Surgical reconstruction of spinal cord circuit provides functional return in humans
2018-01-05ThomasCarlstedtNicholasJamesrtenRisling
Thomas Carlstedt, Nicholas James Mårten Risling
1 The Wolfson Centre for Age-Related Diseases, King’s College London, London, United Kingdom
2 Department of Neuroscience, Karolinska Institutet, Solna, Sweden
Surgical reconstruction of spinal cord circuit provides functional return in humans
Thomas Carlstedt1,*, Nicholas James1, Mårten Risling2
1 The Wolfson Centre for Age-Related Diseases, King’s College London, London, United Kingdom
2 Department of Neuroscience, Karolinska Institutet, Solna, Sweden
This mini review describes the current surgical strategy for restoring function after traumatic spinal nerve root avulsion in brachial or lumbosacral plexus injury in man. As this lesion is a spinal cord or central nervous injury functional return depends on spinal cord nerve cell growth within the central nervous system. Basic science, clinical research and human application has demonstrated good and useful motor function after ventral root avulsion followed by spinal cord reimplantation. Recently, sensory return could be demonstrated following spinal cord surgery bypassing the injured primary sensory neuron. Experimental data showed that most of the recovery depended on new growth reinnervating peripheral receptors.Restored sensory function and the return of spinal reflex was demonstrated by electrophysiology and functional magnetic resonance imaging of human cortex. This spinal cord surgery is a unique treatment of central nervous system injury resulting in useful functional return. Further improvements will not depend on surgical improvements. Adjuvant therapy aiming at ameliorating the activity in retinoic acid elements in dorsal root ganglion neurons could be a new therapeutic avenue in restoring spinal cord circuits after nerve root avulsion injury.
plexus injury; root avulsion; spinal cord surgery; motor sensory recovery; adjuvant therapy
Introduction
A spinal cord injury leads to degeneration of nerve fibres and synapses, death of nerve cells, breakdown of neural connections and functional circuits followed by growth inhibiting glia scar. In order to restore the lost function these events have to be corrected and treated. The initial discovery of spinal cord neuronal growth after injury when presented with a peripheral nerve conduit (David and Aguayo,1981) was followed by a hectic search to find ways in which these new axons could also enter and elongate in central nervous tissue (CNS), ultimately re-establishing new contacts or synapses and hopefully restoring some degree of function. Most research has been focused on what appears to be the primary and most crucial problem in CNS regeneration, which is to promote nerve fibre regeneration or growth across the injury site. A major impediment to regeneration is the various growth inhibiting substances in the injury induced scar (Silver and Miller, 2004; Wanner et al., 2013). Progress has been made in dissecting out various compounds and molecules in the lesion environment that are responsible for the curtailed growth of severed neurites.So far there have not been any solutions reported for the generic CNS lesion which is applicable to humans (Varma et al., 2013).
A spinal nerve root lesion is in effect a spinal cord injury (Carlstedt and Havton, 2012). Interruption of nerve fibres in spinal roots results in degeneration of transverse segmental motor, sensory and autonomic neurites in the central and peripheral nervous system with retraction and disintegration of synapses and neural networks, leading to cessation of functional integration such as local segmental spinal cord re flexes (Carlstedt and Havton, 2012). Within a few weeks there is a conspicuous disintegration of spinal cord motoneurons (Bergerot et al., 2004) as well as secondary sensory neurons in the dorsal horn (Chew et al., 2008)and the development of a glia scar at the site of root traction from the spinal cord (Carlstedt et al., 1989). This lesion can be denoted a longitudinal spinal cord injury in contrast to the classical transverse medullary lesion (Carlstedt and Havton, 2012).
The most common traumatic cause of spinal nerve root injury in man is a traction injury sustained in violent accidents, such as in road traffic injuries (Alnot and Narakas,1995; Birch, 2001). The most common location of such trauma is at the complicated formation of the brachial and lumbosacral nerve plexa which supply the limbs with nerve function. The brachial plexus is most susceptible to such trauma because of the loose suspension of the forelimb with the neck and the trunk. There are also traction injuries affecting the lumbosacral plexus although it is much more protected in the bony pelvis. The ventral root is more fragile than the dorsal root and hence more prone to rupture particularly in a human traumatic situation (Carlstedt,2007).
In the majority of cases of severe brachial plexus traction injury, one or several of the nerve roots have been detached or avulsed from the spinal cord. This means that the arm is lacking neuronal control, but more disturbing is the severe and typical excruciating pain that is associated with such an injury.
This is a devastating injury that can only be repaired if there is regeneration and new neurite growth within the central nervous system before the loss of innervation results in neuronal death occurring due to the detachment of the neurons from their peripheral contacts (Bergerot et al.,2004; Chew et al., 2008).
Reimplanting avulsed motor roots into the spinal cord can restore muscle function (Carlstedt, 2007). Motoneurons in the ventral horn of the spinal cord can re-establish connectivity with muscles if a conduit (the avulsed ventral root or a peripheral nerve graft) is super ficially implanted into the spinal cord in the region of the ventral horn. The first part of this spinal cord motoneuron to muscle re-connectivity occurs as new growth. This appears as a shoot offnot from the interrupted axon, but from a dendrite as an initial central nervous nerve axon; a dendraxon (Lindå et al., 1985). It is well established that dendrites can produce aberrant or supernumerary axons after injury that can extend into the peripheral nervous system (PNS) (Hoang et al., 2005). Within the spinal cord, the myelin sheath and associated nodes of Ranvier of this neurite have a CNS phenotype. Electrophysiology showed the return of muscle function as well as re-established excitatory and inhibitory synaptic contacts after reimplantation spinal cord surgery(Cullheim et al., 1989). Recovery is explained by this original observation of critical and crucial initial CNS type of neurite growth from the lesioned motoneuron before extending distally in peripheral nerves to muscles. This unique finding led to a surgical technique that is now an established clinical treatment and can restore useful motor function in patients, even those with the most severe and complicated brachial plexus avulsion injuries (Carlstedt,2007).
In contrast to the ability of motor neurons to regrow within, and extend beyond, the spinal cord after root avulsion, it is well documented that sensory nerve fibres cannot regenerate back into the spinal cord after injury (Carlstedt,1985a). The primary sensory neuron situated in the dorsal root ganglion is effectively barred in the PNS-CNS transitional region at the astrocyte rich interface with the PNS part of the root. Inhibition can cause the regrowing axonal tips to form synaptoid endings abutting the glia interface.Obviously, sensory function cannot be recovered by reimplanting avulsed sensory roots in these injuries, which for the affected patients means agonizing chronic pain,loss of proprioceptive and exteroceptic sensation, as well as reduced muscle coordination and function (Htut et al.,2007).
Hypothetically, similar to motoneurons also sensory nerve cells in the spinal cord could elongate new pro-cesses into a PNS graft implanted into the dorsal spinal cord to reconnect with the periphery. Indeed, medullary implantation of a nerve graft into the dorsal horn of the spinal cord has been documented to induce extension of processes from spinal cord dorsal horn neurons into the sensory part of a spinal nerve after the dorsal root ganglion has been removed, in effect bypassing the primary sensory neurons (Carlstedt, 1985b). When asking spinal cord neurons to extend new processes into the PNS it is likely that not axons, but dendrites would be recruited by the PNS conduit implanted into the dorsal horn. Experimentally it was shown that intrinsic sensory spinal cord neurons can extend new (non-regenerative) processes into an implanted PNS conduit (Carlstedt, 1985b). Immunohistochemical technique showed that these neurites were dendrites that had extended into the implanted PNS conduit and have functional properties (James et al., 2017). Electrophysiology veri fied that the new growth from sensory spinal cord neurons can transmit impulses. There was also a demonstration of transsynaptically provoked muscle contraction when stimulating those neurites, indicating an integration of this new growth with segmental spinal cord circuits, particularly ventral horn motoneurons had occurred (James et al., 2017). This original experimental observation has led to application.
Surgical Strategy and Outcome
The implantation of a PNS conduit into both the ventral and dorsal part of the spinal cord, for motor and sensory recovery respectively, has been performed in clinical cases of brachial plexus avulsion injury (Figures 1 and 2).Following such procedures proprioception together with muscle function could be demonstrated. The biceps tendon re flex was con firmed clinically as well as the Hoffman (or H-) re flex by means of electrophysiology (Carlstedt et al.,2012). With this extended spinal cord surgery, including also sensory repair, it was obvious that movements had become more controlled without the usual synkinesis seen in cases where only motor conduits had been reconstructed(Carlstedt, 2007). Functional magnetic resonance imaging demonstrated sensory motor activities on active and passive elbow flexion verifying the return of proprioception recordable at cortical level (Carlstedt et al., 2012). This procedure is a type of neurotisation which is palliative and depending on neuronal plasticity. It does not result in full sensory recovery. Surgery alone is inutile in curative attempts to restore a better sensation by means of spinal cord regeneration of primary sensory (dorsal root ganglion)neurons.
Adjuvant Therapy for Spinal Cord Sensory
Regeneration
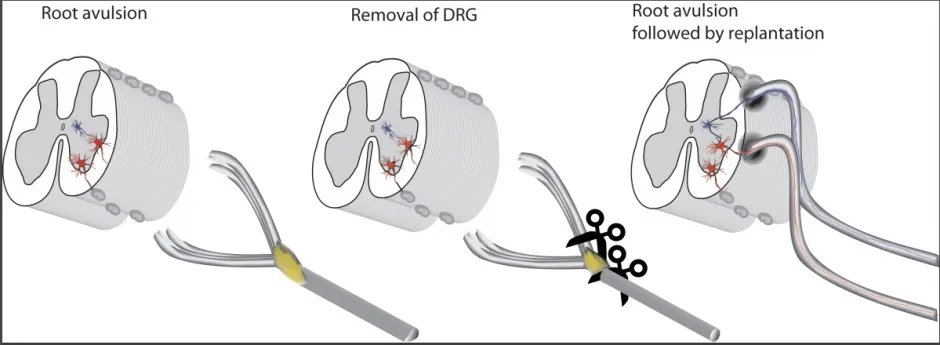
Figure 1 Surgical strategy for recovery of spinal cord circuits after rootavulsion in humans.After dorsal root ganglionectomy conduits of peripheral nervous type-roots or peripheral nerve grafts are implanted into the ventral and dorsal parts of the spinal cord. They are distally connected to respectively motor or sensory parts of spinal nerve after ganglion has been deleted. DRG: Dorsal root ganglion.
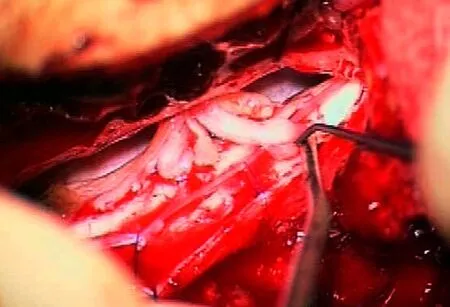
Figure 2 Peroperative view through operating microscope of spinal cord replantation.After a laminoplasty, the dura and arachnoid membrane are opened and the spinal cord exposed. In this case dorsal and ventral roots had been avulsed from the spinal cord and detached out of the medullary canal. Grafts were harvested from sensory nerves (super ficial radial and medial cutaneous nerve of forearm) of the ipsilateral affected arm. Cables of nerve graft were installed into the spinal cord canal through intervertebral foramina. Cables of the nerve grafts were introduced through small slits in pia mater in the ventro- and dorsolateral aspects of the spinal cord and distally connected to motor and sensory parts of detached spinal nerve respectively. The picture demonstrates multiple cable grafts already implanted in the ventral part of the spinal cord for motor recovery. Instruments are seen to insert a graftin the dorsal part of the spinal cord for sensory restoration.
Adjuvant therapy is necessary to complement surgery in order to recover better sensation after dorsal root injury or avulsion from the spinal cord. There are at least two major reasons for the failure of injured dorsal root axons to regrow back into the spinal cord. There is a lack of intrinsic neuronal growth, based largely on inactivity in the phosphoinositide3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, which is negatively regulated by phosphatase and tensin homolog (PTEN) (Park et al.,2010). Another major impediment to regeneration into the spinal cord is the formation of an inhibitory environment by a glial scar.
The retinoic acid signalling system is very powerful in neuron growth and regeneration. Previous work has shown that retinoic acid receptor (RAR) beta 2 signalling stimulates axonal outgrowth in human, mouse and rat (Agudo et al., 2010). RARbeta 2 induces axonal regeneration programs within injured neurons and encourages axonal growth also in the inhibitory central nervous system (Yip et al., 2006). In adult spinal cord explants and dorsal root ganglion neurons where RARbeta 2 is absent, overexpression of RARbeta stimulates neurite outgrowth (Corcoran et al., 2002). In a rat model of cervical dorsal root rhizotomy(C5–T1), a RARbeta agonist was given (Goncalves et al.,2015). After 4 weeks of treatment behavioural tests showed recovery of sensory and skilled locomotor function. Dorsal root fibre regeneration across the dorsal root peripheral/central transitional region (PNS-CNS TR) was shown by biotinylated dextran amine (BDA) labelling, electron microscopy, and by means of tractography, all of which showed a robust ingrowth of neurites. Synaptic recovery was demonstrated by analysis of noxious heat stimuli responses, synaptic density, and mechano- and proprioceptive synapses in the dorsal horn, showing that new connections had been established in the spinal cord (Goncalves et al., 2015). It has earlier been an established concept that in the mature individual retinoic acid response elements are locally activated after neuronal injury and hence the RAR-beta agonist would not have an effect on intact afferents in promoting central sprouting and recovery (Zhelyaznik et al., 2003).
Of paramount importance is how the regenerated dorsal root axons re-entered the spinal cord. In the naïve adult situation, there is a specialized transitional region between the stereotype peripheral nerve compartment of the dorsal root and the central nervous fibre tracts of the spinal cord.This transitional region is characterised by a number of unique structural entities (Berthold and Carlstedt, 1977).Among these are the occurrence of fibrous astrocytic processes surrounding and separating the most proximal peripheral paranodes, as well as a compound PNS-CNS type of node of Ranvier at the crossing of nerve fibres from the PNS to the CNS of organization (Berthold and Carlstedt,1977). In RARbeta agonist treated animals, a glial construct of a similar organization had been re-established at the passing of regenerated dorsal root axons into the spinal cord. This is of conceptual importance as it indicates that this naïve state of structural glial organization has to be recapitulated to allow successful regeneration. This is presently the subject of further studies in particular what the role of the NG2 cells are in this context.
Studies of the mechanisms underlying the switch from a nonpermissive environment in the CNS, as well as an increased regenerative capacity in the dorsal root neurons,demonstrated that the RARbeta agonist modulates the PTEN signalling pathway in both neurons and astrocytes(Goncalves et al., 2015). In neurons RARbeta, induces PTEN to move from the membrane, where it blocks axonal growthviathe PI3K inhibition (Park et al., 2010), into the cytoplasm, where it becomes phosphorylated and hence inactive. In addition, stimulation of RARbeta results in an increased secretion of PTEN in exosomes. These are taken up by astrocytes, resulting in hampered proliferation and glia scar formation, as well as causing them to arrange in a normal appearing scaffold around the regenerating axons,allowing them to grow back into the spinal cord (Goncalves et al., 2015). The dual effect of RARbeta signalling, both neuronal and glial, results in axonal regeneration in the spinal cord after dorsal root injury.
In summary, this surgical strategy is the first and so far the only treatment that restores spinal cord connections and circuits with functional recovery in humans after trauma. There is the potential for new growth and plasticity,rather than regeneration, of spinal cord sensory neurons that can replace the injured primary sensory dorsal root neurons and reconnect to the periphery for reestablishment of some but not all sensory qualities. In order for a more complete return of sensory function after dorsal root avulsion from the spinal cord an adjuvant therapy will be necessary, with RARbeta being one such potential therapeutic target.
Author contributions:All authors contributed equally in performing research,analyzing data as well as writing.
Con flicts of interest:None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:Reviewer: Peter Shortland, Western Sydney University, Australia.
Comments to authors: This review describes recent experiments on trying to promote sensory axon regeneration and restoration of neural circuits in preclinical animal models as a prelude to translational studies in human root avulsion injury.
Agudo M, Yip P, Davies M, Bradbury E, Doherty P, McMahon S,Maden M, Corcoran JP (2010) A retinoic acid receptor beta agonist (CD2019) overcomes inhibition of axonal outgrowth via phosphoinositide 3-kinase signalling in the injured adult spinal cord.Neurobiol Dis 37:147-155.
Alnot JY, Narakas A (1995) Les Paralysies du Plexus Brachial. Paris:Expansion Scienti fique Francaise.
Berthold CH, Carlstedt T (1977) Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. II. General organization of the transitional region in S1 dorsal rootlets. Acta Physiol Scand Suppl 446:23-42.
Birch R (2001) Surgical Disorders of the Peripheral Nerves. 2nd ed.Springer-Verlag London.
Carlstedt T (1985a) Regenerating axons form nerve terminals at astrocytes. Brain Res 347:188-191.
Carlstedt T (1985b) Dorsal root innervation of spinal cord neurons after dorsal root implantation into the spinal cord of adult rats.Neurosci Lett 55:343-348.
Carlstedt T (2007) Central Nerve Plexus Injury. Imperial College Press.
Carlstedt T, Havton L (2012) The longitudinal spinal cord injury:lessons from intraspinal plexus, cauda equina and medullary conus lesions. Handb Clin Neurol 109:337-354.
Carlstedt T, Cullheim S, Risling M, Ulfhake B (1989) Nerve fibre regeneration across the PNS-CNS interface at the root-spinal cord junction. Brain Res Bull 22:93-102.
随着社会经济的快速发展,社会各领域及企业对人才的需求也在不断增加,教育部门也更加重视培养学生的综合能力,并对新教学模式采取积极引进、不断创新的方法。在高中信息技术课堂教学中,随着新课程改革的逐步深入,现代教育理念已经愈加得到人们的支持和认可,信息技术已经成为现代化学习中不可缺少的重要部分,可以有效提高学生的学习效率。高中信息技术教学课程通过翻转课堂这种新的教学方式,将以学生为主体作用得以体现,为这些教改策略的实现提供了新的实践经验和动力。
Carlstedt T, Misra VP, Papadaki A, McRobbie D, Anand P (2012)Return of spinal re flex after spinal cord surgery for brachial plexus avulsion injury. J Neurosurg 116:414-417.
Chew DJ, Leinster VH, Sakthithasan M, Robson LG, Carlstedt T,Shortland PJ (2008) Cell death after dorsal root injury. Neurosci Lett 433:231-234.
Corcoran J, So PL, Barber RD, Vincent KJ, Mazarakis ND, Mitrophanous KA, Kingsman SM, Maden M (2002) Retinoic acid receptor beta2 and neurite outgrowth in the adult mouse spinal cord in vitro. J Cell Sci 115:3779-3786.
Cullheim S, Carlstedt T, Linda H, Risling M, Ulfhake B (1989) Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience 29:725-733.
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214:931-933.
Goncalves MB, Malmqvist T, Clarke E, Hubens CJ, Grist J, Hobbs C,Trigo D, Risling M, Angeria M, Damberg P, Carlstedt TP, Corcoran JP (2015) Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci 35:15731-15745.
Hoang TX, Nieto JH, Havton LA (2005) Regenerating supernumerary axons are cholinergic and emerge from both autonomic and motor neurons in the rat spinal cord. Neuroscience 136:417-423.
Htut M, Misra VP, Anand P, Birch R, Carlstedt T (2007) Motor recovery and the breathing arm after brachial plexus surgical repairs,including re-implantation of avulsed spinal roots into the spinal cord. J Hand Surg Eur Vol 32:170-178.
James ND, Angeria M, Bradbury EJ, Damberg P, McMahon SB, Risling M, Carlstedt T (2017) Structural and functional substitution of deleted primary sensory neurons by new growth from intrinsic spinal cord nerve cells: an alternative concept in reconstruction of spinal cord circuits. Front Neurol 8:358.
Lindå H, Risling M, Cullheim S (1985) ‘Dendraxons’ in regenerating motoneurons in the cat: do dendrites generate new axons after central axotomy? Brain Res 358:329-333.
Park KK, Liu K, Hu Y, Kanter JL, He Z (2010) PTEN/mTOR and axon regeneration. Exp Neurol 223:45-50.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Varma AK, Das A, Wallace Gt, Barry J, Vertegel AA, Ray SK, Banik NL (2013) Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38:895-905.
Wanner IB, Anderson MA, Song B, Levine J, Fernandez A,Gray-Thompson Z, Ao Y, Sofroniew MV (2013) Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33:12870-12886.
Yip PK, Wong LF, Pattinson D, Battaglia A, Grist J, Bradbury EJ,Maden M, McMahon SB, Mazarakis ND (2006) Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord.Hum Mol Genet 15:3107-3118.
Zhelyaznik N, Schrage K, McCaffery P, Mey J (2003) Activation of retinoic acid signalling after sciatic nerve injury: up-regulation of cellular retinoid binding proteins. Eur J Neurosci 18:1033-1040.
How to cite this article:Carlstedt T, James N, Risling M (2017) Surgical reconstruction of spinal cord circuit provides functional return in humans. Neural Regen Res 12(12):1960-1963.
Funding: This work was supported by the Wellcome Trust, Karolinska Institutet, Swedish Defence (No. FOT-AF.9221006) and Darwin Trust of Edinburgh.
*Correspondence to:Thomas Carlstedt, M.D., Ph.D.,carlstedt.thomas@googlemail.com.
orcid:0000-0003-4628-8284(Thomas Carlstedt)
10.4103/1673-5374.221145
2017-11-22
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Conductive polymer scaffolds to improve neural recovery
- The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: perspectives for remyelination therapeutic strategies
- MicroRNAs in Parkinson’s disease and emerging therapeutic targets
- Environmental cues determine the fate of astrocytes after spinal cord injury
- Formin’ bridges between microtubules and actin filaments in axonal growth cones
