MCM2-7复合体参与DNA复制机制及调控特点
2017-08-29王明刚毛德文张荣臻龙富立王秀峰
王明刚,毛德文,张荣臻,龙富立,王秀峰
广西中医药大学第一附属医院肝病一区,广西 南宁 530023
综述
MCM2-7复合体参与DNA复制机制及调控特点
王明刚,毛德文,张荣臻,龙富立,王秀峰
广西中医药大学第一附属医院肝病一区,广西 南宁 530023
脱氧核糖核酸(deoxyribonucheic acid, DNA)无差错复制是基因组信息成功遗传到子代的主要保障,对于生命的遗传信息传递和延续意义重大。微小染色体维持蛋白(minichromosome maintenance proteins, MCM)家族是广泛存在的高保守蛋白,MCM2-7六聚体是DNA复制所必须的。MCMs基因转录与启动子区E2F结构域密切相关,在基因表达层面可受到miRNA-34s和miRNA-1269的影响。在MCM2-7生物学功能过程中可受到Cdt1、cyclinE、p27Kip1、Rb、ATM、ATR蛋白的调控和影响。
DNA复制;微小染色体维持蛋白;调控;微小核糖核酸
脱氧核糖核酸(deoxyribonucleic acid,DNA)是由两条脱氧多核苷酸链反向平行盘绕所形成的双螺旋结构,内部由许多脱氧核苷酸按一定碱基顺序以3′,5′-磷酸二酯键相连构成[1]。DNA是生命遗传信息的主要存储形式。DNA复制是单链模板DNA复制成两个互补DNA链的生物学过程。对于细胞分裂,DNA复制是必须的,且多发生在细胞周期的S期,一个细胞周期DNA复制仅发生一次。DNA无差错复制是基因组信息成功遗传到子代的主要保障,对于生命的遗传信息传递和延续意义重大[2]。
微小染色体维持蛋白(minichromosome maintenance proteins, MCM)家族是高度保守的DNA解旋蛋白复合体,其保守性可追溯到上古时代单细胞形成[3]。在细胞内MCM2、MCM3、MCM4、MCM5、MCM6、MCM7形成六聚体才具有解螺旋酶活性,MCMs蛋白功能正常是DNA复制所必须的[4]。新近研究发现,MCMs蛋白家族除了经典的MCM2-7外,MCM1、MCM8、MCM9、MCM10相继被发现和证实[5-7]。本文总结了近年来经典MCM2-7蛋白复合体参与DNA复制机制及该蛋白复合体被调控的特点。
1 真核细胞DNA复制进程
DNA复制是真核细胞有丝分裂S期的重要生物学事件,其主要分为DNA复制起始和延伸两个部分[8]。DNA 复制源识别复合物 (origin recognition complex, ORC)首先识别并结合到复制起始位点上,作为预复制复合物组装平台(pre-replication complex, pre-RC)[9]。随后招募Cdc6、Cdt1和MCM2-7蛋白,完成pre-RC组装[10]。真核细胞有丝分裂进入S期后,CDK和DDK蛋白将依次磷酸化激活pre-RC蛋白,活化的pre-RC进一步招募Cdc45 和 GINS形成CMG复合物(Cdc45-MCM-GINS complex),CMG复合物具有解螺旋活性可激活起始复制源[11]。后续RPA、RFC、PCNA及 DNA 聚合酶依次结合到被激活的复制源上开始DNA复制进程[12]。
2 MCMs蛋白结构的特点及功能学基础
MCMs蛋白首先在酿酒酵母中被发现[13],结构研究表明MCM蛋白是AAA+ATPase家族的一个亚群,中央结构域是由约200个氨基酸组成的高保守结构区,MCMs蛋白形成六聚体是功能活性的基础[14]。此外,MCM4、MCM6和MCM7在蛋白N端镶嵌了一个锌指结构域,该结构域对MCM形成六聚体起关键作用;MCM2和MCM3中具有核定位序列;MCM2和MCM4的N端具有额外的细胞周期蛋白依赖激酶(cyclin dependent kinase, CDK)结合序列[15]。在MCM2-7六聚体中,MCM4、MCM6和MCM7形成核心,其他MCMs蛋白依次结合,依赖复合体核定位序列进入细胞核和CDK结合序列招募CDK蛋白[16]。
3 MCM2-7参与的DNA复制过程
MCM2-7(MCM2、MCM3、MCM4、MCM5、MCM6、MCM7)蛋白六聚体是较早被发现的MCM蛋白功能复合体,复合体中MCM蛋白的特殊结构是实现核-膜穿梭和磷酸化激活的基础。晶体研究显示MCM2-7复合体呈指环状,中间有足够容纳dsDNA通过的孔道[17]。DNA复制过程中dsDNA从中间孔道通过,解螺旋后的ssDNA从侧边孔道延出[18]。在真核细胞有丝分裂G期MCM2-7复合体与染色体结合,完成预复制复合物组装,进入S期后,MCM2-7复合体被激酶活化,诱发下游聚合酶-引物合成酶装配,同时和DNA聚合酶结合,形成功能性复制叉,开始DNA解螺旋与复制[19]。MCM2-7必须在G期结合到起始点上,缺少MCM2-7的结合,DNA复制将不能进行[20]。
4 MCM2-7的转录调控
MCM2-7基因的转录有明显的周期性规律,具体表现在基因转录mRNA表达的高峰在细胞周期的G1晚期[21],进入S期后mRNA表达将受到抑制[22]。在哺乳动物中,MCMs基因转录与启动子区E2F结构域密切相关,细胞转录因子E2F结合到启动子特定区域是MCMs基因转录所必须的[23]。在正常情况下E2F与Rb蛋白结合,抑制E2F与启动子结合。当细胞进入分裂周期以后,cyclin D结合CDK4与CDK6磷酸化Rb蛋白,Rb蛋白磷酸化后,E2F从复合体中解离,下游转录大量蛋白,包括MCMs蛋白家族,推进细胞周期变化[24]。在细胞周期的G1晚期,Rb蛋白被磷酸化,E2F从复合体中大量解离,结合到启动子区E2F结构域上,转录靶基因(见图1)。
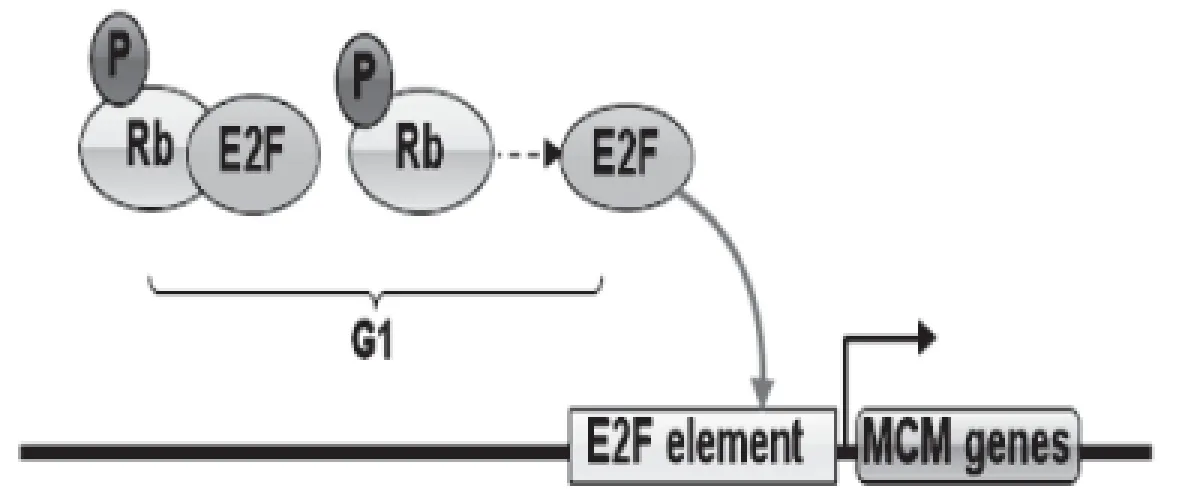
图1 E2F参与的MCM基因转录 Fig 1 E2F transcription involved in MCM gene
5 MCM2-7功能过程的调控
MCM2-7生物学功能的调控可分为间接调控和直接调控。在预复制复合物组装过程中,Geminin蛋白可与Cdt1紧密结合,阻断Cdt1与Cdc6结合及下游招募MCM2-7[25]。CDKs可磷酸化Cdc6,促进Cdc6降解,MCM2-7复合体将不能与染色体结合[26]。在哺乳动物中,cyclin E蛋白是MCM2-7复合体与染色体结合所必须的[27]。另外,MCM2-7复合体可受到一些因子的直接调控,p27Kip1可与MCM7相互作用,诱导细胞周期阻滞[28]。负性调控基因产物Rb蛋白可与MCM7相互作用,影响细胞周期[29]。细胞周期点检验蛋白ATM和ATR可介导MCM2和MCM3蛋白磷酸化,影响MCM2-7复合体的ATP酶活性和解链酶活性[30]。
在正常细胞周期下Cdc6 结合Cdt1招募MCM2-7,完成pre-RC组装。Geminin蛋白可与Cdt1紧密结合,阻断Cdt1与Cdc6结合及下游招募MCM2-7。CDKs可磷酸化Cdc6,致使Cdc6降解,阻断MCM2-7与染色体结合(见图2)。

图2 MCM2-7在结合到染色体前受到的调控Fig 2 Regulation of MCM2-7 binding to chromosomes
6 miRNA参与的MCM2-7表达调控
miRNA是21-25 nt的单链小分子RNA,是非编码RNA的重要组成部分,miRNA具有高度的保守性、时序性和组织特异性[31]。成熟的单链miRNA与蛋白质复合物miRNP结合,引导复合物通过部分互补结合靶基因mRNA 3′UTR,从而抑制靶基因翻译,或通过直接切割互补mRNA,使目标mRNA失效[32]。目前关于miRNA与MCMs表达调控及交互影响的相关研究报道尚少。已证实miRNA-1269可靶向调控MCM2基因表达,影响前列腺癌细胞增殖[33]。基因组关联分析发现p53表达依赖的miRNA-34家族与MCM基因3′UTR有互补序列[34],后在细胞模型中证实miRNA-34家族可靶向调控MCM2-7基因表达,影响DNA复制进程[35]。
MCMs蛋白家族在细胞中广泛存在,其高度的保守性预示着其功能的重要性。MCM2-7复合体是维持基因组稳定和参与DNA复制所必须的。MCMs表达水平与细胞增殖状态及再生能力呈正相关。MCMs生物学过程了MCMs基因转录、MCM2-7复合体形成、核膜穿梭、与起始位点结合、DNA解螺旋及最后与染色体解离等众多环节。目前对各环节的激活原因及调控过程了解并不十分透彻,MCMs的功能和机制研究仍有进一步深入研究的必要,探索非编码RNA与MCMs的交互调控机制将打开更广阔的研究视野。
[1]Bartlett RS, Jette ME, King SN, et al.Fundamental approaches in molecular biology for communication sciences and disorders [J]. J Speech Lang Hear Res, 2012, 55(4): 1220-1231.
[2]Jones MJ, Colnaghi L, Huang TT. Dysregulation of DNA polymerase kappa recruitment to replication forks results in genomic instability [J]. EMBO J, 2012, 31(4): 908-918.
[3]Tye BK.MCM proteins in DNA replication [J]. Annu Rev Biochem, 1999, 68: 649-686.
[4]Tuteja N, Tran NQ, Dang HQ, et al.Plant MCM proteins: role in DNA replication and beyond [J]. Plant Mol Biol, 2011, 77(6): 537-545.
[5]Park J, Long DT, Lee KY, et al.The MCM8-MCM9 complex promotes RAD51 recruitment at DNA damage sites to facilitate homologous recombination [J]. Mol Cell Biol, 2013, 33(8): 1632-1644.
[6]Chang VK, Donato JJ, Chan CS, et al. Mcm1 promotes replication initiation by binding specific elements at replication origins [J]. Mol Cell Biol, 2004, 24(14): 6514-6524.
[7]Quan Y, Xia Y, Liu L, et al. Cell-Cycle-regulated interaction between Mcm10 and double hexameric Mcm2-7 is required for helicase splitting and activation during S phase [J]. Cell Rep, 2015, 13(11):2576-2586.
[8]Warner DF, Evans JC, Mizrahi V.Nucleotide metabolism and DNA replication [J]. Microbiol Spectr, 2014, 2(5): 10.
[9]Bell SP, Stillman B.ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex [J]. Nature, 1992, 357(6374): 128-134.
[10]Diffley JF.Eukaryotic DNA replication [J]. Curr Opin Cell Biol, 1994, 6(3): 368-372.
[11]Dowell SJ, Romanowski P, Diffley JF. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo [J]. Science, 1994, 265(5176): 1243-1246.
[12]Bell SP, Dutta A. DNA replication in eukaryotic cells [J]. Annu Rev Biochem, 2002, 71: 333-374.
[13]Maine GT, Sinha P, Tye BK.Mutants of S. cerevisiae defective in the maintenance of minichromosomes [J]. Genetics, 1984, 106(3):365-385.
[14]Gomez EB, Catlett MG, Forsburg SL.Different phenotypes in vivo are associated with ATPase motif mutations in Schizosaccharomyces pombe minichromosome maintenance proteins [J]. Genetics, 2002, 160(4): 1305-1318.
[15]Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation [J]. Microbiol Mol Biol Rev, 2004, 68(1): 109-131.
[16]Remus D, Blanchette M, Rio DC, et al.CDK phosphorylation inhibits the DNA-binding and ATP-hydrolysis activities of the Drosophila origin recognition complex [J]. J Biol Chem, 2005, 280(48):39740-39751.
[17]Takahashi TS, Wigley DB, Walter JC. Pumps, paradoxes and ploughshares: mechanism of the MCM2-7 DNA helicase [J]. Trends Biochem Sci, 2005, 30(8): 437-444.
[18]Li D, Zhao R, Lilyestrom W, et al.Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen [J]. Nature, 2003, 423(6939): 512-518.
[19]Baltin J, Leist S, Odronitz F, et al.DNA replication in protein extracts from human cells requires ORC and Mcm proteins [J]. J Biol Chem, 2006, 281(18): 12428-12435.
[20]Costa A, Pape T, van Heel M, et al.Structural studies of the archaeal MCM complex in different functional states [J]. J Struct Biol, 2006, 156(1): 210-219.
[21]Kimura H, Kuriyama T.Airway occlusion and airway narrowing [J]. Ryoikibetsu Shokogun Shirizu, 1994,(3): 287-290.
[22]Kimura H, Takizawa N, Nozaki N, et al.Molecular cloning of cDNA encoding mouse Cdc21 and CDC46 homologs and characterization of the products: physical interaction between P1(MCM3) and CDC46 proteins [J]. Nucleic Acids Res, 1995, 23(12): 2097-2104.
[23]Tsuruga H, Yabuta N, Hosoya S, et al.HsMCM6: a new member of the human MCM/P1 family encodes a protein homologous to fission yeast Mis5 [J]. Genes Cells, 1997, 2(6): 381-399.
[24]Andrusiak MG, McClellan KA, Dugal-Tessier D, et al.Rb/E2F regulates expression of neogenin during neuronal migration [J]. Mol Cell Biol, 2011, 31(2): 238-247.
[25]Ballabeni A, Melixetian M, Zamponi R, et al.Human geminin promotes pre-RC formation and DNA replication by stabilizing CDT1 in mitosis [J]. EMBO J, 2004, 23(15): 3122-3132.
[26]Ogino H, Ishino S, Haugland GT, et al. Activation of the MCM helicase from the thermophilic archaeon, thermoplasma acidophilum by interactions with GINS and Cdc6-2 [J]. Extremophiles, 2014, 18(5): 915-924.
[27]Hossain M, Stillman B. Opposing roles for DNA replication initiator proteins ORC1 and CDC6 in control of Cyclin E gene transcription [J]. Elife, 2016, 5: e12785.
[28]Sharma SS, Ma L, Pledger WJ. p27Kip1 inhibits the cell cycle through non-canonical G1/S phase-specific gatekeeper mechanism [J]. Cell Cycle, 2015, 14(24): 3954-3964.
[29]Numata Y, Ishihara S, Hasegawa N, et al.Interaction of human MCM2-7 proteins with TIM, TIPIN and Rb [J]. J Biochem, 2010, 147(6): 917-927.
[30]Martin L, Rainey M, Santocanale C, et al.Hypoxic activation of ATR and the suppression of the initiation of DNA replication through cdc6 degradation [J]. Oncogene, 2012, 31(36): 4076-4084.
[31]Khan HA, Zhao Y, Wang L, et al.Identification of miRNAs during mouse postnatal ovarian development and superovulation [J]. J Ovarian Res, 2015, 8: 44.
[32]Baran-Gale J, Kurtz CL, Erdos MR, et al.Addressing bias in small RNA library preparation for sequencing: a new protocol recovers microRNAs that evade capture by current methods [J]. Front Genet, 2015, 6: 352.
[33]Majid S, Dar AA, Saini S, et al.Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer [J]. Cancer Res, 2010, 70(7): 2809-2818.
[34]Kaller M, Liffers ST, Oeljeklaus S, et al.Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis [J]. Mol Cell Proteomics, 2011, 10(8): M111.010462.
[35]Bai G, Smolka MB, Schimenti JC. Chronic DNA replication stress reduces replicative lifespan of cells by TRP53-dependent, microRNA-assisted MCM2-7 downregulation [J]. PLoS Genet, 2016, 12(1): e1005787.
(责任编辑:马 军)
MCM2-7 complex participates in the mechanism and control of DNA replication
WANG Minggang, MAO Dewen, ZHANG Rongzhen, LONG Fuli, WANG Xiufeng
Department of Hepatology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530023, China
Deoxyribonucleic acid (DNA) replication error-freely is the main security of genomic information successfully inherited to offspring. There is great significance for the life of the genetic information transfer and continuation. The minichromosome maintenance proteins (MCM) family is highly conserved protein. The MCM2-7 hexamer is essential for DNA replication. MCMs gene transcription is closely related to the promoter region E2F domain, at the gene expression level, it can be affected by miRNA-34s and miRNA-1269. In biological function MCM2-7 can be subjected by Cdt1, cyclinE, p27Kip1, Rb, ATM, ATR proteins.
DNA replication; MCMs; Regulate; MicroRNA
广西高等学校高水平创新团队及卓越学者项目;国家中医药管理局“慢性重型肝炎解毒化瘀”重点研究室;国家自然科学基金课题(81460718);广西中医药大学第一附属医院青年基金(QN14010)
王明刚,硕士,研究方向:肝衰竭肝细胞再生机制及中医药的调控研究。E-mail:wmgyx2012@163.com
毛德文,博士,研究方向:肝衰竭的中医药疗效机制研究。E-mail:mdwboshi2005@163.com
10.3969/j.issn.1006-5709.2017.07.024
Q75
A
1006-5709(2017)07-0809-03
2016-08-03
