Ethylbenzene disproportionation and p-xylene selectivity enhancement in xylene isomerization using high crystallinity desilicated H-ZSM-5
2018-05-25MarjanFarshadiCavusFalamaki
Marjan Farshadi ,Cavus Falamaki ,2,3,*
1 Chemical Engineering Department,Mahshahr Campus,Amirkabir University of Technology,Mahshahr 415,Iran
2 Chemical Engineering Department,Amirkabir University of Technology,Tehran 15875-4413,Iran
3 Petrochemical Center of Excellence,Amirkabir University of Technology,Tehran 15875-4413,Iran
1.Introduction
Industrial xylene isomerization units use raffinate feed that may contain an appreciable content of ethyl benzene.The raffinate originates from a zeolite-based para xylene separation unit(like the Eluxyl process of Axens)with a common ethyl benzene concentration of 14 wt%–17 wt%.It is essential to minimize the ethyl benzene concentration in the product of the isomerization unit mainly for preventing its accumulation in the separation–isomerization petrochemical complex[1].The industrial practice is to use dual function catalysts including components of both acidic and metallic(mainly Pt)character.The acidic component is usually a zeolite/gamma alumina composite.The zeolites of industrial practice include protonated ZSM-5[2],ZSM-8,ZSM-11,ZSM-25,ZSM-39,and Mordenite[3].Academic literature,however,suggests the use of TNU-9,SSZ3,EU-1[4,5],ZSM-50[6],NU-87 and beta zeolites[7].The use of platinum allows the implementation of high hydrogen partialpressure to promote the ethylbenzene isomerization reaction by the production of C8naphthenic intermediates[6,8–10].
There have been trials to improve the catalytic performance of the main zeolitic component in order to increase ethylbenzene conversion by using EUO type zeolites by controlled acid site location[11,12]or modified ZSM-5 zeolites by inactivating the external surface through silica chemical vapor deposition and 5,6-benzoquinoline adsorption[1].The latter work used a realistic initial feed including xylene mixture and ethylbenzene at near industrial composition but reported a lower ethylbenzene conversion and paraxylene approach to equilibrium(p-xylene a.t.e.)along ca.10%improvement in total xylene yield with respect to the parent zeolite.The authors of the latter work proposed to add platinum metal to their modified ZSM-5 zeolite to enhance the catalyst performance by inducing ethylbenzene isomerization[8,9].It is reminded that such investigations concerning the is omerization behavior of zeolitic catalysts using ethylbenzene/xylene mixture as feed are scarce and more elaboration is needed.As the main goal concerning industrial interest is to nearly eliminate ethylbenzene from the product while retaining a high p-xylene a.t.e.and yield,it seems that new efficient techniques should be developed.The criteria considered for an efficient catalyst are high p-xylene a.t.e.,high xylene yield and high ethylbenzene conversion.
The present work aims at realizing such an improvement using high alumina ZSM-5 zeolites obtained through desilication.Hierarchical zeolites are gaining emerging interest both in the academy and industry.These fine-tuned structures may be synthesized by bottom-up or top down procedures[13]and actually tailored for achieving desired adsorptive[14,15]and catalytic[16,17]properties.Systematic desilication,dealumination or their combination is applied in the top-down route to produce zeolites for specific applications.
The effect of ZSM-5 zeolite desilication on its catalytic behavior for alkylation[18],FCC reactions[19],n-hexane cracking[20],and hydrocarbon abatement[21]reactions has been reported in the literature.To the knowledge of the authors of this work,the study of the desilication effect on xylene isomerization reactions is scarce and is limited to the work of Fernandez et al.[22].They reported the effect of desilication and desilication/HCl post treatment on the o-xyleneisomerization for ZSM-5 zeolites with Si/Al molar ratios larger than 33.Thelatter work was performed in the absence of ethylbenzene and the ZSM-5 zeolite was not high alumina.They reported a reduction of p-xylene selectivity upon desilication.In addition,the pressure of the xylene feed was 4.3 Torr,which is far more distant to the actual pressure in the industrial scale(8–12 barg).The present work reports for the first time the effect of desilication on the isomerization reactions of ethylbenzene/xylene mixtures with initial compositions close to the feed streams used in the industrial practice.The parent zeolite is considered a high alumina zeolite itself and by desilication,the boundary of Si/Al molar ratio of 10 usually defined for ZSM-5 zeolites will be crossed over.The desilication process is assessed by XRF,XRD,FTIR,TEM,nitrogen adsorption/desorption,NH3-TPD,29Si and27Al MAS NMR analyses.The catalytic behavior for the isomerization reaction is investigated as a function of temperature and WHSV.Based on the product analysis,the effect of desilication on the different possible reactions involved is discussed and an optimal treatment condition is presented.
2.Experimental
2.1.Catalyst preparation
H-ZSM-5 zeolite was purchased from the Pars Pigment and Catalyst Co.,Iran.As claimed by the producer,the Si/Al molar ratio of the parent zeolite is 18,and the Na2O content less than 0.08 wt%.The average particle size of the protonated zeolite is ca.500 nm.
Desilication of the initial zeolite powder was performed by contacting 1 g of the powder with 15 g of NaOH solution of different initial molarities(0.1,0.3,0.5 and 0.7)for 1 h under gentle agitation at 75°C.The final product was filtered out,washed with hot distilled water up to ap Hclose to 7,and finally dried overnight at 110°C.Protonation of the desilicated samples was performed by contacting them with 1 mol·L−1aqueous NH4Cl solution under agitation at 90 °C for 8 h, filtering/washing with distilled water,pre-drying at 110°C and calcination at 450°C for 3 h.
2.2.Catalyst characterization
FTIR analysis of the parent and desilicated zeolite powders was performed using a Spectrum GX apparatus by diluting the powder in KBr pellets(<1 wt%zeolite content).A FEI Tecnai F20 instrument was used for TEM analysis.
NH3-TPD characterization was carried out using a Chemisorb 2750(Micromeritics)instrument.The sample was initially heated under a stream of helium gas(20·cm3min−1)at 150 °C for 2 h to get rid of any adsorbed entities.Still under the purge of helium gas,the sample was cooled down to 100°C.Afterwards a gas stream(5 mol%ammonia and 95 mol%helium,20·cm3min−1)was passed over the sample for 40 min at 100°C.Then,pure helium gas was sent over the sample at the same temperature to eliminate any physisorbed ammonia on the sample and also any remaining ammonia gas in the tubing.In the last stage,a helium stream with a flow rate of 40 cm3·min−1was continuously fed to the sample while the latter was heated with a constant heating rate of 10 °C·min−1up to a temperature of 1000 °C.
Nitrogen adsorption/desorption isotherms were taken with a 3Flex(Micromeritics)instrument.Initial degassing was performed by subjecting the sample at 250°C for 2 h under vacuum.
27Al and29Si MASNMR spectra were obtained using a Bruker Avance III 400 WB instrument.Both27Al and29Si MAS NMR analyses were performed at a 10 kHz spinning frequency.One pulse29Si solid state MAS NMR method was used.A 4 mm MAS BB/1H H4470 probe was implemented.
XRF analysis of the samples was performed with a PW1480(Philips)apparatus.XRD analysis was performed using a PW1800(Philips)instrument.
2.3.Catalytic activity/selectivity tests
The isometric drawing of the catalytic isomerization system is shown in Fig.1.The xylene mixture feed(0.1 wt%toluene,14.1 wt%ethyl benzene,3.7 wt%para xylene,54.3 wt%meta xylene,24.7 wt%ortho xylene and 3.1 wt%C8+)is charged from the xylene mixture vessel through a high pressure(max.50 bar)liquid pump to line A.Hydrogen gasisfed from the hydrogen pressure cylinder through amass flow controller to a T-junction where a liquid/gas mixture formed.The latter is guided to the evaporator(a 0.5 inch stainless steel tube surrounded by a temperature controlled electrical furnace)and afterwards to the fixed bed reactor.The reactor is a stainless steel cylinder of an outer diameter of 12.5 mm and an inner diameter of 10 mm.The reactor is charged with 2 g catalyst in each run.The temperature of the reactor tube is controlled with a second electrical furnace surrounding it.The outlet gas temperature of the evaporator and the reactor bed central temperature are controlled using fixed and mobile temperature transducers.The reactor gas outlet stream passes through an initial air-cooled coil condenser and enters a water cooled separator.Liquid samples may be obtained by opening the ball valve under the separator and discharging.The gas stream(mainly hydrogen and light hydrocarbons),deprived from condensates,exits from the top of the separator and enters the back pressure valve.The catalytic reaction system has been run at an operating pressure of 8 barg throughout the catalytic runs.For each run,the system is leak-checked with high purity N2gas supplied by the N2high pres sure cylinder.Then,the catalyst is activated by passing pure hydrogen at 300°Cfor 90 min.Afterwards,the pressure of line A is set to 8 barg and the liquid xylene mixture is fed to the system.Usually the reaction system acquires stability(as far as the reactor outlet stream composition determined by GC analysis is concerned)after 1.5 h.
Catalytic tests were taken for WHSV's of 2,4,6 and 8 h−1.Three average reaction temperatures of 380,390 and 400°C have been considered.The H2/hydrocarbon molar ratio was 4 throughout the experiments.GC analysis of the product samples was performed using a CP-3800 GC Agilent instrument with a CP-Wax 52 CB column.
The catalyst powder wasuni-axially pressed,crushed and sieved to a size between 100 and 150 μm for the catalytic tests.
3.Results and Discussion
3.1.Catalyst characterization
Table 1 lists the denomination of each protonated sample according to the type of chemical treatment carried out.The same table brings the chemical analysis of the different samples.Fig.2 shows the Si/Al molar ratio of the parent and desilicated samples as a function of NaOH solution molarity applied.It is observed that adistinct linear relationship exists between the Si/Al molar ratio and the basic solution molarity.Accordingly,it may be stated that desilication aiming at a desired Si/Al ratio in the range of 6.2–17.9 can be performed with a high precision and in acontrolled manner.Nonetheless,the crystallinity of the samples should be assessed a priori.Fig.3a and b show the XRD patterns of the protonated samples.It is clearly observed that high crystallinity is preserved after desilication up to a NaOH solution molarity of 0.5 mol·L−1.It is observed that the DS4 sample has undergone appreciable amorphization.

Fig.1.Isometric drawing of the catalytic isomerization system used in this study.

Table 1 Denomination of the protonated samples and their XRF analysis(as wt%)

Fig.2.Si/Almolar ratio of the parent and desilicated zeolitesasa function of NaOHsolution molarity.
Fig.4 shows the FTIR spectraof the high crystallinity samples DS0 to DS3.It is observed that increasing the extent of desilication from sample DS1 to DS3,the original peaks located at 1098.0 and 1227.0 cm−1belonging to the parent H-ZSM-5 zeolite shift to smaller values(1083.0 and 1219.0 cm−1,respectively).The peaks observed in the range of 1083.0–1098.0 cm−1and 1219.5–1227.0 cm−1are attributed to internal asymmetric tetrahedral stretch vibration of Si–O or Al–O bonds[23].It is presumed that the corresponding energy needed for inducing stretch vibration of internal Si–O bonds exceedsthat of Al–O bonds,due to the lighter Al atom.Upon desilication,the content of Al–O bonds increases.Accordingly,it is expected that upon desilication,the corresponding peaks shift to the right,i.e.towards smaller wave numbers(smaller energies).Such a relation between the Si/Al ratio and the position of FTIR peaks has been reported in the past[23].
The relative integrated intensity of the broad peak in the region of 3000–3750 cm−1(silanol groups of different kinds)remains approximately constant upon desilication for the different samples.However,sample DS2 shows an emerging peak at 3673 cm−1.The latter peak is more pronounced in the spectrum of the DS3 sample.Such a peak has been observed by other researchers in the past[24]and is attributed to the presence of Al–OH groups.The latter groups may belong to the zeolite framework(tetrahedral)or might be of extra-framework character(octahedral).Their appearance is somehowacceptable,but a firm assignment of the Al atom coordination state needs auxiliary analysis methods.This argument will be further treated in the MAS NMR analysis discussion.
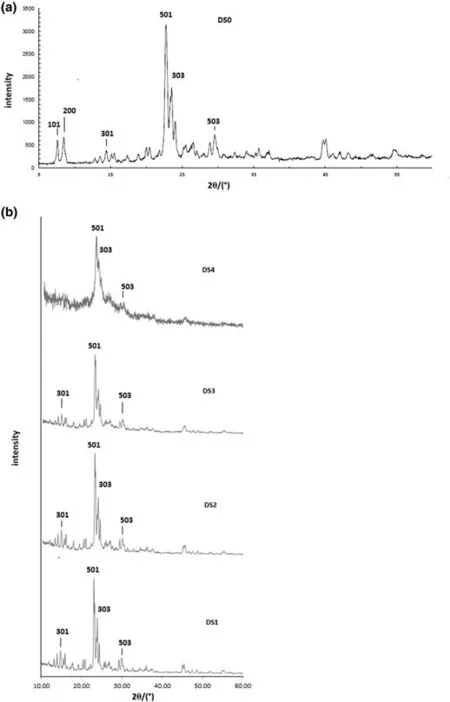
Fig.3.XRD diffractograms of the parent(a)and desilicated zeolites(b).

Fig.5.Nitrogen adsorption/desorption isotherms of different samples.Curvesbelonging to sample DS0 are shown as not connected points.

Fig.4.FTIR spectra of the parent and desilicated zeolites.

Table 2 Quantitative results obtained from the nitrogen adsorption/desorption isotherms of the protonated samples
Fig.5 shows the adsorption/desorption isotherms of the different samples.A continuous and gradual increase of adsorption capacity with increasing desilication extent is observed.A quantitative assessment of the results is presented in Table 2.The specific surface area of the parent zeolite is 380.8 m2·g−1,which increases with the decrease of Si/Al ratio up to 469.7 m2·g−1for sample DS3.Further desilication is accompanied with a substantial decrease of specific surface area(sample DS4).A continuous increase of specific pore volume with desilication extent is observed.Interestingly,the pore volume of sample DS4 is about 2.4 fold that of the parent zeolite.Considering the calculated specific micro and mesopore volumes,it is observed that for the highly crystalline samples(DS0 to DS3),a constant increase of mesopore volume from 0.0618 to 0.3985 cm3·g−1occurs with increasing the extent of desilication.The micropore volume gradually decreases from sample DS0 to DS3 and significantly from DS3 to DS4.On the other hand,the same trend is observed for the micropore surface area.Accordingly,it may be stated that micropores are gradually eliminated upon desilication.This is accompanied with a continuous increase of mesopore volume and mesopore surface area(the same table).Sample DS4 is very different from the other ones,actually because it is of very lowcrystallinity.The significant decrease of micropore volume and surface area observed during the transition from DS3 to DS4 testifies the collapse of the zeolite framework,previously observed through XRD analysis.

Fig.6.TEM pictures of(a)DS0(b)DS0(c)DS1(d)DS3 and(e)DS4 samples.

Fig.7.NH3-TPD analysis of the protonated samples.
A better understanding and visualization of porosity evolution may be gained resorting to the TEM pictures shown in Figs.6a to 6e.Fig.6a shows that the parent H-ZSM-5 zeolite particles consist of inter-grown polycrystalline entities.Fig.6b illustrates a high resolution picture of some exposed H-ZSM-5 crystallites.They appear almost transparent to the electron beam.Considering sample DS1(Fig.6c),it is observed that desilication has resulted in the creation of some inhomogeneity within the crystals.Disordered zones of different intensity of opacity are more pronounced in the case of sample DS3(Fig.6d).The observed inhomogeneity is the result of dissolution of the internal zeolite framework as a result of contact with NaOH solution.Sample DS4 seems somehow spoiled out(Fig.6e),and the high alkalinity employed has also affected the outer edges and surfaces of the ZSM-5 crystals,making them unrecognizable as faceted crystals.These results corroborate with the explanation of the nitrogen adsorption/desorption data concerning the creation of mesoporosity within the zeolite crystals.Based on Fig.6e,the amorphization of the initial zeolite upon treatment with 0.7 mol·L−1NaOH solution may be a result of the collapse of the regular zeolite structure.Recall that sample DS4 showed substantial decrease in specific surface area with respect to sample DS3(Table 2).
The results of NH3-TPD analysis of the different protonated samples are illustrated in Fig.7.Table 3 lists the quantitative information(deconvolution results)pertaining to the spectra shown in Fig.7.Generally,4 types of acid sites could be discerned which are denominated as weak(78 °C–114 °C),medium(170 °C–225 °C),strong(400 °C–440 °C)and super acid sites(670 °C–870 °C),based on the temperature range in which they appear.The parent protonated sample exhibits actually no significant amount of super acid sites and the relative amount of its strong and medium acidity sites is not very different(23%and 28%,respectively).With the on-going desilication process(sample DS1),the general trend of the desorption spectrum remains approximately intact.However,the pertaining temperature of the strong acid sites increases from 415 to 430°C.Further intensification of the desilication process(sample DS2),results in a total increase of the acid sites content(Fig.7)and a clear increase in the relativeamount of the super acid sites.The latter increase in the absolute and relative content of the super acid sites is accompanied with a slight lowering of their strength,as the peak maximum temperature reduces from 870 to 790°C.A significant increase in the relative and absolute amount of the medium acid sites is also observed.Further desilication(sample DS3)results again in a clear increase of total acidity and eruption of the peak belonging to the super acid sites.The relative amount of the latter sites increases to a maximum value of 7%.Just considering the effect of Si/Al ratio for a highly crystalline H-ZSM-5 zeolite,it is rational to expect an increase of total acidity with decreasing the ratio[25].Further desilication(sample DS4)results in the decrease of the total acidity and pronounced broadening of the peaks belonging to the medium,strong and super acid sites.Attribution of a specific chemical species to what has been denominated as super acids is a difficult task at this stage and needsa complementary analysis like solid state NMR which follows.
Fig.8a shows the29Si MAS NMR spectra of the DS0,DS2 and DS3 protonated samples.Increasing the extent of desilication from DS1 to DS2 increases slightly the relative content of Si(1Al)(~−105).Further desilication(sample DS3)results in a distinct increase of the Si(1Al)species content.Generally,the trend observed with increasing the extent of desilication is rational as Si–O bonds in Si–O–Si entities are more susceptible to chemical attack compared to the Si–O–Al ones.This concept has been proposed in the past by several researchers andit is attributed to the negative charge imparted by the tetrahedral Al atoms to the Si–O bond resulting in partial Columbic repulsion of negatively charged hydroxyl groups[24,26].Fig.8b shows the27Al MASNMR spectra of the samples DS0 to DS4.It is observed that the parent zeolite contains a minor amount of octahedral extra-framework aluminum species.The desilicated sample DS1 shows a slightly smaller extra frame work aluminum.Increasing the extent of desilication results in a slightly gradual increase of this species.Sample DS4 shows a rather distinct increase of extra-framework aluminum,which may be attributed to the destruction of the zeolitic framework and production of an amorphous phase.Accordingly,it is deduced that upon desilication,some aluminum atoms are also leached out of the zeolite framework.Nonetheless,it is presumed that not all of them can egress the tortuous zeolitelabyrinth and remain asoctahedral aluminum sites within the micro and mesopore channels of the desilicated sample.The increase of the content of these species with the extent of desilication corroborates with the NH3-TPD results and FTIR analysis.There exists a debate regarding the distribution of the extracted Al atoms in the literature.Sadowska et al.[26]report no enrichment and Verboekend and Perez-Ramirez report an enrichment of external zeolite crystal surface in Al[27].Such assessments rely on the core Si/Al ratio calculated based on the29Si and27Al MAS NMR analysis results and need extra XPS analysis.However,Si/Al ratio calculation based on NMR analysis has been reported to be prone to error due to partial amorphization of ZSM-5 crystals during desilication[24].Due to the strong interaction between octahedral aluminum species and the Broensted acid sites(protons)detected upon NH3-TPD analysis,it seems mostly improbable that these aluminum species have enough mobility to diffuse out of the channels in order to enrich the surface.

Table 3 Quantitative assessment of the NH3-TPD spectra for the protonated parent and desilicated/protonated samples

Fig.8.29Si MAS NMR(a)and 27Al MAS NMR(b)spectra of the different protonated samples.
Based on the27Al MAS NMR analysis,it may be stated that the super acid sites contain octahedral aluminum.Assignment of a specific chemical species like oxo-aluminum species((AlO)n)+,as reported by Ghasemian et al.[17]in the NH3-TPD spectrum in a temperature range of 700–750 °C for the clinoptilolite zeolite,is not possible at this stage.
3.2.Catalytic study of ethylbenzene/xylene mixture isomerization reaction
Fig.9 shows the catalyst selectivity of the different samples for para xylene in terms of approach to equilibrium(a.t.e.)according to the following relation:

where C denotes the concentration of para xylene and T is the reaction temperature.The equilibrium concentration of para xylene at the reaction temperature under consideration is calculated according to the thermodynamic approach of Chirico and Steele[28].

Fig.9.p-xylene a.t.e.(a)as a function of kind of zeolite and reaction temperature for a WHSV of 2 h−1 and(b)as a function of WHSV and reaction temperature for sample DS3.(pressure=0.8 MPa).
Considering sample DS0,it is observed that,depending on there action temperature,p-xylene a.t.e.lies between 69%to 76%.Generally,this value increases with increasing reaction temperature.Sample DS1 exhibits an improved p-xylene a.t.e.,which changes between 79%to 81%in the temperature span under consideration.Further desilication(sample DS2)results in aslightly lower performance(p-xylenea.t.e.between 76%to 79%).However,sample DS2 shows a clear enhancement in p-xylene a.t.e.compared to the parent zeolite.Sample DS3 shows the highest enhancement of p-xylene a.t.e.,its value ranging between 77%and 83%.Further desilication(sample DS4)results in a drop of p-xylene a.t.e.Recall that sample DS4 is highly amorphized.Summing up,it may be stated that desilication improves selectivity of the isomerization reaction versus p-xylene for the high crystallinity samples.
It should be mentioned that,to the knowledge of the authors of the present work,the only group that has reported the yield of para xylene(as wt%of para xylene in the product),is that of Fernandez et al.[22].They reported a para xylene concentration in the product of ca.9.3 wt%for their parent and desilicated samples(WHSV=4.1–4.9 h−1,673 K,4.3 Torr),respectively.This is while,in our case,the para xylene concentration in the product is 19.32 wt%,for the DS3 sample(WHSV=4 h−1,673 K,0.8 MPa).

Ethylbenzene conversion is defined as below:where Cethylbenzeneis the concentration of ethylbenzene in wt%.Fig.10 shows EBCas a function of the kind of catalyst,reaction temperature and WHSV.While the maximum conversion of ethylbenzene(EBc)on the parent zeolite is ca.53%(at 400 °C and WHSV=2 h−1),all the desilicated samples showa minimum conversion which is higher than 53%.Sample DS1 is able to convert it up to 73%(again at 400°C and WHSV=2 h−1).Sample DS2 exhibits an improved catalytic behavior in this regard and shows a maximum conversion of 85%at the same reaction conditions.Further increasing the extent of desilication(sample DS3)results in a distinct improvement of the zeolite capacity in converting ethylbenzene to ca.90%at the same reaction conditions.Sample DS4,however,shows an attenuated ethylbenzene conversion respect to sample DS3.
The tendency of the different catalysts to produce aromatic comp ounds with more than 8 carbon atoms(C8+)is shown in Fig.11,as a function of reaction temperature and WHSV.The parent zeolite itself results in a p rod uction of C8+hydrocarbons in the range of 3 wt%to 4 wt%.Desilication results in an increase of C8+concentration in the product.This value is maximal for sample DS4.
For the sake of place,we bring only the product concentrations of benzene and toluene for a temperature of 390°C in Table 4.It is observed that,compared to the parent zeolite,the desilicated samples result in increased production of benzene and toluene.Fig.12a and b shows the benzene and toluene yield as a function of WHSV at 390°C for the different samples.(See Fig.13.)

Fig.10.Ethylbenzeneconversion(a)asa function of kind of zeolite and reaction temperature for a WHSVof 2 h−1 and(b)asafunction of WHSVand reaction temperature for sample DS3.(pressure=0.8 MPa).

Fig.11.Concentration ofcompounds in the product(a)as a function of kind of zeolite and reaction temperature for a WHSV of 2 h−1 and(b)as a function of WHSV and reaction temperature for sample DS3.(pressure=0.8 MPa).
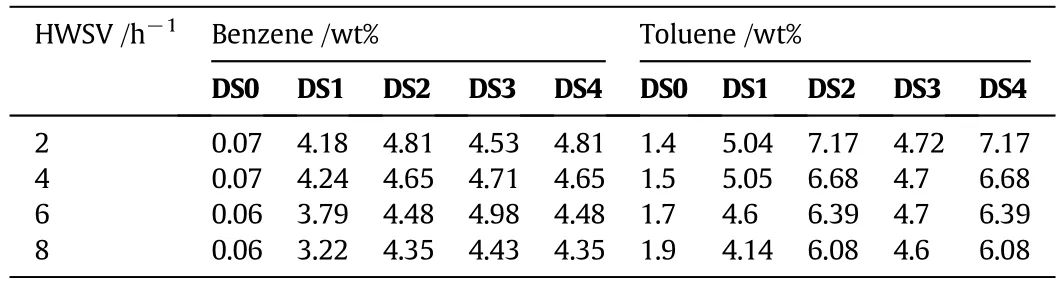
Table 4 Benzene and toluene product concentration as a function of kind of catalyst and at a reaction temperature of 390°C
Fig.13 shows the sum of xylene isomers mass percent in the product for the different catalysts as a function of temperature and WHSV.Considering the smallest WHSV(larger residence time)of 2 h−1and the highest temperature of 400°C(largest reaction rate),the performance of the different catalysts will be compared.The parent zeolite results in a maximum xylene yield of 85%.Sample DS1 shows a slightly smaller yield of 81%and sample DS2 a distinct smaller one equal to 78%.Sample DS3 shows again an improved yield of 82%and sample DS4,the lowest level of 78%.Accordingly,as far as the xylene yield is concerned,it may be stated that sample DS3 exhibits the best catalytic performance among the desilicated zeolites.Considering p-xylene a.t.e.and ethylbenzene conversion results discussed previously,it may be stated that the DS3 sample shows the best catalyst performance among the parent and all the desilicated samples.
What follows is an explanation of catalytic test results based on the previous discussionsconcerning the physico/chemical characteristics of the desilicated zeolites and the known related catalytic reaction chemistry.It has been proposed that isomerization of xylene isomers in protonated MFI type zeolites proceeds mainly via an inter-molecular mechanism due to steric hindrances preventing the production of bulky tri-alkyl benzene molecules[29−31].Accordingly,an initial benzenium ion is produced by the transfer of a proton from the protonated zeoliteto the initially neutral aromatic xylene ring.It was shown in the previous paragraphs that desilication resulted in an increase of total acidity(mainly due to a lower Si/Al ratio)and,more interestingly,the creation of very strong acidity(samples DS1 to DS3).This fact most probably promotes the isomerization-activity of the catalyst.However,this is not enough for obtaining a higher para xylene selectivity.Based on Table 2,desilication is also accompanied with the production of mesoporosity and increase of the size of mesopores.The size of the sinusoidal and straight channels of the parent ZSM-5 zeolite are(0.51 nm×0.55 nm)and(0.54 nm×0.56 nm),respectively.This is while the kinetic diameter of para,meta and ortho xylenes are 0.58,0.68 and 0.68 nm,respectively[32].An important fact which should be taken into consideration,is that upon desilication a series of interconnected micropores and mesopores within each individual zeolite crystal may form[33,34].Thisreducesthe effectivepath for the reactant and product molecules leading to an enhanced mass transport.In other terms,due to the smaller tortuosity(τ),larger voidage(ε),and larger diffusivity due to larger pores(Dparaxylene),the effective diffusivity of para xylene increases significantly(Dparaxylene,effective=Dparaxylene.ε/τ).The diffusivity of meta and ortho xylenes within the modified zeolite channels is most probably still smaller than para xylene due to configuration(geometric)reasons.The net outcome is an enhanced production and selectivity of para xylene.

Fig.12.Benzene(a)and toluene(b)yield as a function of WHSV at 390°C for the different samples.

Fig.13.Xylene yield(a)as a function of kind of zeolite and reaction temperature for a WHSV of 2 h−1 and(b)as a function of WHSV and reaction temperature for sample DS3.(pressure=0.8 MPa).
It was shown that desilication had a clear and positive effect in lowering the ethylbenzene content in the product,crossing over the thermodynamic limitation posed by the mere C8H10isomerization reactions.In other words,desilication induces other type of reactions for ethylbenzene conversion.Ethylbenzene isomerization reactionsusually need a metallic catalyst like Pt,absent in our case.Other plausible reactions for ethylbenzene conversion are disproportionation and transalkylation reactions[35].The former reaction results in the formation of bulky di-ethylbenzene molecules along benzene molecules.The trans-alkylation reactions may result in the production of bulky molecules like di-methyl–ethylbenzene and ethyl–methyl benzene,along the production of benzene and toluene molecules.Referring to Fig.11 and Table 4,such reactions seem to be promoted through desilication.As mentioned earlier,desilication is accompanied with the creation of extra internal volume and increase of pore size.Up to sample DS3,this is also accompanied with an increased acidity(namely activity)of the catalyst.Accordingly,steric hindrance for the production of the aforesaid bulky molecules may be alleviated,eventually promoting intramolecular mechanisms.Pore size increase also promotes the diffusivity of bulky molecules.As a corollary,they gain a higher probability for egressing the porous labyrinth before undergoing subsequent decomposition reactions.In other words,our results show partial elimination of restricted transition-state selectivity in favor of ethylbenzeneconversion reactions.
Xylene disproportionation and trans-alkylation reactions result in the lowering of xylene yield and therefore they are undesired.An important point is that upon desilication,especially considering the optimum sample DS3,the extent of these reactions due to the creation of new surface and larger poresisminimal.Xyleneyield at the optimum conditions of lowest WHSV(2 h−1)and reaction temperature of 400 °C for sample DS3 is only 3%smaller than that of the parent zeolite.In other words,the effect of desilication is mainly the enhancement of disproportionation and trans-alkylation reactions for ethylbenzene and not for the xylene isomers,accordingly having only small effect on the xylene yield.It should be added that the stronger acid sites created through desilication also promote the disproportionation reaction.Thisisan important practical outcome.In industrial practice,high ethylbenzene conversion through reforming reactions(isomerization)that lead to its transformation into xylenes is usually limited and the total conversion of ethylbenzene hardly exceeds 30%.Therefore,the authors of this work propose the use of bifunctional catalysts comprehending desilicated H-ZSM-5 zeolite and a metal like Pt for isomerization units.
4.Conclusions
High alumina ZSM-5 zeolite desilication(initial Si/Al ratio 18)could be performed successfully up to a Si/Al ratio of 9.67 while keepingahigh level of crystallinity.The increase of desilication extent was accompanied with the creation and gradual increase of the content of super acid sites.There exists a limit for the desilication treatment(Si/Al molar ratio of 9.67)for obtaining improved catalytic performance.Desilication promotes ethylbenzene conversion by disproportionation and trans-alkylation reactions while the same reactions are limited for the xylene isomers.The para xylene approach to equilibrium improved by more than 7%at 400 °C and a WHSV equal to 2 h−1for the optimum sample with respect to the parent zeolite.At the same conditions,the optimum sample exhibited the maximum ethylbenzene conversion of 89%,i.e.more than 40%w.r.t.the parent zeolite.This is while the xylene yield decreases only 3%at the same conditions.The procedure for the synthesis of the hierarchical zeolite may be of high industrial importance due to the high ethylbenzene conversion capacity.
Acknowledgments
This work was financed by BIPC,Mahshahr,Iran under the contract number 08-133/57665.BIPC is highly acknowledged for the support provided.The authors thank by heart Rahman Mahmoudi,Shokufeh Farahani,Majid Mollavali,Amin Moavi and Mohammad Ali Sharififor their cooperation and fruitful human ambient they created throughout the work.Bahir Duraki is greatly acknowledged for his help in NMR analysis.
[1]T.C.Tsai,I.Wang,C.K.Huang,S.D.Liu,Study on the ethyl benzene and xylene conversion over modified ZSM-5,Appl.Catal.A Gen.321(2007)125–134.
[2]J.Zhou,Z.Liu,L.Li,Y.Wang,H.Gao,W.Yang,Z.Xie,Y.Tang,Hierarchical mesoporous ZSM-5 zeolite with increased external surface acid sites and high catalytic performance in o-xylene isomerization,Chin.J.Catal.34(2013)1429–1433.
[3]S.Gui,Y.Hao,L.Zhou,Z.Jing,Y.Qiao,H.Gu,Y.Li,B.Cheng,J.Wang,Catalyst supported with noble metals for the isomerization of alkylaromatics,United State Patent,5,1998 759 950.
[4]N.M.Tukur,S.Al-Khattaf,Comparison studies of xylene isomerization and disproportionation reactions between SSZ-33,TNU-9,mordenite and ZSM-5 zeolite catalysts,Chem.Eng.J.166(2011)348–357.
[5]X.Li,P.Ren,Y.Zhang,X.Liu,X.Sun,M.Gao,M.Jia,Z.Lü,T.Dou,Synthesis,characteristics of hierarchical EU-1 zeolite for xylene isomerization probe reaction,Chin.J.Chem.Eng.24(2016)1577–1583.
[6]E.Guillon,S.Lacombe,T.Sozinho,P.Magnoux,S.Gnep,P.Moreau,M.Guisnet,Howto improve the selectivity of zeolite catalysts in C8 aromatic cut isomerization,Oil Gas Sci.Technol.Rev.IFP 64(2009)731–744.
[7]F.J.Llopis,G.Sastre,A.Corma,Xylene isomerization and aromatic alkylation in zeolites NU-87,SSZ-33,β,and ZSM-5:Molecular dynamics and catalytic studies,J.Catal.227(2004)227–241.
[8]J.M.Silva,M.F.Ribeiro,F.Ramoa Ribeiro,E.Benazzi,Influence of platinum on the transformation of an ethylbenzene-o-xylene mixture on HZSM-5,Appl.Catal.A Gen.125(1995)1–14.
[9]A.A.Susu,C.T.Ako,Ethylbenzene isomerization on bifunctional platinum/alumina product yields and pseudo mass action kinetics,Appl.Catal.16(1985)179–192.
[10]K.Toch,J.W.Thybaut,B.D.Vandegehuchte,C.S.L.Narasimhan,L.Domokos,G.B.Marin,A single-event microkinetic model for ethylbenzene dealkylation/xylene isomerization on Pt/H-ZSM-5 zeolite catalyst,Appl.Catal.A Gen.425-426(2012)130–144.
[11]F.Moreau,P.Moreau,N.S.Gnep,P.Magnoux,S.Lacombe,M.Guisnet,Ethylbenzene isomerization over bifunctional p latinum alumina—EUO catalysts:Location of active sites,Microporous Mesoporous Mater.90(2006)327–338.
[12]P.Moreau,Isomerization of ethylbenzene on Pt/zeolite bifuctional catalysts with intermediate-size pores,University of Poitiers(LACCO),2005.
[13]D.Verboekend,J.Perez-Ramirez,Towards a sustainable manufacture of hierarchical zeolites,ChemSusChem 7(2014)753–764.
[14]R.Mahmoudi,C.Falamaki,Ni2+-ion-exchanged dealuminated clinoptilolite:A superior adsorbent for deep desulfurization,Fuel 173(2016)277–284.
[15]R.Mahmoudi,C.Falamaki,Systematic study on the effect of desilication of clinoptilolite zeolite on its deep-desulfurization characteristics,Nanochem.Res.1(2016)64–72.
[16]S.Fathi,M.Sohrabi,C.Falamaki,Improvement of HZSM-5 performance by alkaline treatments:Comparative catalytic study in the MTG reactions,Fuel 116(2014)529–537.
[17]N.Ghasemian,C.Falamaki,M.Kalbasi,M.Khosravi,Enhancement of the catalytic performance of H-clinoptilolite in propane–SCR–NO x process through controlled dealumination,Chem.Eng.J.252(2014)112–119.
[18]S.Abello,A.Bonilla,J.Perez-Ramirez,Mesoporous ZSM-5 zeolite catalysts prepared by desilication with organic hydroxides and comparison with NaOH leaching,Appl.Catal.A Gen.364(2009)191–198.
[19]J.Ding,H.Liu,P.Yuan,G.Shi,X.Bao,Catalytic properties of a hierarchical zeolite synthesized from a natural aluminosilicate mineral without the use of a secondary mesoscale template,ChemCatChem 5(2013)2258–2269.
[20]M.Milina,S.Mitchell,N.L.Michels,J.Kenvin,J.Perez-Ramirez,Interdependency between porosity,acidity,and catalytic performance in hierarchical ZSM-5 zeolites prepared by post-synthesis modification,J.Catal.308(2013)398–407.
[21]B.Puertolas,L.Garcia-Andujar,T.Garcia,M.V.Navarro,S.Mitchell,J.Perez-Ramirez,Bifunctional Cu/H-ZSM-5 zeolite with hierarchical porosity for hydrocarbon abatement under cold-start conditions,Appl.Catal.B Environ.154-155(2014)161–170.
[22]C.Fernandez,I.Stan,J.P.Gilson,K.Thomas,A.Vicente,A.Bonilla,J.Perez-Ramirez,Hierarchical ZSM-5 zeolites in shape-selective xylene isomerization:Role of mesoporosity and acid site speciation,Chem.Eur.J.16(2010)6224–6233.
[23]L.Shirazi,E.Jamshidi,M.R.Ghasemi,The effect of Si/Al ratio of ZSM-5 zeolite on its morphology,acidity and crystal size,Cryst.Res.Technol.43(2008)1300–1306.
[24]B.Gil,L.Mokrycki,B.Sulikowski,Z.Olejniczak,S.Walas,Desilication of ZSM-5 and ZSM-12 zeolites:Impact on textural,acidic and catalytic properties,Catal.Today 152(2010)24–32.
[25]M.Osman,L.Atanda,M.M.Hossain,S.Al-Khattaf,Kinetics modeling of disproportionation and ethylation of ethylbenzene over HZSM-5:Effects of SiO2/Al2O3ratio,Chem.Eng.J.222(2013)498–511.
[26]K.Sadowska,A.Wach,Z.Olejniczak,P.Kustrowski,G.Datka,Hierarchical zeolites:Zeolite ZSM-5 desilicated with NaOH and NaOH/tetrabutylamine hydroxide,Microporous Mesoporous Mater.167(2013)82–88.
[27]D.Verboekend,J.Perez-Ramirez,Desilication mechanism revisited:Highly mesoporous all-silica zeolites enabled through pore-directing agents,Chem.Eur.J.17(2011)1137–1147.
[28]R.D.Chirico,W.V.Steele,Thermodynamic equilibria in xylene isomerization.5.Xylene isomerization equilibria from thermodynamic studies and reconciliation of calculated and experimental product distributions,J.Chem.Eng.Data 42(1997)784–790.
[29]M.Guisnet,N.S.Gnep,S.Morin,Mechanism of xylene isomerization over acidic solid catalyst,Microporous Mesoporous Mater.35-36(2000)47–59.
[30]K.H.Roebschleager,E.G.Christoffel,Reaction mechanism of ethylbenzene isomerization,Ind.Eng.Chem.Prod.Res.Dev.18(1979)347–352.
[31]H.Vinek,J.A.Lercher,Production and reaction of xylenes over H-ZSM-5,J.Mol.Catal.64(1991)23–29.
[32]D.H.Olson,W.O.Hang,Structure-selective relationship in xylene isomerization and selective toluene disproportionation,ACS Symp.Ser.248(1984)275–307.
[33]C.H.Christensen,K.Johannsen,I.Schmidt,C.H.Chritensenm,Catalytic benzene alkylation over mesoporous zeolite single crystals:Improving activity and selectivity with a newfamily of porous materials,J.Am.Chem.Soc.125(2003)13370–13371.
[34]J.Shi,Y.Wang,W.Yang,Y.Tang,Z.Xie,Recent advances of pore system construction in zeolite-catalyzed chemical industry processes,Chem.Soc.Rev.44(2015)8877–8903.
[35]H.K.Min,S.B.Hong,Mechanistic investigations of ethylbenzene disproportionation over medium-pore zeolites with different framework topologies,J.Phys.Chem.C 115(2011)16124–16133.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review☆
- Cultivation of microalgae for biodiesel production:A reviewon upstream and downstream processing☆
- Numerical study and acceleration of LBM-RANS simulation of turbulent flow☆
- GPU-based discrete element simulation on flow stability of flat-bottomed hopper☆
- Tuning sol size to optimize organosilica membranes for gas separation☆
- Oil–water pre-separation with a novel axial hydrocyclone☆
