白藜三醇通过下调microRNA-21减轻快速电刺激所致心房肌细胞电重构*
2017-09-03沈冰冰朱启仁王志荣张卓琦
张 松, 沈冰冰, 李 飞, 朱启仁, 王志荣, 张卓琦△
(1徐州医科大学附属医院心内科, 江苏 徐州 221002; 2邳州市人民医院, 江苏 邳州 221300)
白藜三醇通过下调microRNA-21减轻快速电刺激所致心房肌细胞电重构*
张 松1, 沈冰冰1, 李 飞2, 朱启仁1, 王志荣1, 张卓琦1△
(1徐州医科大学附属医院心内科, 江苏 徐州 221002;2邳州市人民医院, 江苏 邳州 221300)
目的: 研究白藜三醇(resveratrol,RSV)对快速电刺激(rapid electrical stimulation,RES)导致乳鼠心房肌细胞电重构时微小RNA-21(microRNA-21,miR-21)表达的影响,探讨RSV通过miR-21参与电重构的可能机制。方法: 采用胰酶、Ⅰ型胶原酶双酶法及差速贴壁法分离培养乳鼠心房肌细胞。通过RES建立乳鼠心房肌细胞房颤模型,心房肌细胞随机分为4组:空白对照(control)组、RSV组、RES组和RSV+RES组。为了证实RSV是否通过调控miR-21的表达参与电重构,除了上述4组,另增加过表达和沉默miR-21组:RES+阴性对照组(RES+NC组)、RES+miR-21 mimics组、RES+miR-21 mimics+RSV组、RES+miR-21 inhibitor组和RES+miR-21 inhibitor+RSV组。CCK-8法检测心房肌细胞活性以确定RSV最佳作用浓度及时间,qPCR法检测各组细胞内miR-21及L型钙离子通道CACNA1C、CACNB2 mRNA的表达水平,Western blot检测L型钙离子通道Cav1.2和Cavβ2的蛋白表达水平。结果: 与control组相比,RES组miR-21表达明显上调(P<0.05),加入RSV预处理后miR-21表达下调(P<0.05)。与RES+miR-21 mimics组相比,RES+miR-21 mimics+RSV组miR-21表达下调(P<0.05),而CACNA1C和CACNB2 mRNA及Cav1.2和Cavβ2蛋白表达量增加(P<0.05)。与RES组比,RES+miR-21 inhibitor和RES+miR-21 inhibitor+RSV组的miR-21表达下调(P<0.05),CACNA1C和CACNB2 mRNA及Cav1.2和Cavβ2蛋白表达量增加,但RES+miR-21 inhibitor组与RSV+RES组比,miR-21表达、CACNA1C和CACNB2 mRNA及Cav1.2和Cavβ2蛋白表达量的差异无统计学显著性。结论: 在快速电刺激乳鼠心房肌细胞模拟房颤模型中,RSV干预可能通过下调miR-21表达而调控其下游靶基因这一途径来减轻心房肌细胞电重构。
白藜三醇; 微小RNA-21; 快速电刺激; 电重构; 乳鼠心房肌细胞
心房颤动(atrial fibrillation,AF)是临床上最常见的慢性持续性心律失常,具有较高的发病率、致死率、致残率,其发生和维持的机制非常复杂[1]。目前研究认为AF的发生机制包括电重构、结构重构、细胞内钙离子活动异常、氧化应激和炎症反应等[2-3]。其中,心房重构是房颤发生与维持的重要机制。随着近年来对AF发生机制研究的深入,已有越来越多的研究表明微小RNA(microRNA,miRNA,miR)参与心房重构的调控。其中,miR-21的表达量在房颤发生中明显上调,通过调控钙离子通道蛋白参与心房重构[4]。
白藜三醇(resveratrol,RSV)是主要存在于葡萄、虎杖等植物中的一种非黄酮类多酚化合物。大量研究表明,RSV具有抗炎、抗氧化、抗血小板聚集及心律失常[5]等众多心血管保护作用[6]。同时,研究表明RSV可通过下调miR-21减轻缺氧/复氧损伤,从而保护心肌细胞[7]。为研究RSV对快速电刺激(rapid electrical stimulation,RES)导致乳鼠心房肌细胞电重构时miR-21表达的影响,探讨RSV通过miR-21参与电重构的可能机制,本研究采用体外培养的乳鼠心房肌细胞,电刺激法建立心房细胞AF模型。通过观察心房肌细胞miR-21及靶基因mRNA、蛋白水平变化,探讨RSV通过miR-21参与电重构的可能机制,为进一步临床应用RSV治疗房颤提供理论依据。
材 料 和 方 法
1 实验动物
新生SD大鼠(1~3日龄)来源于徐州医科大学实验动物中心。
2 药物、试剂和仪器
RSV和Ⅰ型胶原酶均购于Sigma;胎牛血清(fetal bovine serum,FBS)购于Gibco; CCK-8试剂盒购于Dojindo;miR-21、CACNA1C和CACNB2 引物由上海捷瑞生物工程有限公司设计及合成;miR-21过表达/沉默序列(表1)由苏州吉玛基因股份有限公司合成;抗Cav1.2和Cavβ2蛋白兔源多克隆 I 抗购于Alomone Labs;辣根过氧化物酶标记山羊抗兔IgG II 抗购于北京中山金桥生物技术有限公司。C-Pace细胞培养刺激仪购于Bioprobes;倒置显微镜和体视显微镜购于Olympus。
3 主要方法
3.1 乳鼠心房肌细胞分离及培养 取新生SD大鼠心脏,参考文献[8]进行培养,采用双酶法及差速贴壁法获得乳鼠原代心房肌细胞。
3.2 CCK-8法检测心房肌细胞活力以确定白藜三醇最佳作用浓度及时间 将原代心房肌细胞按每孔1×105个细胞接种于96孔板,RSV分5、10、15、20和25 μmol/L 5个浓度组,CCK-8试剂盒检测经不同浓度梯度RSV预处理不同时间的心房肌细胞活性。检测步骤:避光条件下每孔加入CCK-8和新鲜DMEM混合液(比例为1∶10)110 μL,将培养板继续置于37 ℃、5% CO2条件下培养,2 h后酶标仪测定在450 nm处的吸光度[9],计算细胞存活率。
3.3 乳鼠心房肌细胞房颤模型制备及分组 原代心房肌细胞培养于6孔板中,培养至第3天后予以RSV预处理,RSV的剂量是15 μmol/L。将与6孔板配套的带有刺激电极的盖板覆盖于6孔板上,使电极到达培养基液面以下。盖板通过导线连接于细胞刺激器,置于培养箱中继续培养。参考文献[10-12]方法,将细胞刺激器设置为脉冲5 ms,频率4 Hz,电压15 V,持续电刺激24 h以建立心房细胞房颤模型。心房肌细胞随机分为4组:对照(control)组、RSV组、RES组和RES+RSV组。为了证实白藜三醇是否通过调控miR-21的表达参与电重构,除了上述4组,另增加过表达或沉默miR-21组:RES+NC组、RES+miR-21 mimics组、RES+miR-21 mimics+RSV组、RES+miR-21 inhibitor组和RES+miR-21 inhibitor+RSV组。
3.4 过表达或沉默miR-21 按上述方法培养的乳鼠心房肌细胞,48 h后细胞贴壁完全,状态良好,可进行转染。转染操作按Lipofectamine 2000试剂说明,将miR-21 mimics/inhibitor转染至细胞。
3.5 RT-qPCR法检测心房肌细胞miR-21及L型钙离子通道CACNA1C、CACNB2 mRNA的表达水平 各组处理结束后,Trizol试剂一步法提取心房肌细胞总RNA。采用两步法进行RT-qPCR反应。miR-21茎环引物、上下游引物,以及各靶基因及内参照U6、GAPDH上下游引物均由上海捷瑞公司设计与合成,具体序列见表1。结果采用2-ΔΔCt法进行相对定量分析。
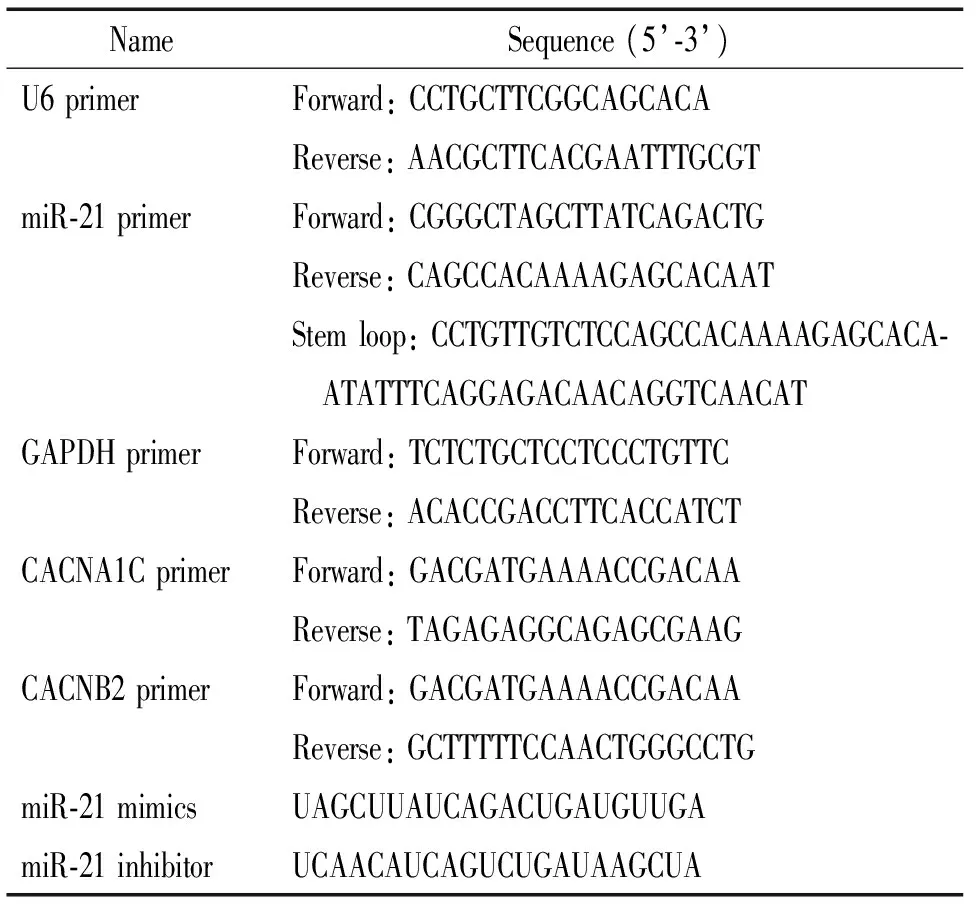
表1 引物及miR-21 mimics/inhibitor序列
3.6 Western blot检测心房肌细胞 Cav1.2和Cavβ2蛋白的表达水平 将以上9组细胞经预冷的PBS漂洗2遍后,用含有蛋白酶抑制剂的细胞裂解液在冰上裂解30 min;12 000×g离心15 min,收集上清液;经BCA法测浓度后,蛋白高温变性,加样,5%的浓缩胶和8%的分离胶进行跑胶,按湿转方法转膜, I 抗(Cav1.2以1∶1 000稀释,Cavβ2以1∶1 000稀释)4 ℃孵育过夜,TBST洗膜5 min×3次, II 抗孵育1.5 h,洗膜3次,ECL法显色。用ImageJ图像分析软件进行半定量分析。蛋白质的相对含量以目的蛋白与GAPDH条带光密度值的比值表示。
4 统计学处理
用GraphPad Prism 5.0软件进行统计学分析。计量资料均以均数±标准差(mean±SD)来表示。各组间差异的比较采用单因素方差分析(one-way ANOVA),组间两两比较采用q检验,以P<0.05为差异有统计学意义。
结 果
1 CCK-8筛选RSV最佳预处理时间及浓度
CCK-8实验结果显示,与不同浓度RSV预处理6 h和12 h相比,不同浓度RSV预处理24 h和48 h使乳鼠心房肌细胞活力减低(P<0.05)。因此白藜三醇最佳预处理时间设定为12 h,见图1。

Figure 1.The cell viability after 6 h, 12 h, 24 h and 48 h pretreatment with different concentrations of RSV. Mean±SD.n=5.
图1 不同浓度的RSV预处理6、12、24和48 h后细胞活力的变化
与对照组相比,白藜三醇25 μmol/L组乳鼠心房肌细胞活性明显减低(P<0.01),其它的4个浓度组与对照组相比差异无统计学显著性;与白藜三醇15 μmol/L组相比,白藜三醇20和25 μmol/L组乳鼠心房肌细胞活力减低(P<0.05)。因此白藜三醇作用于乳鼠心房肌细胞的最佳浓度设定为15 μmol/L,见图2。
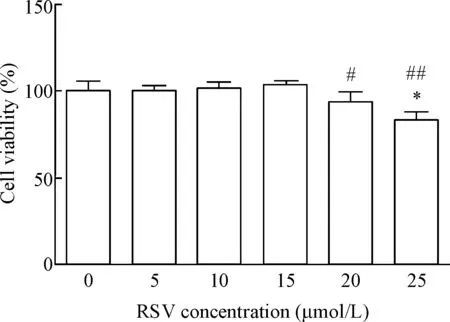
Figure 2.The cell viability after 12 h pretreatment with different concentrations of RSV. Mean±SD.n=5.*P<0.05vs0 μmol/L group;#P<0.05,##P<0.01vs15 μmol/L group.
图2 不同浓度RSV预处理12 h后细胞活力的变化
2 RT-qPCR检测原代心房肌细胞miR-21及L型钙离子通道CACNA1C、CACNB2 mRNA的表达
与control组比, RES组miR-21表达上调(P<0.05),RSV预处理后可明显下调miR-21的表达(P<0.05);沉默miR-21后,RES+miR-21 inhibitor组miR-21表达下调(P<0.05),且与RES+RSV组没有明显差异;过表达miR-21后,RES+miR-21 mimics组miR-21表达明显上调,加入RSV预处理后同样可下调miR-21的表达(P<0.05),见图3。

Figure 3.The effects of RSV on the expression of miR-21 in primary atrial myocytes were detected by RT-qPCR. U6 was used as an internal control. Mean±SD.n=3.**P<0.01vscontrol;##P<0.01vsRES;&P<0.05vsRES+RSV;△△P<0.01vsRES+miR-21 mi-mics;▲P<0.05vsRES+RSV+miR-21 mimics.
图3 RT-qPCR检测RSV对原代心房肌细胞miR-21表达的影响
与control组比,RES组的CACNA1C表达量减少(P<0.05),加入RSV干预后,CACNA1C的表达量增加(P<0.05);沉默miR-21后,RES+miR-21 inhibitor组CACNA1C的表达量增加(P<0.05),且与RES+RSV组相比差异无统计学显著性;过表达miR-21后,RES+miR-21 mimics组的CACNA1C表达量减少,加入RSV预处理CACNA1C的表达量增加(P<0.05);CACNA1C的表达量与miR-21表达水平呈负相关,见图4。
RT-qPCR检测不同分组L型钙离子通道基因CACNB2的mRNA的表达,CACNB2的mRNA表达趋势与CACNA1C的mRNA表达趋势类似,见图5。
3 Western blot检测原代心房肌细胞 Cav1.2和Cavβ2蛋白的表达
各组Cav1.2和Cavβ2蛋白表达与CACNA1C和CACNB2 mRNA表达的趋势一致,见图6。
讨 论
房颤的发生和发展过程中伴随着心房电重构,电重构的过程又能导致房颤恶化,即房颤导致房颤[13]。

Figure 4.The effects of RSV on the mRNA expression of CACNA1C were detected by RT-qPCR. GAPDH was used as an internal control. Mean±SD.n=3.**P<0.01vscontrol;##P<0.01vsRES;&&P<0.01vsRES+RSV;△△P<0.01vsRES+miR-21 mimics;▲P<0.05vsRES+RSV+miR-21 mimics.
图4 RT-qPCR检测RSV对CACNA1C mRNA表达的影响
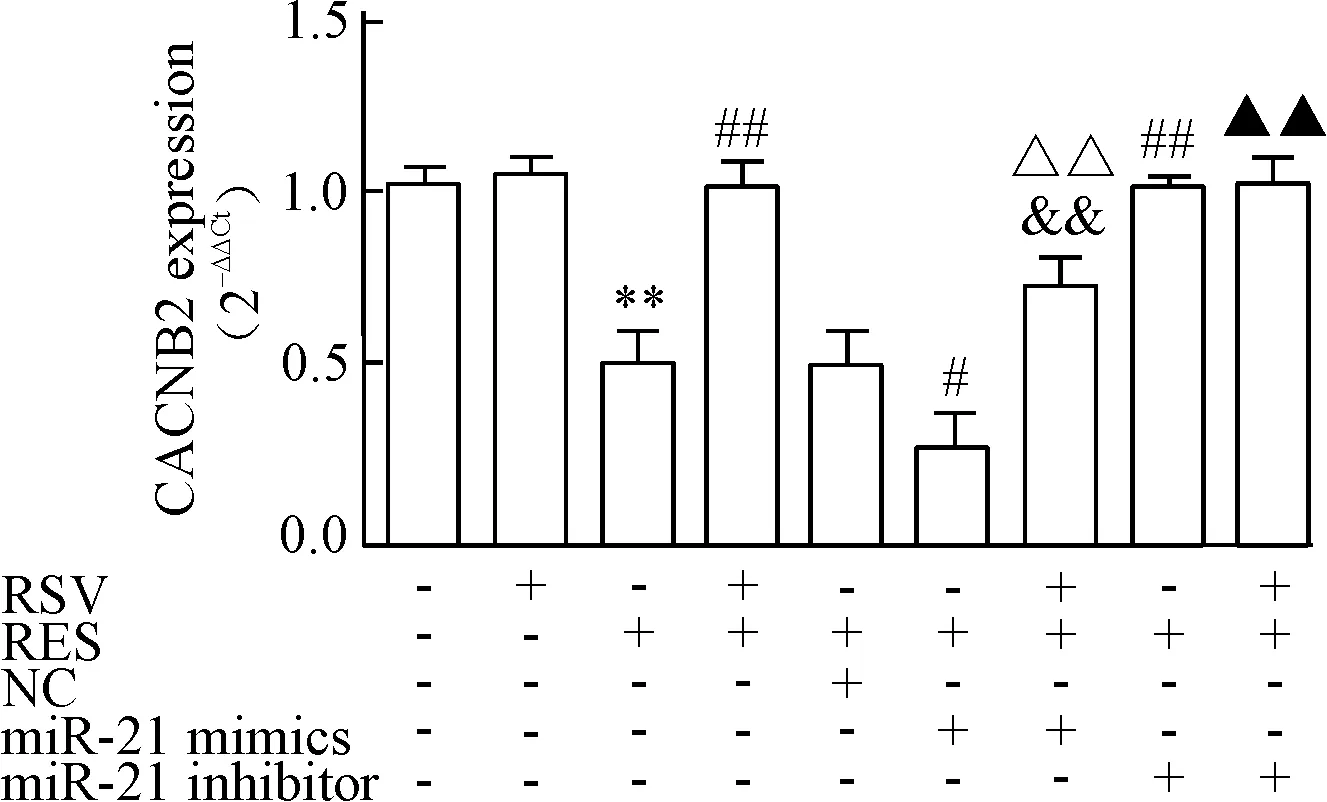
Figure 5.The effects of RSV on the mRNA expression of CACNB2 were detected by RT-qPCR. GAPDH was used as an internal control. Mean±SD.n=3.**P<0.01vscontrol;#P<0.05,##P<0.01vsRES;&&P<0.01vsRES+RSV;△△P<0.01vsRES+miR-21 mimics;▲P<0.05vsRES+RSV+miR-21 mimics.
图5 RT-qPCR检测RSV对CACNB2 mRNA表达的影响
心房电重构主要表现为心房有效不应期和动作电位时程呈进行性缩短,传导速度减慢,不应期离散度增加,以及频率适应性减退等[14-15],而这些主要是通过离子通道的改变实现的,钾离子通道电流、钙离子通道电流等均参与动作电位的形成。房颤的治疗原则包括:控制心室率、抗凝治疗、缓解症状、治疗基础心脏病和诱发因素、恢复并维持窦性心律。目前临床上治疗房颤的药物疗效并不十分理想,而导管消融术很难将多个分布的局灶全部发现,且复发率高,两者均有明显的局限性[16-17],因此探寻新的治疗方法尤为重要。电重构发生在房颤的早期阶段,在此阶段针对房颤发生机制的上游治疗正越来越得到重视。
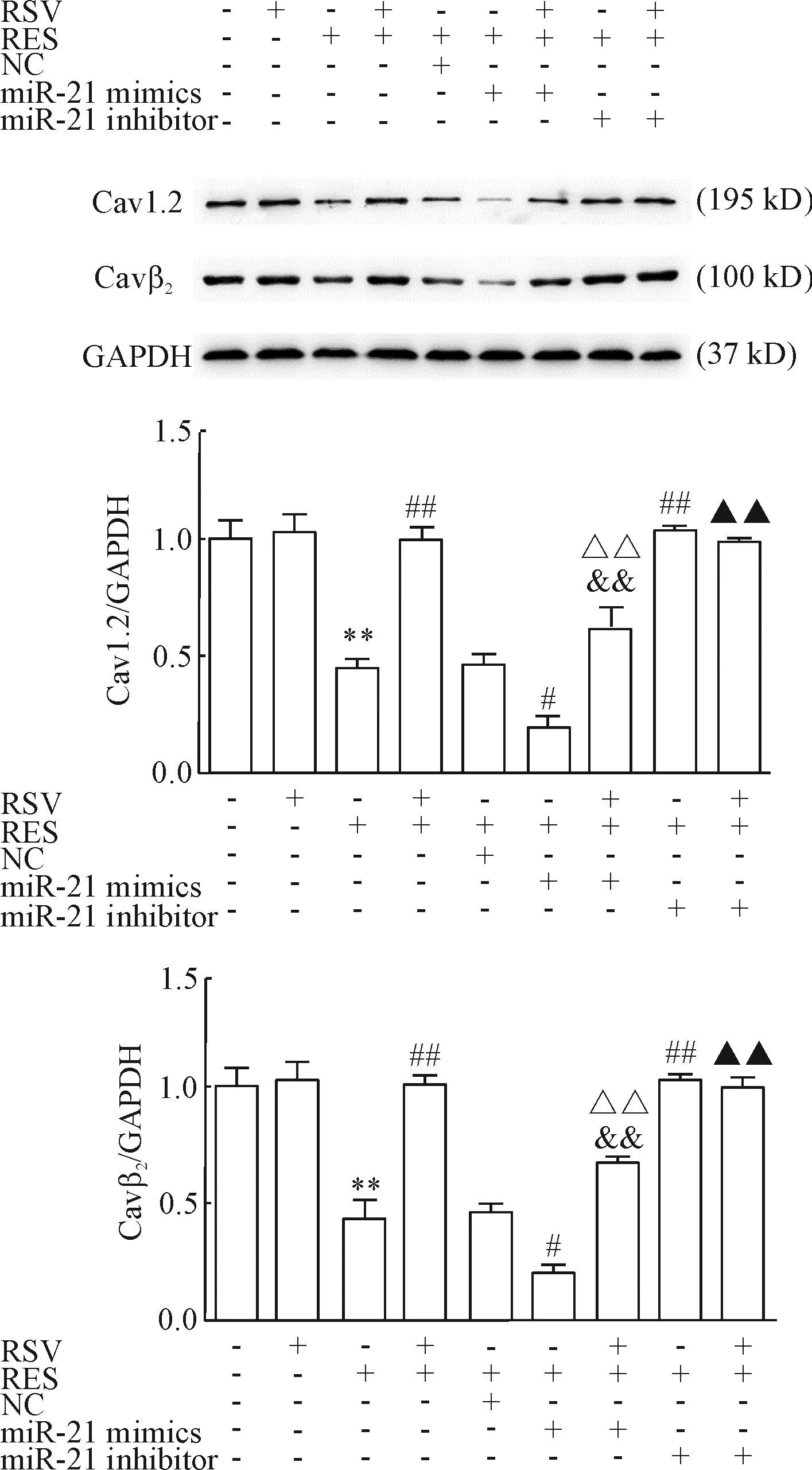
Figure 6.The effects of RSV on the protein expression of Cav1.2 and Cavβ2were detected by Western blot. GAPDH was used as an internal control. Mean±SD.n=3.**P<0.01vscontrol;#P<0.05,##P<0.01vsRES;&&P<0.01vsRES+RSV;△△P<0.01vsRES+miR-21 mimics;▲▲P<0.01vsRES+RSV+miR-21 mimics.
图6 Western blot检测RSV对Cav1.2和Cavβ2蛋白表达的影响
miRNA是一类长约22个核苷酸的单链、内源性、非编码的小分子RNA。近年来随着对房颤发生机制研究的深入,越来越多研究表明miRNA参与心房重构的调控。Luo等[18]通过研究证实miRNA参与心脏电重构,他们结合实验方法设计了生物信息学分析,来证实miRNA具有调节编码人类心脏离子通道蛋白基因的潜能。房颤的发生涉及多个离子通道蛋白的改变,而 miRNA参与调控多个心脏电重构相关蛋白的表达[19],因此miRNA表达失衡引起离子通道蛋白功能失调可能是房颤发生的电生理基础,也可能成为抗心律失常药物的作用靶点[20]。同时也有研究认为,miRNA可能通过调控离子通道蛋白的表达,从而表现出促房颤或抗房颤的作用[21]。
RSV一种非黄酮类多酚化合物。本实验室前期研究已证实RSV可以通过减轻心房纤维化[22]、抑制心房重构[23]、减少氧化应激损伤[24]等发挥抗心律失常作用。同时有研究表明,RSV可调控miRNA的表达发挥其保护作用[25]。在本研究中,我们通过体外培养的乳鼠心房肌细胞,电刺激法建立心房细胞AF模型,课题组前期已证实miR-21在此模型中明显上调[26]。本实验发现RSV预处理可以下调miR-21的表达,并且编码L型钙离子通道的基因CACNA1C、CACNB2 的mRNA及Cav1.2、Cavβ2的蛋白表达量均增加,与miR-21表达呈负相关,这些结果表明CACNA1C和CACNB2 可能是miR-21的靶基因,这与Barana等[4]使用萤光素酶报告分析结果一致。为进一步探讨白藜三醇是否通过调控miR-21的表达参与电重构,另增加过表达或沉默miR-21组,结果显示:过表达miR-21后,与RES+miR-21 mimics组相比,加入RSV干预可下调miR-21表达,且CACNA1C、CACNB2的mRNA及Cav1.2、Cavβ2的蛋白表达量增加。沉默miR-21后,RES+miR-21inhibitor及RES+miR-21inhibitor+RSV组miR-21表达均下调,同时CACNA1C、CACNB2 mRNA及Cav1.2、Cavβ2蛋白表达量增加,且与RES+RSV组相比无明显差异。本研究结果显示,RSV可能通过下调miR-21表达而调控其下游靶基因CACNA1C、CACNB2 及其编码的Cav1.2、Cavβ2蛋白水平来减轻快速电刺激所致心房肌细胞电重构,为房颤的上游治疗提供了新的方向,为临床应用白藜三醇治疗房颤提供理论依据和实验数据。
[1] Gramley F, Lorenzen J, Jedamzik B, et al. Atrial fibrillation is associated with cardiac hypoxia[J]. Cardiovasc Pathol, 2010, 19(2):102-111.
[2] Schroen B, Heymans S. Small but smart: microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing[J]. Car-diovasc Res, 2012, 93(4):605-613.
[3] Luo X, Yang B, Nattel S. MicroRNAs and atrial fibrillation: mechanisms and translational potential[J]. Nat Rev Cardiol, 2015, 12(2):80-90.
[4] Barana A, Matamoros M, Dolz-Gaiton P, et al. Chronic atrial fibrillation increases microRNA-21 in human atrial myocytes decreasing L-type calcium current[J]. Circ Arrhythm Electrophysiol, 2014, 7(5):861-868.
[5] 卞洲艳, 唐其柱, 易方方, 等. 白藜芦醇对大鼠心肌梗死后室性心律失常及长期存活率的影响[J]. 中华心律失常学杂志, 2009, 13(1):66-69.
[6] Kaneko H, Anzai T, Morisawa M, et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization[J]. Atherosclerosis, 2011, 217(2):350-357.
[7] Mukhopadhyay P, Mukherjee S, Ahsan K, et al. Restoration of altered microRNA expression in the ischemic heart with resveratrol[J]. PLoS One, 2010, 5(12):e15705.
[8] Qin Y, Zhang Z, Chen J, et al. Ca2+disorder caused by rapid electrical field stimulation can be modulated by CaMKIIδ expression in primary rat atrial myocytes[J]. Biochem Biophys Res Commun, 2011, 409(2):287-292.
[9] 张晓萍, 彭景燕, 熊君宇, 等. 罗哌卡因预处理对其诱导ND7/23细胞毒性的影响[J]. 中华麻醉学杂志, 2011, 31(4):463-464.
[10]Yang Z, Shen W, Rottman JN, et al. Rapid stimulation causes electrical remodeling in cultured atrial myocytes[J]. J Mol Cell Cardiol, 2005, 38(2):299-308.
[11]Inoue N, Ohkusa T, Nao T, et al. Rapid electrical stimulation of contraction modulates gap junction protein in neonatal rat cultured cardiomyocytes: involvement of mitogen-activated protein kinases and effects of angiotensin II-receptor antagonist[J]. J Am Coll Cardiol, 2004, 44(4):914-922.
[12]Brundel BJ, Henning RH, Ke L, et al. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation[J]. J Mol Cell Cardiol, 2006, 41(3):555-562.
[13]Harada M, Luo X, Qi XY, et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation[J]. Circulation,2012, 126(17):2051-2064.
[14]Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats[J]. Circulation,1995, 92(7):1954-1968.
[15]Morillo CA, Klein GJ, Jones DL, et al. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation[J]. Circulation, 1995, 91(5):1588-1595.
[16]Sovari AA, Dudley SC. Antioxidant therapy for atrial fibrillation: lost in translation?[J]. Heart, 2012, 98(22):1615-1616.
[17]Murray KT, Mace LC,Yang Z. Nonantiarrhythmic drug therapy for atrial fibrillation[J]. Heart Rhythm, 2007, 4(3 Suppl):S88-S90.
[18]Luo X, Zhang H, Xiao J, et al. Regulation of human cardiac ion channel genes by microRNAs: theoretical perspective and pathophysiological implications[J]. Cell Physiol Biochem, 2010, 25(6):571-586.
[19]Luo X, Pan Z, Shan H, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation[J]. J Clin Invest, 2013, 123(5):1939-1951.
[20]Gomes da Silva AM, Silbiger VN. miRNAs as biomarkers of atrial fibrillation[J]. Biomarkers, 2014, 19(8):631-636.
[21]Lu Y, Zhang Y, Wang N, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation[J]. Circulation, 2010, 122(23):2378-2387.
[22]杜海歌, 张超群, 徐 晤, 等. 白藜三醇通过HIF途径对快速心房起搏猪心房结构重构的抑制作用[J]. 徐州医学院学报, 2012, 32(3):149-153.
[23]周晓峰, 王志荣, 张卓琦, 等. 白藜芦醇对快速起搏右心房诱发的持续性心房颤动猪心房结构重构的影响[J]. 中国心血管杂志, 2013, 18(6):455-458.
[24]葛力萁, 李承宗, 程明月, 等. 探讨白藜三醇抑制快速电刺激乳鼠心肌细胞氧化应激损伤及其机制[J]. 中国循环杂志, 2015, 30(7):684-688.
[25]Lancon A, Kaminski J, Tili E, et al. Control of micro-RNA expression as a new way for resveratrol to deliver its beneficial effects[J]. J Agric Food Chem, 2012, 60(36):8783-8789.
[26]李艳茹, 张 琼, 张 松, 等. 心房颤动电重构机制中相关microRNA的表达差异[J]. 徐州医学院学报, 2016, 36(5):291-295.
(责任编辑: 卢 萍, 罗 森)
Resveratrol reduces electrical remodeling in atrial fibrillation by down-regulating microRNA-21 in neonatal rat atrial myocytes
ZHANG Song1, SHEN Bing-bing1, LI Fei2, ZHU Qi-ren1, WANG Zhi-rong1, ZHANG Zhuo-qi1
(1DepartmentofCardiology,TheAffiliatedHospitalofXuzhouMedicalCollege,Xuzhou221002,China;2PizhouPeople’sHospital,Pizhou221300,China.E-mail:zhuoqizhang@sina.com)
AIM: To detect the effects of resveratrol (RSV) on the expression of microRNA-21 (miR-21) in primarily cultured neonatal rat atrial myocytes with electric remodeling induced by rapid electrical stimulation (RES). Furthermore, to find out the possible mechanism of miR-21 regulating electrical remodeling. METHODS: The neonatal rat atrial myocytes were isolated by double-enzyme (trypsin and collagenase I) digestion and differential adhesion method. The atrial fibrillation (AF) model was induced by RES. Atrial myocytes were randomly divided into 4 groups: control group, RSV group, RES group, and RSV+RES group. To further detect whether RSV regulated electric remodeling by miR-21, except the 4 groups, we add miR-21 over-expression group and miR-21 inhibitor group: RES+negative control (NC) group, RES+miR-21 mimics group, RES+miR-21 mimics+RSV group, RES+miR-21 inhibitor group, and RES+miR-21 inhibitor+RSV group. The optimal concentration and pretreatment time of resveratrol were determined by CCK-8 assay. The expression of miR-21 and the mRNA expression of L-type calcium channels CACNA1C and CACNB2 in atrial myocytes were detected by qPCR. The protein expression of L-type calcium channels Cav1.2 and Cavβ2in the atrial myocytes was analyzed by Western blot. RESULTS: The expression of miR-21 in RES group was significantly increased compared with control group, while preconditioning with RSV decreased the expression of miR-21. Compared with RES+miR-21 mimics group, the expression of miR-21 in RES+miR-21 mimics+RSV group was significantly decreased. Meanwhile, the mRNA expression of CACNA1C and CACNB2, and the protein levels of Cav1.2 and Cavβ2were increased (P<0.05). Compared with RES group, the expression of miR-21 in RES+miR-21 inhibitor group and RES+miR-21 inhibitor+RSV group was decreased, while the mRNA expression of CACNA1C and CACNB2, and the protein levels of Cav1.2 and Cavβ2were increased. However, no difference of the expression of miR-21, the mRNA expression of CACNA1C and CACNB2, and the protein levels of Cav1.2 and Cavβ2among RSV+RES, RES+miR-21 inhibitor and RES+miR-21 inhibitor+RSV groups was observed (P<0.05).CONCLUSION: In AF model induced by RES, RSV may reduce electric remodeling by inhibiting the expression of miR-21 and regulating the downstream target genes.
Resveratrol; MicroRNA-21; Rapid electrical stimulation; Electric remodeling; Neonatal rat atrial myocytes
1000- 4718(2017)08- 1353- 06
2017- 03- 07
2017- 03- 17
国家自然科学基金资助项目(No. 30800219);徐州市医学青年后备人才项目
R541.7; R965
A
10.3969/j.issn.1000- 4718.2017.08.002
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 13775886473; E-mail: zhuoqizhang@sina.com
