD(0.78 nm)>α-CD(0.57 nm)>calix(0.20 nm).The results indicated that the membranes containing α-CD or calix showed slight decrease on permeation flux due to the small cavity.While the membrane containingβ-CD with the large cavity had ahigh permeation flux.However,there was no significant difference on the separation factor of these modified membranes.It demonstrated that the cavity of the cyclodextrin had a significant influence on the permeation flux of the membrane.
3.1.2.Chitosan
Chitosan(CS)(Fig.12)is a cationic polysaccharide,which has some desirable properties such as high hydrophilicity,good chemical resistance,biodegradability,and good membrane-forming property[63].Meanwhile,as a rigid polymer,CS has a large free volume and allows easy segmental mobility in glassy state,which results in high permeability.More importantly,CS is a natural polymer which can be easily obtained by alkaline deacetylation of chitin.Because of these advantages,CS becomes one of the most common membrane materials.Lu et al.[64]reported PVA/CS blend membranes which were prepared by incorporation of PVA and CSin varying proportions for the separation of benzene/cyclohexane mixture.Compared with pure PVA and CS membranes,the PVA/CS blend membranes had a better pervaporation performance.For a 50 wt%benzene/cyclohexane mixture,the PVA/CS blend membrane with 50 wt%CS showed a total permeation flux of 51.41 g·m−2·h−1and a separation factor of 49.9.The intermolecular hydrogen bonding between PVA molecules and CS molecules resulted in looser arrangement of the two polymer chainsin PVA/CSblend membranes.Consequently,the diffusivity of components was enhanced in PVA/CS blend membranes.Meanwhile,the separation factor was greatly improved with the existence of sufficient amount of hydrophilic groups in blend membranes.
3.2.Inorganic particles as the dispersion phase
Organic–inorganic hybrid membranes possess both advantages of polymeric materials and inorganic particles,which have attracted much attention in recent years[65–69].Inorganic particles can improve the anti-swelling,mechanical strength,chemical resistance and thermal stability of membranes.Nowadays,inorganic particles used as fillers for separating aromatic/aliphatic hydrocarbon mixtures include metal ions,carbon materials,zeolite molecular sieves and silicon dioxide.Although the incorporation of inorganic fillers in polymers could improve the separation performance of membranes,there are still some problems which need further research,such as the controlling of particle size and dispersion,reducing particle agglomeration,improving the compatibility between polymers and inorganic particles,losing of particles during the pervaporation process,and increasing the loading of inorganic particles in polymers.All of these conditions may have a significant influence on the separation ability of membranes.
3.2.1.Metal ions
Transition metal ions can form δ bond with carbon atoms,and the dorbitals in these transition metal ions enable them to form d–π conjugation interaction with unsaturated hydrocarbons.Because of these properties,transition metal ions can be used as fillers to prepare hybrid membranes for separating aromatic/aliphatic hydrocarbon mixtures[70–72].Wu et al.[73–76]prepared AgCl/PMMA hybrid membranes by in situ polymerization.The effect of surfactants on the size and morphology of AgCl nanoparticles,the structure and pervaporation performance of the hybrid membranes were investigated.They prepared some hybrid membranes containing small molecule surfactants such as dioctyl sodium succinate(AOT)and a triblock copolymer polyoxyethylene–polyoxypropylene–polyoxyethylene(F127).AOT surfactant has poor compatibility with the polymers,which results in the formation of two phases between AOT and the polymers.Thus,large particles reached several micrometers in size and appeared in the membrane after polymerization because of the aggregation of the particles[70].When the surfactant was replaced by F127,small AgCl nanoparticles were formed in the microemulsion and were distributed uniformly in the hybrid membrane after polymerization.It was because of the favorable affinity between the surfactant and PMMA.However,because the solubility of F127 in the MMA solution was weak,the amount of AgCl nanoparticles in the microemulsion was limited,which further decreased the separation performance of the hybrid membranes.In order to solve this problem,polymerizable 2-acrylamido-2-methyl propane sulfonic acid(AMPS)as another surfactant was added in microemulsion to prepare AgCl/poly(GMA-co-MMA-co-AMPS)copolymer hybrid membranes.

Fig.12.The chemical structure of chitosan.
In order to control the size and loading of AgCl nanoparticles,Wu et al.proposed a method namely ionic liquid microemulsion to prepare AgCl/poly(MMA-co-AM)and AgCl/poly(MMA-co-ST)hybrid membranes.During the preparation process,they used the ionic liquid 1-dodecyl-3-methyl imidazoium chloride(C12mim Cl)as the surfactant,methyl methacrylate-acrylamide(MMA-co-AM)and methyl methacrylate-styrene(MMA-co-ST)as the oil phase,respectively.With the concentration of C12mim Cl increasing,the permeation flux and separation factor increased.When the concentration of C12mim Cl was more than 5 mol·L−1,the separation factor slightly decreased because of the formation of cavities in the hybrid membranes after removing C12mim Cl.The π bonding between ST and benzene molecules could improve the DS of AgCl/poly(MMA-co-ST)hybrid membranes,which promoted the diffusion of benzene molecules in the polymer matrix,thus enhancing the separation performance.
Ag nanoparticles can also be doped into polymeric materials to prepare hybrid membranes.The aggregation of Ag in the membranes has a significant influence on the separation performance.Mahmoudi et al.reported that combining Ag nanoparticles with graphene oxide(GO)nanosheets may result in the uniform distribution of the metal particles in the membrane[77].Meanwhile,adding metals or metal oxide nano-particles onto the GO surface or into the lamellar structure of GO can effectively prevent the aggregation of GO.Dai et al.[78]prepared GO–Ag nanoparticle composites through impregnation reduction using different reactants.Then,a series of GO–Ag/PI hybrid membranes were prepared by in situ polymerization to separate benzene/cyclohexane mixture.Experiment results showed that PI hybrid membranes containing Ag–GO–Cexhibited the best separation performance among hybrid membranes.This was due to that in the Ag–GO–C nanoparticle composites,the distribution of small Ag nanoparticles on the GO surface and in the hybrid membranes was more homogeneously compared with the membranes prepared using other reactants.Moreover,the pervaporation performance of the hybrid membranes initially increased but eventually decreased with Ag content increasing in Ag–GO nanoparticle composites.At high Ag content in Ag–GO nanoparticle composites,the great aggregation of Ag nanoparticles in hybrid membranes would hinder the diffusion of benzene molecules in the polymer matrix.The best performance of GO–Ag–C/PI hybrid membranes was obtained at 15 wt%Ag content in Ag–GO nanoparticle composites.The permeation flux and separation factor were ~1600 g·m−2·h−1and ~35,respectively,when the feed was 50 wt%benzene/cyclohexane mixture.
3.2.2.Carbon-based materials
Graphite consists of the repetitions of hexagonal carbon ring,which is similar to the structure of benzene molecule.The σ and π bond interaction between graphite and benzene molecules can improve the pervaporation performance of membranes for separating aromatic/aliphatic hydrocarbon mixtures.Lu et al.[79] filled PVA and CS blending membranes with carbon graphite(CG)to prepare CG–PVA/CS hybrid membranes.The free volume of CG–PVA/CS hybrid membranes significantly increased with the incorporation of CG.The DS of CG–PVA/CShybrid membranes in benzene/cyclohexane mixture increased with the increase of CG content due to the loosening of polymer chain arrangement and the decrease of crystallization degree.The results indicated that the separation factor was mainly dominated by solubility selectivity rather than diffusivity selectivity.Moreover,both the loading of CG and the mass ratio of PVA to CS in hybrid membranes would affect the separation factor and permeation flux of the CG–PVA/CS hybrid membranes.They also studied the influence of crystalline flake graphite on the separation of benzene/cyclohexane mixture[80,81].Compared with pure PVA membranes which had a separation factor of 16.9 and a permeation flux of 23.1 g·m−2·h−1,PVA–graphite hybrid membranes showed an increasing separation factor with 6-fold(91.6)and an increasing permeation flux with 4-fold(91.3 g·m−2·h−1)for a 50 wt%benzene/cyclohexane mixture.This may be due to the fact that graphite has much higher flexibility,and the defect-free voids at the PVA–graphite interface are formed,which loosens the chain packing of PVA and increases the free volume of membrane[80].
Recently,graphene oxide(GO)as an atomic layer thick nanosheet containing oxygen-rich functional groups,offers a potential for making nanocomposite materials with high chemical stability,excellent antifouling and strong hydrophilicity properties.Our group[82]prepared a poly(vinyl alcohol)–graphene oxide(PVA–GO)nanohybrid layer onto an asymmetric PAN ultra filtration membrane to form a“porefilling”membrane by a dynamic pressure-driven assembly method.The size of GO sheet was reduced to match the aperture of the substrate by ultrasonic dispersion.Therefore,the GO sheet could penetrate into the pores of the substrate to form a “pore- filling”structure.The doping of GO nanosheet into PVA molecules increased the π electron acceptor,thereby improving the separation performance of hybrid membranes.Meanwhile,the “pore- filling”structure of the PVA–GO hybrid membrane helped to suppress excessive swelling of the membrane,and its stability was effectively improved.The pervaporation experiments showed that the separation performance of hybrid membrane had no significant change even after immersion of the membrane in the 50 wt%toluene/n-heptane mixture for 480 h.
Apart from graphite and its derivatives,carbon molecular sieve(CMS)as an outstanding candidate is also doped into polymeric materials to prepare hybrid membranes.CMS has appropriate pore size,narrowpore size distribution,and interconnected pore.At the same time,CMS possesses high adsorption selectivity towards organic compounds,which promotes its wide application in adsorption and separation processes.It is important that CMSis not affected by swelling and can operate at high temperatures and under harsh solvent environments.Therefore,CMS-based membranes have great applications in the field of gas separation[83,84],removal of dilute organic compounds from water[85],dehydration of organic solvents[86,87],separation of organic liquid mixtures[88]and separation of biofuel[89].Sun et al.[88]prepared PVA–CMS hybrid membranes to separate benzene/cyclohexane mixture.The hydrogen bond interaction among PVA polymer chains was decreased by the filling of CMS to make the chains more flexible.The crystallinity of PVA membrane was thusdecreased and the free volume in the membrane was increased.PVA–CMS hybrid membrane could effectively reduce mass transfer resistance and improve the permeation of benzene molecules.However,when the filling of CMS was excessive,the hybrid membranes inhibited the permeation of benzene molecules because excessive CMS took up the free volume and adjoining CMS particles blocked the pores on the CMS.
Carbon nanotube(CNT)is another important member in the family of carbon materials.It has many superior properties such as high flexibility,lowmass density,high-specific surface area,and the effective π–π stacking interaction with aromatic compounds[90,91].Therefore,CNT can be a candidate to separate aromatic/aliphatic hydrocarbon mixtures.Peng et al.[92]prepared PVA/CNT hybrid membranes to separate benzene/cyclohexane mixture.The CNT was dispersed by β-CD.Compared with pure PVA membranes and β-CD/PVA membranes,the Young's modulus and thermal stability of β-CD-CNT/PVA hybrid membranes were significantly improved.The results showed that π–π stacking interaction existed between CNT and benzene molecules,which enhanced the adsorption and diffusion of benzene molecules in the membranes.In addition,CS is another choice to disperse CNT due to the emulsifying capacity of CS and the unique solubility behavior of CS.Compared with β-CD-CNT,the dispersion and solubility behavior of CNT in membrane can be remarkably improved through substantial wrapping of CS.Thus,Peng et al.[93]prepared hybrid membranes using PVA and CNT wrapped with CS to separate benzene/cyclohexane mixture.The free volume of PVA–CNT nanohybrid membranes was investigated by molecular dynamics(MD)simulation.The pervaporation performance of PVA–CNT(CS)(CNT content:2.0 wt%)nanohybrid membrane is much better than that of β-CD-CNT/PVA hybrid membrane.It may be attributed to the suitable pore size of PVA–CNT(CS)(0.269 nm)which is between the size of benzene molecule(0.263 nm)and cyclohexane molecule(0.303 nm).
In recent years,multi walled carbon nanotube(MWCNT)is also used to prepare hybrid membranes for the separation of benzene/cyclohexane mixture.Wang et al.[94]used two chemical methods to modify MWCNT surface in order to change the surface polarity of the MWCNT and improve its distribution in PMMA.MWCNT-PMMA hybrid membranes containing aminated MWCNTs(MWCNT-NH2)and carboxylic MWCNTs(MWCNT-COOH)were prepared.MWCNT-NH2exhibited high surface polarity,which therefore contributed to the distribution of MWCNT-NH2in PMMA.The highest separation factor for the hybrid membranes containing 1.0 wt%MWCNT-NH2was about 21,which was about seven times that of membranescontaining pristine MWCNTs.It is maybedue to the strong complexation between benzene molecules and MWCNT-NH2.MWCNTs can also be modified by metal ions such as Ag+.Shen et al.[72]doped functionalized MWCNTs which were grafted with Ag+on the pyridine ring by a complexation reaction into a CS membrane to prepare MWCNTs-Ag+/CS hybrid membranes for separating benzene/cyclohexane mixture.The pervaporation performance of MWCNTs-Ag+/CS hybrid membranes was higher than that of MWCNTs/CS hybrid membranes and pristine CS membranes.
Active carbon is widely reported in the field of adsorption or separation of organic compounds[95],desulfurization of fuel[96]and adsorption or separation of gas[97,98]because of it shigh adsorption selectivity,the molecular sieving properties and capacity towards most hydrophobic organic compounds.Aouinti et al.[99]doped super activated carbon(Maxsorb SPD30)into PVC to prepare hybrid membranes for the separation of toluene/n-heptane mixture.The hybrid membranes had high affinity with toluene molecules.With the increase of Maxsorb SPD30 content,the permeation flux of hybrid membranes increased rapidly,while the Young's modulus decreased slightly.When the loading of Maxsorb SPD30 was up to 40 wt%,the best separation performance was obtained.
3.2.3.Zeolite molecular sieves
With a series of advantages such as high mechanical strength,good thermal and chemical stability,zeolite molecular sieve has been used as a kind of fillers to prepare hybrid membranes[100,101].Zhang et al.[71]used Rh-loaded H-β-zeolite(Rh/H-β-zeolite)as an inorganic particle doped into PVC to prepare hybrid membranes for the pervaporation separation of benzene/cyclohexane mixture.The pervaporation performance of Rh/H-β-zeolite hybrid membranes was much better than that of pure PVC membranes,which was due to the strong interaction between benzene molecules and Rh/H-β-zeolite.As the loading of Rh/H-β-zeolite increased,the separation factor of hybrid membranes increased firstly and then decreased.The membrane containing 7 wt%of Rh/H-β-zeolite had the highest separation factor of 26.44 for a 50 wt%benzene/cyclohexane mixture.When the feed concentration of benzene increased,the permeation flux increased while the separation factor decreased due to plasticization or swelling of membranes.
3.2.4.Silicon-based materials
Peng et al.[102]used PVA and γ-glycidoxypropyltrimethoxysilane(GPTMS)to prepare organic–inorganic hybrid membranes by an in situ sol–gel approach for the separation of benzene/cyclohexane mixture.Fig.13 shows the structure of PVA–GPTMS.Compared with pure PVA membranes,PVA–GPTMS hybrid membranes had higher thermal stability and pervaporation performance.When the content of GPTMS was 28 wt%,for a 50 wt%benzene/cyclohexane mixture,PVA–GPTMS hybrid membrane had a permeation flux of 137.1 g·m−2·h−1and a separation factor of 46.9.In addition,they also investigated the relationship between apparent fractional free volume and permeation flux of the membranes[103].The results showed that PVA–GPTMS hybrid membranes possessed small free volume cavities with an average radius of about 0.26–0.30 nm allowing only benzene molecule diffusion,and large free volume cavities with an average radius ranging between 0.39 and 0.42 nm,which allowed both benzene and cyclohexane molecule diffusion.Thus,the pervaporation performance of PVA–GPTMS hybrid membranes increased dramatically with the incorporation of GPTMS.
In addition,clay which is mainly composed of silicate can be used as another silicon-based material to prepare hybrid membranes.Aouinti et al.[104]used Maghnite H,Maghnite H+,Wyoming,Kaolin and Nanocor clay particles as fillers to prepare hybrid PVC membranes for separating toluene/n-heptane mixture.The addition of Wyoming and Kaolin to PVC led to a decrease of permeation flux due to the barrier effect of Wyoming and Kaolin particles.However,the permeation flux increased 200%and 700%for Maghnite and Nanocor clay particles as fillers.It was because the size of Nanocor clay particle is nanoscale,and it has a good affinity with toluene molecules.Moreover,organophilic bentonite as another silicon-based filler can also be doped into polymeric materials to prepare hybrid membranes.Kuila and Ray[53]blended organophilic bentonite with 25%SA and 75%CMC mixture to prepare a series of blend membranes.In comparison to pure SA/CMC membranes,the separation factor of the blend membranes filled with organophilic bentonite filler was greatly improved to 212,while the permeation flux showed a decrease from 1540 to 713 g·m−2·h−1.
3.3.Metal–organic frameworks(MOFs)as the dispersion phase

Fig.13.The chemical structure of PVA–GPTMS[102].
Metal–organic frameworks(MOFs),as new and effective materials,have attracted more attentions in the field of membrane materials[105].MOF-based hybrid membranes are widely applied in gas separation[106,107],nano filtration[108,109],reverse osmosis[110]and pervaporation[111,112].MOFs can also be used as fillers in the separation of aromatic/aliphatic hydrocarbon mixtures,because their unsaturated metal ions can form d–π conjugation interaction with aromatic molecules.Meanwhile,organic linkers can also interact with aromatic molecules by π–π conjugation.As a result,the incorporation of MOFs into polymeric membranes is in favor of enhancing the affinity of hybrid membranes to aromatic molecules through these interactions,thereby improving the separation factor and permeation flux of membranes.Our group[111]doped Cu3(BTC)2as a kind of facilitated transport fillers into PVA to prepare Cu3(BTC)2/PVA hybrid membranes for the pervaporation separation of toluene/n-heptane mixture.The crystal structure of Cu3(BTC)2was shown in Fig.14(a).When the Cu3(BTC)2loading was 0.75 wt%,for a 50 wt%toluene/n-heptane mixture,the hybrid membranes had a separation factor of 17.9 and a permeation flux of 133.3 g·m−2·h−1,which were much higher than the pure PVA PEBA matrix.The highest separation factor of 5.1 and the permeation flux of 771 g·m−2·h−1were obtained for 10 wt%toluene/n-heptane mixture when the Co(HCOO)2loading was 4 wt%.
3.4.Metal–organic polyhedras(MOPs)as the dispersion phase
Although the compatibility of MOF and polymer is improved,the dispersion of MOFs in polymer still needs to be further improved because of the agglomeration of the MOF particles.It can be solved through adding metal–organic polyhedras(MOPs)as fillers.MOPmolecules can dissolve in specific solvent and thus form a monodisperse solution.In addition,W3000 is an amphiphilic dendritic polymer grafted with long unsaturated fatty acid chains and polyethylene glycol chains.A high affinity is found between the aromatic compounds and polar groups and unsaturated bonds in W3000[113].Based on the advantages of both,our group synthesized porous nanocage MOP-t Bu[Cu24(5-t Bu-1,3-BDC)24(S)24]as fillers to prepare MOP-t Bu/W3000 hybrid membranes for separating aromatic/aliphatic hydrocarbon mixtures[114].For a 50 wt%toluene/n-heptane mixture,the separation factor and permeation flux of the hybrid membranes were 19.0 and 229.6 g·m−2·h−1,respectively,which were higher than those of the pure W3000 membrane.In order to investigate the separation mechanism of the MOP hybrid membranes and the effect of polarity of membranes with a separation factor of 8.9 and a permeation flux of 14 g·m−2·h−1.This is due to the d–π conjugation interaction between the unsaturated metal ionsof Cu3(BTC)2and aromatic molecules,or the π–π interaction between the benzene ring structure of ligands and aromatic molecules.In addition,the pore structure of Cu3(BTC)2provided more mass transfer channels which could obviously improve the permeation flux of Cu3(BTC)2/PVA hybrid membranes.Simultaneously,Co(HCOO)2was incorporated into PEBA to prepare hybrid membranes for separating toluene/n-heptane mixture[112].Fig.14(b)shows the crystal structure of Co(HCOO)2.When the particle size of Co(HCOO)2increased from 300 to 1000 nm,the permeation flux of the Co(HCOO)2/PEBA hybrid membranes increased while the separation factor decreased due to the weaker compatibility between large particles and functional group,three MOPs with same structure but different functional groups[Fig.15(a)]were prepared[115].They were doped into polymer to prepare hybrid membranes.As shown in Fig.15(b)and(c),the surface of MOPs/Boltorn W3000 hybrid membrane was very smooth which implied the good dispersion of MOP molecules in the polymer.The homodisperse of MOPs in polymer ensures the sufficient loading of nanoparticles and decreases the interface defect between polymer and MOPs,contributing to enhance separation performance of hybrid membranes.The experiment and simulation results indicated that the MOP hybrid membranes with sulfonate group had the best comprehensive pervaporation performance due to its better affinity towards the toluene molecules.It provided the evidence that the effect of polarity of functional group on the MOP fillers had a significant effect on the separation performance of membranes.The order of the separation performance of these hybrid membranes was MOPSO3NanHm/W3000>MOP-OH/W3000>MOP-t Bu/W3000,which corresponded to the polarity of functional groups in these MOPs.
4.Conclusions
This article summarizes the recent development of pervaporation membranes for separating aromatic/aliphatic hydrocarbon mixtures from the perspective of membrane material.The characteristics and separation performances of the membrane materials have been reviewed and compared.It can be found from this article that the development of pervaporation technique for separating aromatic/aliphatic mixtures is still slow.The separation performance of the membrane exhibited serious trade-off phenomenon.It should be further improved to satisfy the practical requirement in industry.More efforts are needed in the future research from the following aspects:
(1)Promising membrane materials should be designed with high aromatic permselective property.According to the solution diffusion mechanism of pervaporation,the adsorption selectivity has a very significant influence on the separation performance of membrane.At present,polymer is still the main material for the separation of aromatic/aliphatic mixtures.More types of facilitated transfer particlescan besynthesized and incorporated into the polymer to improve the separation performance of the membrane,such as MOFs,MOPs,COFs,and their hybrids.The seporous cage materials may improve the selectivity and permeability of membrane.
(2)Effective preparation method should be developed to simplify fabrication procedure and regulate micro structure of membrane.From the perspective of application,the preparation process of the membrane should be green,economical and facile so as to obtain a better reproducibility and low cost.Furthermore,the micro structure of membrane can be controlled by the preparation method.During the aromatic/aliphatic separation be further clarified so as to guide the design and preparation of efifcient membrane materials.

Fig.14.The crystal structures of(a)Cu3(BTC)2 and(b)Co(HCOO)2.

Fig.15.(a)The crystal structures of MOPs with different functional groups;SEM images of(b)the surface of ceramic substrate(30 K),(c)the surface of MOPs/Boltorn W3000 composite membrane(30 K)[115].
Nomenclature
AM acrylamide
AMPS 2-acrylamido-2-methyl propane sulfonic acid
AOT dioctyl sodium succinate
BAPP 2,2-bis[4-(4-aminophenoxy)phenyl]propane
BDAF 2,2-bis[4-(4-aminophenoxy)phenyl]hexa fluoropropane
BDDDMAC benzyldodecyldimethylammonium chloride
BHBP 4,4′-bis(2-hydroxyethoxy)biphenyl
B.p. boiling point
BTAPPF bis-2,2′-[4-{2′-trifluoromethyl 4′-(4″-aminophenyl)phenoxy}phenyl] fluorenylidene
BTAPPHI bis-2,2′-[4-{2′-trifluoromethyl 4′-(4″-aminophenyl)phenoxy}phenyl]hexa fluoro isopropylidene
BTAPPI bis-2,2′-[4-{2′-trifluoromethyl 4′-(4″-aminophenyl)phenoxy}phenyl]isopropylidene
BTAPPPI 3,3-bis-[4-{2′-trifluoromethyl 4′-(4″-aminophenyl)phenoxy}phenyl]-2-phenyl-2,3-dihydro-isoindole-1-one
BTC benzene-1,3,5-tricarboxylate
CA cellulose acetate
C12mim Cl 1-dodecyl-3-methyl imidazoium chloride process,the membranes are prone to excessive swelling,leading to a decrease in stability.The excessive swelling can be suppressed through regulating the microstructure of membrane.
(3)More modeling and simulation studiesneed to bed one to explain the transport mechanism of the membrane as well as their application.The separation performance of the new materials to aromatic/aliphatic mixturescan be simulated to screen the membrane material in a large range.It could be helpful for us to obtain an effective membrane material.Moreover,the separation mechanism of the membrane for aromatic/aliphatic mixtures should
CG carbon graphite
CMC sodium carboxymethyl cellulose
CMS carbon molecular sieve
CNT carbon nanotube
COFs covalent organic frameworks
CS chitosan
calix butyl calixarene
DABA 3,5-diamino-benzoic acid
DABC trans-4,4′-diaminodibenzo-18-crown-6
DBD dielectric barrier discharge
DDBT 2,8(6)-dimethyl-3,7-diaminobenzothiophene-5,5-dioxane
DS degree of swelling
DSDA 3,3′,4,4′-diphenylsulfone tetracarboxylic dianhydride
F127 polyoxyethylene–polyoxypropylene–polyoxyethylene
GA glutaraldehyde
GMA glycidyl methacrylate
GO graphene oxide
GPTMS γ-glycidoxypropyltrimethoxysilane
HBP hyperbranched polymer
HQE hydroquinone bis(2-hydroxyethyl)ether
Hydrin a tercopolymer of ethylene oxide/epichlorohydrin/allyl glycidyl ether
IA isophthalic acid
IGC inverse gas chromatography
MA methyl acrylate
MASPE methacrylic acid[3-sulfo-propyl ester]potassium salt
Maxsorb SPD30 super activated carbon
MD molecular dynamics
MDA 4,4′-diaminodiphenyl methane
MDI 4,4′-methylene-bis(phenylisocyanate)
MMA methyl methacrylate
MOFs metal–organic frameworks
MOPs metal–organic polyhedras
MOP-OH Cu24(5-OH-1,3-BDC)24(S)24
MOP-SO3NanHmCu24(5-SO3NanHm-1,3-BDC)24(S)24
MOP-t Bu Cu24(5-t Bu-1,3-BDC)24(S)24
M.p. melting point
MSE molecular surface engineering
MWCNT multi walled carbon nanotube
MWCNT-COOH carboxylic multi walled carbon nanotube
MWCNT-NH2aminated multi walled carbon nanotube
NBR acrylonitrile butadiene rubber
PA polyamide
PAH polyaromatic hydrocarbons
PAI-SO2poly(amide-imide)
PALS positron annihilation lifetime spectroscopy
PAN polyacrylonitrile
PBG poly(γ-benzyl-L-glutamate)
PBI polybenzimidazole
PMDA pyromellitic dianhydride
PDMS polydimethylsiloxane
PE polyether
PEA poly(ether amide)
PEBA poly(ether-block-amide)
PEFCs polymer electrolyte fuel cells
PEG-DMA polyethylene glycol dimethacrylate
PEI polyetherimide
PEO526OHMA poly(ethylene glycol)methacrylate
PIs polyimides
PMHS poly(methylhydrosiloxane)
PMMA poly(methyl methacrylate)
PTET-60 poly(trimethyleneco-ethylene terephthalate)
PTMO poly(oxytetramethylene)
PU polyurethane
PUI polyurethaneimide
PUU polyurethaneurea
PVA poly(vinyl alcohol)
PVAc poly(vinyl acetal)
PVC poly(vinyl chloride)
SA sodium alginate
SBR styrene butadiene rubber
ST styrene
TA terephthalic acid
TDI 2,4-tolylene diisocyanate
TIPA 5-tert-butyl-isophthalic acid
UV ultraviolet
α-CD α-cyclodextrin
β-CD β-cyclodextrin
δ Hansen solubility parameter
δDdispersive force contribution
δHhydrogen bonding contribution
δPpolar contribution
2D 2 dimensional
4,4′-SDA 4,4′-diaminodiphenylsul fide
4MPD 2,3,5,6-tetramethyl-1,4-phenylene diamine
6FDA 4,4′-hexa fluoroisopropylidene diphthalic anhydride
[1]C.Ribeiro,B.Freeman,D.Kalika,S.Kalakkunnath,Aromatic polyimide and polybenzoxazole membranes for the fractionation of aromatic/aliphatic hydrocarbons by pervaporation,J.Membr.Sci.390-391(2012)182–193.
[2]J.Villaluenga,A.Tabe-Mohammadi,A reviewon the separation of benzene/cyclohexane mixtures by pervaporation process,J.Membr.Sci.169(2000)159–174.
[3]B.Smitha,D.Suhanya,S.Sridhar,M.Ramakrishna,Separation of organic–organic mixtures by pervaporation—A review,J.Membr.Sci.241(2004)1–21.
[4]L.Jiang,Y.Wang,T.Chung,X.Qiao,J.Lai,Polyimides membranes for pervaporation and biofuels separation,Prog.Polym.Sci.34(2009)1135–1160.
[5]P.Shao,R.Y.M.Huang,Polymeric membrane pervaporation,J.Membr.Sci.287(2007)162–179.
[6]J.Wijmans,R.Baker,The solution-diffusion model:A review,J.Membr.Sci.107(1995)1–21.
[7]H.Ye,J.Li,Y.Lin,J.Chen,C.Chen,Synthesis of polyimides containing fluorine and their pervaporation performances to aromatic/aliphatic hydrocarbon mixtures,J.Macromol.Sci.A 45(2008)172–178.
[8]H.Vinhthang,S.Kaliaguine,Predictive models for mixed-matrix membrane performance:A review,Chem.Rev.113(2013)4980–5028.
[9]G.M.Geise,H.B.Park,A.C.Sagle,B.D.Freeman,J.E.Mcgrath,Water permeability and water/salt selectivity tradeoff in polymers for desalination,J.Membr.Sci.369(2011)130–138.
[10]L.M.Robeson,The upper bound revisited,J.Membr.Sci.320(2008)390–400.
[11]P.D.Chapman,T.Oliveira,A.G.Livingston,K.Li,Membranes for the dehydration of solvents by pervaporation,J.Membr.Sci.318(2008)5–37.
[12]M.Shestakova,M.Sillanpää,Removal of dichloromethane from ground and wastewater:A review,Chemosphere 93(2013)1258–1267.
[13]V.S.Cunha,M.L.L.Paredes,C.P.Borges,A.C.Habert,R.Nobrega,Removal of aromatics from multicomponent organic mixtures by pervaporation using polyurethane membranes:Experimental and modeling,J.Membr.Sci.206(2002)277–290.
[14]H.R.Mortaheb,F.Ghaemmaghami,B.Mokhtarani,A reviewon removal of sulfur components from gasoline by pervaporation,Chem.Eng.Res.Des.90(2012)409–432.
[15]K.Rychlewska,K.Konieczny,Pervaporative desulfurization of gasoline—separation of hydrocarbon/thiophene mixtures using polydimethylsiloxane(PDMS)-based membranes,Desalin.Water Treat.57(2016)1247–1254.
[16]A.Jonquières,R.Clément,P.Lochon,Permeability of block copolymers to vapors and liquids,Prog.Polym.Sci.27(2002)1803–1877.
[17]D.Katarzynski,F.Pithan,C.Staudt,Pervaporation of multi component aromatic/aliphatic mixtures through copolyimide membranes,Sep.Sci.Technol.43(2008)59–70.
[18]C.P.Ribeiro,B.D.Freeman,D.S.Kalika,S.Kalakkunnath,Pervaporative separation of aromatic/aliphatic mixtures with poly(siloxane-co-imide)and poly(ether-coimide)membranes,Ind.Eng.Chem.Res.52(2013)8906–8916.
[19]C.P.Ribeiro,B.D.Freeman,D.S.Kalika,S.Kalakkunnath,Separation of aromatic/aliphatic mixtures by pervaporation using ortho-functionalized polyimide membranes,ACS Symp.1078(2011)81–105.
[20]S.-H.Hsiao,C.-P.Yang,S.-C.Huang,Polyimides from 1,5-bis(4-amino-2-tri fluoromethylphenoxy)naphthalene and aromatic tetracarboxylic dianhydrides,Eur.Polym.J.40(2004)1063–1074.
[21]Y.Liu,R.Wang,T.Chung,Chemical cross-linking modification of polyimide membranes for gas separation,J.Membr.Sci.189(2001)231–239.
[22]Y.K.Kim,H.B.Park,Y.M.Lee,Preparation and characterization of carbon molecular sieve membranes derived from BTDA–ODA polyimide and their gas separation properties,J.Membr.Sci.255(2005)265–273.
[23]K.Tanaka,M.N.Islam,M.Kido,H.Kita,K.i.Okamoto,Gas permeation and separation properties of sulfonated polyimide membranes,Polymer 47(2006)4370–4377.
[24]X.Qiao,T.Chung,K.P.Pramoda,Fabrication and characterization of BTDA-TDI/MDI(P84)co-polyimide membranes for the pervaporation dehydration of isopropanol,J.Membr.Sci.264(2005)176–189.
[25]T.Chung,W.Guo,Y.Liu,Enhanced Matrimid membranes for pervaporation by homogenous blends with polybenzimidazole(PBI),J.Membr.Sci.271(2006)221–231.
[26]L.Yang,Y.Kang,Y.Wang,L.Xu,H.Kita,K.-i.Okamoto,Synthesis of crown ethercontaining copolyimides and their pervaporation properties to benzene/cyclohexane mixtures,J.Membr.Sci.249(2005)33–39.
[27]C.M.Bell,I.Huang,M.Zhou,R.Baker,J.Wijmans,A vapor permeation processes for the separation of aromatic compounds from aliphatic compounds,Sep.Sci.Technol.49(2014)2271–2279.
[28]G.Kung,L.Jiang,Y.Wang,T.Chung,Asymmetric hollowfibers by polyimide and polybenzimidazole blends for toluene/iso-octane sep aration,J.Membr.Sci.360(2010)303–314.
[29]D.Katarzynski,C.Staudt,Temperature-dependent separation of naphthalene/n-decane mixtures using 6FDA–DABA-copolyimide membranes,J.Membr.Sci.348(2010)84–90.
[30]I.Bettermann,C.Staudt,Permeation of binuclear,sulphur containing aromatic compounds,Desalination 250(2010)1144–1146.
[31]D.Katarzynski,C.Staudt,Separation of multi component aromatic/aliphatic mixtures by pervaporation with copolyimide membranes,Desalination 189(2006)81–86.
[32]N.Schmeling,R.Konietzny,D.Sieffert,P.Rölling,C.Staudt,Functionalized copolyimide membranes for the separation of gaseous and liquid mixtures,Beilstein J.Org.Chem.6(2010)789–800.
[33]S.Roychowdhury,D.Mitra,Fabrication of aromatic polyimide membrane to study the pervaporative separation of phenanthrene/n-tetradecane mixtures(model diesel)and process optimization using response surface methodology,Chem.Eng.Commun.204(2017)64–78.
[34]S.Maji,S.Banerjee,N.C.Pradhan,Separation of benzene/cyclohexane mixture using semi fluorinated aromatic poly(ether amide)membranes with and without cardo unit in the main chain,Sep.Purif.Technol.70(2009)128–135.
[35]S.Maji,S.Banerjee,Preparation of newsemi fluorinated aromatic poly(ether amide)s and evaluation of pervaporation performance for benzene/cyclohexane 50/50 mixture,J.Membr.Sci.349(2010)145–155.
[36]S.Maji,S.Banerjee,Synthesis and characterization of newmeta connecting semi fluorinated poly(ether amide)s and their pervaporation properties for benzene/cyclohexane mixtures,J.Membr.Sci.360(2010)380–388.
[37]T.Wu,N.Wang,J.Li,L.Wang,W.Zhang,G.Zhang,S.Ji,Tubular thermal crosslinked-PEBA/ceramic membrane for aromatic/aliphatic pervaporation,J.Membr.Sci.486(2015)1–9.
[38]A.Yildirim,N.Hilmioglu,S.Tulbentci,Separation of benzene/cyclohexane mixtures by pervaporation using PEBA membranes,Desalination 219(2008)14–25.
[39]A.Wolińska-Grabczyk,Effect of the hard segment domains on the permeation and separation ability of the polyurethane-based membranes in benzene/cyclohexane separation by pervaporation,J.Membr.Sci.282(2006)225–236.
[40]H.Ye,J.Li,Y.Lin,J.Chen,C.Chen,Preparation and pervaporation performances of PEA-based polyurethaneurea and polyurethaneimide membranes to benzene/cyclohexane mixture,J.Macromol.Sci.A 45(2008)563–571.
[41]B.Chen,G.Li,L.Wang,R.Chen,F.Yin,Proton conductivity and fuel cell performance of organic-inorganic hybrid membrane based on poly(methyl methacrylate)/silica,Int.J.Hydrog.Energy 38(2013)7913–7923.
[42]O.Okeowo,S.Y.Nam,J.R.Dorgan,Nonequilibrium nanoblend membranes for the pervaporation of benzene/cyclohexane mixtures,J.Appl.Polym.Sci.108(2008)2917–2922.
[43]H.H.Schwarz,G.Malsch,Polyelectrolyte membranes for aromatic–aliphatic hydrocarbon separation by pervaporation,J.Membr.Sci.247(2005)143–152.
[44]E.Boscaini,M.L.Alexander,P.Prazeller,T.D.Märk,Investigation of fundamental physical properties of a polydimethylsiloxane(PDMS)membrane using a proton transfer reaction mass spectrometer(PTRMS),Int.J.Mass Spectrom.239(2004)179–186.
[45]J.Chen,J.Li,Y.Lin,C.Chen,Pervaporation performance of polydimethylsiloxane membranes for separation of benzene/cyclohexane mixtures,J.Appl.Polym.Sci.112(2009)2425–2433.
[46]H.Zhou,Y.Su,X.Chen,J.Luo,S.Tan,Y.Wan,Plasma modification of substrate with poly(methylhydrosiloxane)for enhancing the interfacial stability of PDMS/PAN composite membrane,J.Membr.Sci.520(2016)779–789.
[47]Q.An,J.Qian,Q.Zhao,C.Gao,Polyacrylonitrile-block-poly(methyl acrylate)membranes 2:Swelling behavior and pervaporation performance for separating benzene/cyclohexane,J.Membr.Sci.313(2008)60–67.
[48]Z.Li,B.Zhang,L.Qu,J.Ren,Y.Li,A novel atmospheric dielectric barrier discharge(DBD)plasma graft- filling technique to fabricate the composite membranes for pervaporation of aromatic/aliphatic hydrocarbons,J.Membr.Sci.371(2011)163–170.
[49]M.Iravaninia,M.Mirfendereski,T.Mohammadi,Pervaporation separation of toluene/n-heptane mixtures using a MSE-modified membrane:effects of operating conditions,Chem.Eng.Res.Des.90(2012)397–408.
[50]M.Rezakazemi,M.Iravaninia,S.Shirazian,T.Mohammadi,Transient computational fluid dynamics modeling of pervaporation separation of aromatic/aliphatic hydrocarbon mixtures using polymer composite membrane,Polym.Eng.Sci.53(2013)1494–1501.
[51]T.Uragami,K.Tsukamoto,T.Miyata,Permeation and separation characteristicsof a mixture of benzene/cyclohexane through cellulose alkyl ester membranes during pervaporation,Macromol.Chem.Phys.206(2005)642–648.
[52]Y.Bai,J.Qian,Q.Zhao,Y.Xu,S.Ye,Compatibility of PTET-60/CA blends and separation performance of their membranes for benzene/cyclohexane mixture by pervaporation,J.Appl.Polym.Sci.102(2006)2832–2838.
[53]S.B.Kuila,S.K.Ray,Separation of benzene–cyclohexane mixtures by filled blend membranes of carboxymethyl cellulose and sodium alginate,Sep.Purif.Technol.123(2014)45–52.
[54]Y.N.Sang,J.R.Dorgan,Non-equilibrium nanoblends via forced assembly for pervaporation separation of benzene from cyclohexane:UNIFAQ-FV group contribution calculations,J.Membr.Sci.306(2007)186–195.
[55]N.Wang,L.Wang,R.Zhang,J.Li,C.Zhao,T.Wu,S.Ji,Highly stable“porefilling”tubular comp osite membrane by self-crosslinkable hyperbranched p olym ers for toluene/n-hep tane sep aration,J.Membr.Sci.474(2015)263–272.
[56]S.Kononova,R.Kremnev,E.Suvorova,Y.Baklagina,B.Volchek,P.Uchytil,B.Shabsels,K.Romashkova,K.Setnickova,J.Reznickova,Pervaporation membranes with poly(γ-benzyl-L-glutamate)selective layers for separation of toluene–nheptane mixtures,J.Membr.Sci.477(2015)14–24.
[57]S.Kononova,R.Kremnev,Y.Baklagina,B.Volchek,E.Vlasova,B.Shabsels,K.Romashkova,D.Romanov,S.Arkhipov,A.Bogomazov,Interrelation between the structural and transport properties of pervaporation membranes with diffusion layers based on poly-γ-benzyl-L-glutamate,Crystallogr.Rep.56(2011)502–507.
[58]M.T.H.Suding,C.Staudt,Sulfur-containing cop olyimides for the membranebased separation of aromatic/aliphatic mixtures,J.Appl.Polym.Sci.127(2013)5065–5074.
[59]T.Aouak,A.A.Alghamdi,A.A.Alrashdi,M.Ouladsmane,M.M.Alam,Z.AlOthman,M.Naushad,Miscibility enhancement of poly(vinyl chloride)/polystyrene blend:application to membrane separation of benzene from benzene/cyclohexane mixture by pervaporation,Sep.Sci.Technol.51(2016)2440–2454.
[60]F.Peng,Z.Jiang,C.Hu,Y.Wang,L.Lu,H.Wu,Pervaporation of benzene/cyclohexane mixtures through poly(vinyl alcohol)membranes with and without βcyclodextrin,Desalination 193(2006)182–192.
[61]P.Rölling,M.Lamers,C.Staudt,Cross-linked membranes based on acrylated cyclodextrins and polyethylene glycol dimethacrylates for aromatic/aliphatic separation,J.Membr.Sci.362(2010)154–163.
[62]V.Dubey,L.K.Pandey,C.Saxena,Pervaporation of benzene/cyclohexane mixtures through supramolecule containing poly(vinyl acetal)membranes,Sep.Purif.Technol.50(2006)45–50.
[63]J.M.Yang,W.Y.Su,T.L.Leu,M.C.Yang,Evaluation of chitosan/PVA blended hydrogel membranes,J.Membr.Sci.236(2004)39–51.
[64]L.Lu,F.Peng,Z.Jiang,J.Wang,Poly(vinyl alcohol)/chitosan blend membranes for pervaporation of benzene/cyclohexane mixtures,J.Appl.Polym.Sci.101(2006)167–173.
[65]P.Kumar,V.V.Guliants,Periodic mesoporous organic–inorganic hybrid materials:Applications in membrane separations and adsorption,Microporous Mesoporous Mater.132(2010)1–14.
[66]Y.Gu,R.M.Dorin,U.Wiesner,Asymmetric organic–inorganic hybrid membrane formation via block copolymer–nanoparticle co-assembly,Nano Lett.13(2013)5323–5328.
[67]V.C.Souza,M.G.N.Quadri,Organic-inorganic hybrid membranes in separation processes:A 10-year review,Braz.J.Chem.Eng.30(2013)683–700.
[68]V.Meynen,H.L.Castricum,A.Buekenhoudt,Class II hybrid organic–inorganic membranes creating newversatility in separations,Curr.Org.Chem.18(2014)2334–2350.
[69]W.R.Kang,A.S.Lee,S.Park,S.H.Park,K.Y.Baek,K.B.Lee,S.H.Lee,J.H.Lee,S.S.Hwang,J.S.Lee,Free-standing,polysilsesquioxane-based inorganic/organic hybrid membranes for gas separations,J.Membr.Sci.475(2015)384–394.
[70]J.Shen,X.Zheng,H.Ruan,L.Wu,J.Qiu,C.Gao,Synthesis of AgCl/PMMA hybrid membranes and their sorption performance of cyclohexane/cyclohexene,J.Membr.Sci.304(2007)118–124.
[71]X.Zhang,L.Qian,H.Wang,W.Zhong,Q.Du,Pervaporation of benzene/cyclohexane mixturesthrough rhodium-loadedβ-zeolite- filled polyvinyl chloride hybrid membranes,Sep.Purif.Technol.63(2008)434–443.
[72]J.Shen,Y.Chu,H.Ruan,L.Wu,C.Gao,B.V.D.Bruggen,Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes,J.Membr.Sci.462(2014)160–169.
[73]L.Wu,T.Wang,W.Xiang,Regulation of AgCl in reverse microemulsion and its effect on the performance of AgCl/PEO-PPO-PEO/PMMA hybrid membranes,Compos.Sci.Technol.80(2013)8–15.
[74]L.Wu,T.Wang,Z.Jiang,Formation of AgCl nanoparticle in reverse microemulsion using polymerizable surfactant and the resulting copolymer hybrid membranes,J.Membr.Sci.429(2013)95–102.
[75]L.Wu,J.Shen,C.Du,T.Wang,Y.Teng,B.V.D.Bruggen,Development of AgCl/poly(MMA-co-AM)hybrid pervaporation membranes containing AgCl nanoparticles through synthesis of ionic liquid microemulsions,Sep.Purif.Technol.114(2013)117–125.
[76]L.Zhou,X.Dai,J.Du,T.Wang,L.Wu,Y.Tang,J.Shen,Fabrication of poly(MMA-co-ST)hybrid membranes containing AgCl nanoparticles by in situ ionic liquid microemulsion polymerization and enhancement of their separation performance,Ind.Eng.Chem.Res.54(2015)3326–3332.
[77]E.Mahmoudi,L.Y.Ng,M.M.Ba-Abbad,A.W.Mohammad,Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates,Chem.Eng.J.277(2015)1–10.
[78]S.Dai,Y.Jiang,T.Wang,L.Wu,X.Yu,J.Lin,S.Shi,Enhanced performance of polyimide hybrid membranes for benzene separation by incorporating three dimensional silver–graphene oxide,J.Colloid Interface Sci.478(2016)145–154.
[79]L.Lu,H.Sun,F.Peng,Z.Jiang,Novel graphite- filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexanemixtures,J.Membr.Sci.281(2006)245–252.
[80]F.Peng,L.Lu,H.Sun,F.Pan,Z.Jiang,Organic−inorganic hybrid membranes with simultaneously enhanced flux and selectivity,Ind.Eng.Chem.Res.46(2007)2544–2549.
[81]F.Peng,L.Lu,C.Hu,H.Wu,Z.Jiang,Significant increase of permeation flux and selectivity of poly(vinyl alcohol)membranes by incorporation of crystalline flake graphite,J.Membr.Sci.259(2005)65–73.
[82]N.Wang,S.Ji,J.Li,R.Zhang,G.Zhang,Poly(vinyl alcohol)–graphene oxide nanohybrid “pore- filling”membrane for pervaporation of toluene/n-heptane mixtures,J.Membr.Sci.455(2014)113–120.
[83]X.Ma,Y.S.Lin,X.Wei,J.Kniep,Ultrathin carbon molecular sieve membrane for propylene/propane separation,AICHE J.62(2016)491–499.
[84]O.Salinas,X.Ma,E.Litwiller,I.Pinnau,Ethylene/ethane permeation,diffusion and gas sorption properties of carbon molecular sieve membranes derived from the prototype ladder polymer of intrinsic microporosity(PIM-1),J.Membr.Sci.504(2016)133–140.
[85]F.Peng,Z.Jiang,C.Hu,Y.Wang,H.Xu,J.Liu,Removing benzene from aqueous solution using CMS- filled PDMS pervaporation membranes,Sep.Purif.Technol.48(2006)229–234.
[86]L.Li,Z.Xiao,Z.Zhang,S.Tan,Pervaporation of acetic acid/water mixtures through carbon molecular sieve- filled PDMS membranes,Chem.Eng.J.97(2004)83–86.
[87]K.S.Liao,Y.J.Fu,C.C.Hu,J.T.Chen,D.W.Lin,K.R.Lee,K.L.Tung,Y.C.Jean,J.Y.Lai,Microstructure of carbon molecular sieve membranes and their application to separation of aqueous bioethanol,Carbon 50(2012)4220–4227.
[88]H.Sun,L.Lu,F.Peng,H.Wu,Z.Jiang,Pervaporation of benzene/cyclohexane mixtures through CMS- filled poly(vinyl alcohol)membranes,Sep.Purif.Technol.52(2006)203–208.
[89]P.Tin,H.Lin,R.Ong,T.Chung,Carbon molecular sieve membranes for biofuel separation,Carbon 49(2011)369–375.
[90]C.A.Hunter,J.K.M.Sanders,The nature of π-π interactions,J.Am.Chem.Soc.112(1990)5525–5534.
[91]J.Zhao,J.Lu,J.Han,C.-K.Yang,Noncovalent functionalization of carbon nanotubes by aromatic organic molecules,Appl.Phys.Lett.82(2003)3746–3748.
[92]F.Peng,C.Hu,Z.Jiang,Novel ploy(vinyl alcohol)/carbon nanotube hybrid membranes for pervaporation separation of benzene/cyclohexane mixtures,J.Membr.Sci.297(2007)236–242.
[93]F.Peng,F.Pan,H.Sun,L.Lu,Z.Jiang,Novel nanocomposite pervaporation membranes composed of poly(vinyl alcohol)and chitosan-wrapped carbon nanotube,J.Membr.Sci.300(2007)13–19.
[94]T.Wang,L.Zhao,Y.Chen,L.Ding,S.Feng,L.Wu,Y.Wang,Influence of modification of MWCNTs on the structure and performance of MWCNT-Poly(MMA-AM)hybrid membranes,Polym.Adv.Technol.25(2014)288–293.
[95]J.M.Duval,B.Folkers,M.H.V.Mulder,G.Desgrandchamps,C.A.Smolders,Separation of a toluene/ethanol mixture by pervaporation using active carbon- filled polymeric membranes,Sep.Sci.Technol.29(1994)357–373.
[96]R.A.Amaral,A.C.Habert,C.P.Borges,Activated carbon polyurethane membrane for a model fuel desulfurization by pervaporation,Mater.Lett.137(2014)468–470.
[97]T.Trinh,T.van Erp,D.Bedeaux,S.Kjelstrup,C.Grande,A procedure to find thermodynamic equilibrium constants for CO2and CH4adsorption on activated carbon,Phys.Chem.Chem.Phys.17(2015)8223–8230.
[98]M.R.A.Mamun,M.R.Karim,M.M.Rahman,A.M.Asiri,S.Torii,Methane enrichment of biogas by carbon dioxidefixation with calcium hydroxide and activated carbon,J.Taiwan Inst.Chem.Eng.58(2015)476–481.
[99]L.Aouinti,D.Roizard,M.Belbachir,PVC–activated carbon based matrices:A promising combination for pervaporation membranes useful for aromatic–alkane separations,Sep.Purif.Technol.147(2015)51–61.
[100]K.J.Kim,S.H.Park,W.W.So,S.J.Moon,Pervaporation separation of aqueous organic mixtures through sulfated zirconia-poly(vinyl alcohol)membrane,J.Appl.Polym.Sci.79(2001)1450–1455.
[101]B.Liu,Y.Cao,T.Wang,Q.Yuan,Preparation of novel ZSM-5 zeolite- filled chitosan membranes for pervaporation separation of dimethyl carbonate/methanol mixtures,J.Appl.Polym.Sci.106(2007)2117–2125.
[102]F.Peng,L.Lu,H.Sun,Y.Wang,J.Liu,Z.Jiang,Hybrid organic−inorganic membrane:Solving the tradeoff between permeability and selectivity,Chem.Mater.17(2005)6790–6796.
[103]F.Peng,L.Lu,H.Sun,Y.Wang,H.Wu,Z.Jiang,Correlations between free volume characteristics and pervaporation permeability of novel PVA–GPTMS hybrid membranes,J.Membr.Sci.275(2006)97–104.
[104]L.Aouinti,D.Roizard,G.H.Hu,F.Thomas,M.Belbachir,Investigation of pervaporation hybrid polyvinylchloride membranes for the separation of toluene–n-heptane mixtures—case of clays as filler,Desalination 241(2009)174–181.
[105]E.Adatoz,A.K.Avci,S.Keskin,Opportunities and challenges of MOF-based membranes in gas separations,Sep.Purif.Technol.152(2015)207–237.
[106]V.Na fisi,M.Hägg,Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2capture,J.Membr.Sci.459(2014)244–255.
[107]C.Casadocoterillo,Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2separation,RSCAdv.5(2015)102350–102361.
[108]J.Campbell,J.D.S.Burgal,G.Szekely,R.P.Davies,D.C.Braddock,A.Livingston,Hybrid polymer/MOF membranes for organic solvent nano filtration(OSN):Chemical modification and the quest for perfection,J.Membr.Sci.503(2016)166–176.
[109]R.Zhang,S.Ji,N.Wang,L.Wang,G.Zhang,J.R.Li,Coordination-driven in-situ selfassembly strategy for the preparation of metal–organic framework hybrid membranes,Angew.Chem.Int.Ed.53(2014)9933–9937.
[110]J.Duan,Y.Pan,F.Pacheco,E.Litwiller,Z.Lai,I.Pinnau,High-performance polyamide thin- film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8,J.Membr.Sci.476(2015)303–310.
[111]Y.Zhang,N.Wang,S.Ji,R.Zhang,C.Zhao,J.R.Li,Metal–organic framework/poly(vinyl alcohol)nanohybrid membrane for the pervaporation of toluene/nheptane mixtures,J.Membr.Sci.489(2015)144–152.
[112]Y.Zhang,N.Wang,C.Zhao,L.Wang,S.Ji,J.R.Li,Co(HCOO)2-based hybrid membranes for the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures,J.Membr.Sci.520(2016)646–656.
[113]U.Domańska,Z.Żołek-Tryznowska,Solubility of hyperbranched polymer,Boltorn W-3000,in alcohols,ethers and hydrocarbons,J.Chem.Thermodyn.42(2010)1304–1309.
[114]C.Zhao,N.Wang,L.Wang,H.Huang,R.Zhang,F.Yang,Y.Xie,S.Ji,J.R.Li,Hybrid membranes of metal–organic molecule nanocages for aromatic/aliphatic hydrocarbon separation by pervaporation,Chem.Commun.50(2014)13921–13923.
[115]C.Zhao,N.Wang,L.Wang,S.Sheng,J.Yu,H.Fan,F.Yang,S.Ji,J.R.Li,Functionalized metal-organic polyhedra hybrid membranes for aromatic hydrocarbons recovery,AIChE J.62(2016)3706–3716.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
- Study on extraction kinetics of α-cyclopentylmandelic acid enantiomers with hydroxyethyl-β-cyclodextrin as chiral selector☆
