Effective and simple recovery of 1,3-propanediol from a fermented medium by liquid–liquid extraction system with ethanol and K3PO4
2018-05-25DaianaWischralHongxinFuFernandoPellegriniPessoaNeiPereiraJrShangTianYang
Daiana Wischral *,Hongxin Fu ,Fernando L.Pellegrini Pessoa ,Nei Pereira Jr ,Shang-Tian Yang
1 School of Chemistry,Department of Biochemical Engineering,Federal University of Rio de Janeiro,Av.Horácio Macedo 2030,Bloco E.,Rio de Janeiro,RJ 21949-900,Brazil
2 William G.Lowrie Department of Chemical and Biomolecular Engineering,The Ohio State University,151 West Woodruff Avenue,Columbus,OH 43210,USA
3 School of Chemistry,Department of Chemical Engineering,Federal University of Rio de Janeiro,Av.Horácio Macedo 2030,Bloco E.,Rio de Janeiro,RJ 21949-900,Brazil
1.Introduction
The application of 1,3-propanediol in the polymer industry has increased significantly in the last fewyears,because of the attractive physical and chemical properties[1].The market demand for 1,3-propanediol has increased because of the enhanced production of polytri methylene terephthalate(PTT),applied in the manufacturing of carpetsand special fibres,which should reach 150000 tonsor 560 million USD by 2019[2,3].Due to the rising oil prices and concerns of environmental pollution of petroleum-based synthesis processes,the bioproduction of 1,3-propanediol from glycerol or glucose has received increased attention in recent years[1].The final concentration of 1,3-propanediol in the fermentation broth usually varies from 6.7 g·L−1to 70 g·L−1,and can reach 100 g·L−1when a metabolically engineered bacterium is used[2,3].The price of 1,3-propanediol obtained by a biochemical technique depends on the final purity grade required for its application and the substrate cost[4,5].In general,the downstream cost represents between 50%–70%of the production cost[6,7].It is thus important to develop an efficient and low-cost separation method for bioproduction of 1,3-propanediol.
Xiu et al.(2007)[8]described the 1,3-propanediol recovery from fermentation broth using salting-out extraction.The need to improve the purity of 1,3-propanediol obtained in salting-out extraction is a barrier against its successful commercialization.The fermentation broth is a mix of components:water,residual glycerol,1,3-propanediol,acetate,lactate,proteins,polysaccharides,nucleic acids,and salts.This complex mix,high hydrophobicity and high boiling point(214°C at atmospheric pressure)of 1,3-propanediol make salting-out extraction exceedingly dif ficult to optimize[7,9].
Extraction systems that present salting-out effects result in a top phase(solvent rich),and bottom phase(salt rich).An important characteristic of this system is the high polarity of a top phase,such as methanol,ethanol or acetone[2,10].This method has been investigated for polar solutes extraction,such as alcohol or acids in water[2].The hydrophilic organic solvent,utilized for the salting-out effect,is easily recovered by evaporation and offers less toxicity risk in comparison to traditional solvents and reactive extractions[11].
Xiu and Zeng(2008)[4]reported a salting-out extraction system to recover 1,3-propanediol from a fermented medium with a partition coefficient of 4.77 and recovery yield of 93.7%.The authors utilized 46%(v/v)ethanol as the organic phase and saturated ammonium sulphate as the aqueous phase,which also removed the cells and proteins from the fermented medium with a removal rate of 99.7%and 79.0%,respectively.The salting-out extraction presented a good yield and resolution,quick phase separation,the possibility of continuous operation,high efficiency,simple scale-up and lowenergy consumption.In addition,salting-out extraction is a promising and sustainable technique because there is the possibility of recycling both components(solvent and salt)[12–14].
In thisstudy,we further evaluated and optimized salting-out extraction for 1,3-propanediol recovery from fermentation broth by screening for the best combination of salt and solvent,and optimizing their concentrations to obtain the highest partition coefficient and recovery yield ever reported.
2.Materials and Methods
2.1.Salting-out extraction of 1,3-propanediol
The fermentation broth used in this study was obtained from fedbatch culture of Clostridium beijerinckii DSM 791 A1 under anaerobic conditions at 37°C in a 1-L stirred-tank bioreactor containing 500 ml of previously optimized medium:5.0 g·L−1K2HPO4;0.5 g·L−1yeast extract;0.005 g·L−1sodium acetate and 30 g·L−1glycerol[3,15].Unless otherwise noted,the fed-batch fermentation broth contained 26.1 g·L−11,3-propanediol,4.9 g·L−1cells,4.8 g·L−1butyric acid,2.8 g·L−1acetic acid and 2.8 g·L−1glycerol.Before being used in salting-out extraction,cells present in the fermentation broth were removed by centrifugation at 9100×g for 5 min.Five grammes of the clarified broth and 2 g of a solid salt(K2HPO4,Na2CO3,K2CO3,(NH4)2SO4,NaHPO4,K3PO4or C6H5NaO7)were mixed in a 10-ml tube and vortexed for 1 min.Then,2 g of solvent(methanol,ethanol,isopropanol or acetone)was added into the tube and vortexed for 2 min.The mixture was left standing for 12 h at 25°C to allowphase separation.The mass ratio of 2:2:5 for salt:solvent:1,3-propanediol solution was selected according to the phase diagram reported previously for salting-out extraction systems[16–21].Such massratio should give a two-phase system above the equilibrium line.
After complete phase separation,the volumes and 1,3-propanediol concentrations in the top(solvent)and bottom(salt)phases were recorded and used to estimate the partition coefficient(K)and recovery yield or efficiency(Y)as follows[22]:

where Ctopand Cbottomare the concentrations(g·L−1)of 1,3-propanediol,Vtopand Vbottomare the volumes in the top and bottom phases,respectively.The Ctopwas determined by high-performance liquid chromatography(HPLC)and Cbottomwas obtained by mass balance(because it was difficult to measure directly due to its high salt concentration).
Based on the K and Y values,the best combination of salt(K3PO4)and solvent(ethanol)was selected.The salting-out extraction was then further optimized in the concentrations of salt,solvent,and 1,3-propanediol for the recovery of 1,3-propanediol from the fermentation broth using a central composite rotatable design(CCRD)with 1,3-propanediol,solvent and salt concentrations as independent variables and response surface methodology with K and Y as the response variables.
The global desirability function was applied to simultaneously maximize both response variables(K and Y).The results predicted by desirability function were then evaluated experimentally in replicates,which were conducted applying the predicted optimal conditions.The experimental design and the statistical evaluation of the results were made by using STATISTICA software,version 6.0(StatSoft,Inc.)including analysis of variance(ANOVA).
2.2.Analytical methods
Concentrations of 1,3-propanediol,glycerol,acids(butyric and acetic),ethanol,acetone,isopropanol and methanol were determined using an HPLC(Shimadzu RID-10A)following the method previously described[3].The analysis was conducted with 0.0035 mol·L−1H2SO4(mobile phase)at 0.6 ml·min−1and 65 °C and external standards of each chemical were used for the identification and quantification.
3.Results and Discussion
Two phases were formed in salting-out extraction.The top phase contained mainly the solvent and 1,3-propanediol originally present in the fermentation broth,while the bottom phase contained water and salts.In addition,cells and proteins originally present in the fermentation broth were separated and appeared in the interphase between top and bottom phases.Most of acids(butyric and acetic)were also removed to the top phase because of their higher affinity with the solvent while glycerol and salts appeared in the bottom aqueous phase.The impurities(acids and ethanol)in the top phase could be removed by distillation,because there is an evident difference of boiling point between the impurities(<165 °C)and 1,3-propanediol(214°C).
3.1.Screening for best salt/solvent combination for salting-out extraction
The salt and solvent characteristics affected the formation of phases.Initially,a screening was performed to find the best combination of salt and solvent for salting-out extraction of 1,3-propanediol from the fermentation broth.At the mass ratio of 2:2:5 for salt:solvent:fermentation broth,the combination of K3PO4and ethanol gave the highest partition coefficient(K)and recovery yield(Y)(Tables 1 and 2).The formation of the two phases was affected by the hydration ability of the solvent,which is related to the solvent structure.In general,a higher polarity solvent,such as methanol and ethanol,can bind more water molecules and is thus superior for 1,3-propanediol extraction[17].

Table 1 Partition coefficient(K)from screening of salts/solvents for 1,3-propanediolreco very from the fermentation broth by salting-out extraction

Table 2 Recovery yield percentage(Y,%)from screening of salts/solvents for 1,3-propanediol recovery from the fermentation broth by salting-out extraction
3.2.Optimization of salt,solvent and 1,3-propandiol concentrations
The partition coefficient(K)might increase with increasing the alcohol and salt concentrations[17].In addition,the recovery yield(Y)may also depend on salt content and alcohol concentration.To further improve K and Y in salting out extraction,the 1,3-propanediol concentration in the fermentation broth as well as the mass ratios of K3PO4and ethanol in the two-phase system were optimized in the ranges of 18 wt%–38 wt%for K3PO4,16 wt%–36 wt%for ethanol,and 5 g·L−1–35 g·L−1for 1,3-propanediol concentration in the fermentation broth(to add up to 100 wt%),respectively.The values for each factor and level are given in Table 3,and the design matrix with results is listed in Table 4.

Table 3 CCRD factors and levelsused in the optimization of salting-out extraction for 1,3-propanediol recovery

Table 4 CCRD design and K and Y obtained in the optimization of salting-out extraction for 1,3-propanediol recovery
ANOVA is a statistical technique where the sum of squares,degrees of freedom and mean of squares are used to calculate the Fisher probability(F)and then to obtain p-value,which indicates the significance of the evaluated factors,their interactions and lack of it.Therefore,ANOVA analyses were conducted for each dependent factor(K in Table 5 and Y in Table 6)to analyse the concentrations of K3PO4,ethanol,and 1,3-propanediol solution using pure error and 95%of confidence interval(p≤0.05).
The p-values for both K and Y clearly indicated that the amount of K3PO4was more significant(lower p-value)than the amount of ethanol(higher p-value)in affecting 1,3-propanediol recovery by salting-out extraction.The experimental results can be best fitted with a quadratic model with the determination coefficient(R2)0.965 for K and 0.995 for Y,which means that 96.5%and 99.5%of the variances were explained by the K and Y models,respectively,which are given below:


Table 5 ANOVA analyses for K3PO4,ethanol and 1,3-propanediol optimization of the partition coefficient(K)

Table 6 ANOVA analyses for K3PO4,ethanol and 1,3-propanediol optimization of the recovery yield(Y)

where k is K3PO4concentration(wt%),e is ethanol concentration(wt%),and p is 1,3-propanediol concentration(g·L−1).
The response surface,plotted from the CCRD using STATISTICA 6.0 software,can be used to showthe relationship between the dependent factors(K and Y)and the independent factors(K3PO4,ethanol,and 1,3-propanediol concentration).These response surfaces,depicted in Fig.1,show that a maximum point was reached for both K and Y in the range studied.Therefore,it is possible to use the desirability technique to optimize the response variables in this system.The global desirability value,simultaneously optimized for three independent factors,was 1.00 when considering a 95%confidence interval,which means that this function obtained by optimization fulfils 100%of the maximum value possible for both dependent factors(K and Y).The optimum composition of the extraction system was 34 wt%salt,28 wt%solvent,and 23.0 g·L−11,3-propanediol in the fermentation broth.
The predicted values according to the desirability function and the results for K and Y from the experiments applying the predicted optimal conditions are shown in Table 7.According to these values,the experimental results were inside the confidence limits(predicted values by desirability function),which means that these findings were in agreement with the model[Eqs.(3)and(4)].After optimization using the response surface methodology,the partition coefficient increased 4.6 times(from 7.15 to 32.99),and the recovery yield increased 1.1 times(from 88 to 97),as compared to the results from the initial screening studies(Tables 1 and 2).
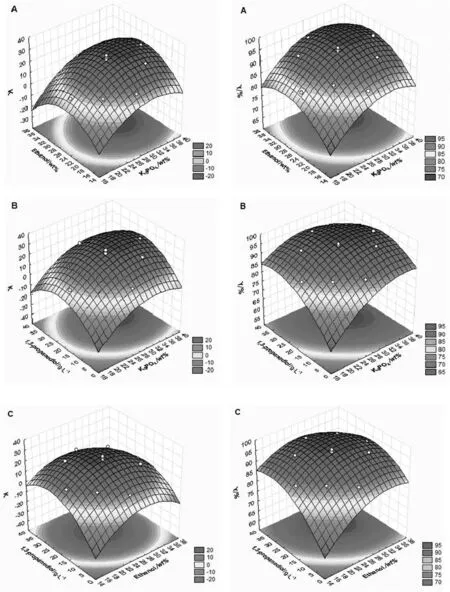
Fig.1.Response surfaces for partition coefficient(K)and recovery yield(Y)for 1,3-propanediolsalting-out extraction with K3PO4/ethanol.A:ethanol/K3PO4,B:1,3-propanediol/K3PO4 and C:1,3-propanediol/ethanol.

Table 7 Validation of the optimum conditions predicted for 1,3-propanediol recovery from the broth:34 wt%K3PO4,28 wt%ethanol,and 38 wt%fermentation broth containing 23.0 g·L−1 1,3-propanediol
3.3.Comparison to other studies
The results from this study(33 for K and 97%for Y)are better than those reported using salting-out extraction systems with ethanol for 1,3-propanediol recovery(see Table 8).Li et al.(2011)[18]haveobtained 38 for K and 98%for Y,using salting-out extraction with methanol/K2HPO4and fermentation broth using Klebsiella pneumonia CGMCC 2028 with 65.0 g·L−1of 1,3-propanediol.The salting-out extraction with ethanol/K2HPO4was also evaluated by Fu et al.(2013)[22]with fermentation broth,using K.pneumonia CGMCC 2028,and the maximum value of 11 for K and 94%for Y were acquired with continuous extraction.The higher partition coefficient for K3PO4,compared with that obtained in previous studies with K2HPO4,could be explained by the higher salting-out effect of PO43−and the optimized combination of salt:solvent:1,3-propandiol solution[21].
Product recovery processes including liquid–liquid extraction,gas stripping,and pervaporation have been developed for biochemical productions.In situ extractive fermentation could result in an emulsionand precipitate layer,causing incomplete phase separation and broth contamination risk[24].Xue et al.(2016)[25]found interesting results for butanol recovery( final product mixture with >500 g·L−1of butanol),from ABE fermentation by Clostridium acetobutylicum,applying two-stage gas stripping-pervaporation.Continuing the studies,Xue et al.(2017)[26]reported that the concentrated ABE products recovered from the gas stripping-pervaporation process could be directly alkylated to C5–C15 or longer-chain ketones in a continuous mode.However,we tried to apply gas stripping for 1,3-propanediol in situ separation(data not shown)but was unsuccessful,which could be explained by its properties:lowconcentration in the broth,complex mix of components,high hydrophobicity,and high boiling point.The salting-out extraction using liquid–liquid systems is also more interesting for 1,3-propanediol recovery in comparison to other separation methods such as distillation,because liquid–liquid extraction is more selective and energy efficient[1].

Table 8 Comparison of partition coefficients(K)and recovery yields(Y)for salting-out extraction of 1,3-propanediol from fermentation broth with different salts
The salting-out extraction is favoured for fermentation product extraction also because the concentration of the target compound usually is low.Moreover,cells in the fermentation broth could be easily removed if the operation temperature is approximately 25°C,consistent with other studies also showing that the removal of cells and proteins with high yields was possible with salting-out extraction[16,18].Finally,salting-out extraction could be readily integrated with repeated batch fermentation for 1,3-propanediol bioproduction since it would be applied after fermentation,not in situ.It should be noted that both salt and solvent used in salting-out extraction can be recovered and reutilized,making this process eco-friendly.Currently,salting-out extraction is mainly used for carboxylic acids[27]and diols[28]separation,such as 2,3-butanediol[12,17],1,3-propanediol[13,16,18],lactic acid[29,30]and butyric acid[31].
4.Conclusions
The recovery of 1,3-propanediol from a fermentation broth by salting-out extraction was studied with several salt/solvent combinations,and the best result was found with a K3PO4/ethanol system.The concentrations of ethanol,K3PO4and 1,3-propanediol in the system were further optimized using the response surface methodology to obtain the highest partition coefficient of 33 and recovery yield of 97%.The salting-out extraction is thus a promising method for recovering fermentation-produced 1,3-propanediol and has several advantages over other separation methods.
Acknowledgements
The authors acknowledge CNPq,FAPERJ and CAPES through the PDSE and Program and Human Resources Program 13 of the National Petroleum Agency(ANP-PRH 13)for scholarship.
[1]W.Sabra,C.Groeger,A.P.Zeng,Microbial cell factories for diol production,Adv.Biochem.Eng.Biotechnol.(2015)165–197.
[2]J.J.Malinowski,Evaluation of liquid extraction potentials for downstream separation of 1,3-propanediol,Biotechnol.Tech.13(1999)127–130.
[3]D.Wischral,J.Zhang,C.Cheng,M.Lin,L.M.De Souza,F.L.P.Pessoa,N.Pereira Jr.,S.-T.Yang,Production of 1,3-propanediol by Clostridium beijerinckii DSM 791 from crude glycerol and corn steep liquor:Process optimization and metabolic engineering,Bioresour.Technol.212(2016)100–110.
[4]W.D.Deckwer,Microbial conversion of glycerol production to 1,3-propanediol,FEMS Microbiol.Rev.16(1995)143–149.
[5]B.G.Hermann,M.Patel,Today's and tomorrow's bio-based bulk chemicals from white biotechnology:A techno-economic analysis,Appl.Biochem.Biotechnol.136(2007)361–388.
[6]Z.L.Xiu,A.P.Zeng,Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol,Appl.Microbiol.Biotechnol.78(2008)917–926.
[7]P.Anand,R.K.Saxena,R.G.Marwah,A novel downstream process for 1,3-propanediol from glycerol-based fermentation,Appl.Microbiol.Biotechnol.90(2011)1267–1276.
[8]Z.L.Xiu,Z.Li,B.Jianng,Y.Sun,D.Zhang,Aqueous two-phase extraction of 1,3-propanediol from fermentation broth,China Pat,200710010201.X,2007.
[9]R.Saxena,P.Anand,S.Saran,J.Isar,Microbial production of 1,3-propanediol:Recent developments and emerging opportunities,Biotechnol.Adv.27(2009)895–913.
[10]A.Greve,M.R.Kula,Cost structure and estimation for the recycling of salt in a protein extraction process system,Bioprocess Eng.6(1990)173–177.
[11]A.Louwrier,Model phase separations of proteins using aqueous/ethanol components,Biotechnol.Tech.12(1998)363–365.
[12]B.Jiang,Z.G.Li,J.Y.Dai,D.J.Zhang,Aqueous two phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system,Process Biochem.44(2009)112–117.
[13]Z.Li,B.Jiang,D.Zhang,Z.Xiu,Aqueous two-phase extraction of 1,3-propanediol from glycerol-based fermentation broths,Sep.Purif.Technol.66(2009)472–478.
[14]J.N.Sun,B.Rao,L.Y.Zhang,Y.L.Shen,Extraction of acetoin from fermentation broth using an acetone/phosphate aqueous two-phase system,Chem.Eng.Commun.199(2012)1492–1503.
[15]D.Wischral,C.A.Barcelos,N.Pereira Jr.,F.L.P.Pessoa,1,3-Propanediol:Statistical optimization of medium to improve production by Clostridium beijerinckii DSM 791,J.Adv.Biotechnol.5(2015)614–623.
[16]O.Aydogan,E.Bayraktar,U.Mehmetoglu,T.Kaeding,Selection and optimization of an aqueous two-phase system for the recovery of 1,3-propandiol from fermentation broth,Eng.Life Sci.10(2010)121–129.
[17]J.Y.Dai,Y.L.Zhang,Z.L.Xiu,Salting-out extraction of 2,3-butanediol from Jerusalem artichoke-based fermentation broth,Chin.J.Chem.Eng.19(2011)682–686.
[18]Z.G.Li,H.Teng,Z.L.Xiu,Extraction of 1,3-propanediol from glycerol-based fermentation broths with methanol/phosphate aqueous two-phase system,Process Biochem.46(2011)586–591.
[19]Y.Sun,L.Yan,H.Fu,Z.Xiu,Selection and optimization of a salting-out extraction system for recovery of biobutanol from fermentation broth,Eng.Life Sci.13(2013)464–471.
[20]H.Fu,S.-T.Yang,Z.Xiu,Phase separation in a salting-out extraction system of ethanol–ammonium sulphate,Sep.Purif.Technol.148(2015)32–37.
[21]S.Shahriari,C.M.S.S.Neves,M.G.Freire,J.A.P.Coutinho,Role of the Hofmeister series in the formation of ionic-liquid-based aqueous biphasic systems,J.Phys.Chem.116(2012)7252–7258.
[22]H.Fu,Y.Sun,Z.Xiu,Continuous countercurrent salting-out extraction of 1,3-propanediol from fermentation broth in a packed column,Process Biochem.48(2013)1381–1386.
[23]Z.G.Li,Y.Q.Sun,W.L.Zheng,H.Teng,Z.L.Xiu,A novel and environment-friendly bioprocessof 1,3-propanediol fermentation integrated with aqueoustwo-phase extraction by ethanol/sodium carbonate system,Biochem.Eng.J.80(2013)68–75.
[24]C.Xue,J.Zhao,L.Chen,S.T.Yang,F.Bai,Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum,Biotechnol.Adv.35(2017)310–322.
[25]C.Xue,F.Liu,M.Xu,J.Zhao,L.Chen,J.Ren,F.Bai,S.T.Yang,A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production,Biotechnol.Bioeng.113(2016)120–129.
[26]C.Xue,M.Liu,X.Guo,E.P.Hudson,L.Chen,F.Bai,F.Liu,S.T.Yang,Bridging chemicaland bio-catalysis:High-value liquid transportation fuel production from renewable agricultural residues,Green Chem.19(2017)660–669.
[27]H.Fu,Y.Sun,Hu Teng,D.Zhang,Z.L.Xiu,Salting-out extraction of carboxylic acids,Sep.Sci.Technol.139(2015)36–42.
[28]H.Fu,J.Dai,Y.Sun,D.Zhang,Z.L.Xiu,Partition behavior of hydrophilic diols in an ethanol/ammonium sulfate salting-out extraction system,Eng.Life Sci.15(2016)797–803.
[29]O.Aydogan,E.Bayraktar,U.Mehmetoglu,Aqueous two-phase extraction of lactic acid:Optimization by response surface methodology,Sep.Sci.Technol.46(2011)1164–1171.
[30]B.C.Wei,Z.Y.Song,Y.Q.Sun,Z.L.Xiu,Salting-out extraction of lactic acid from fermentation broths,Chin.J.Chem.Eng.12(2012)44–48.
[31]H.Fu,X.Wang,Y.Sun,L.Yan,J.Shen,J.Wang,S.T.Yang,Z.L.Xiu,Effects of saltingout and salting-out extraction on the separation of butyric acid,Sep.Sci.Technol.180(2017)44–50.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
