进展期胃癌腹腔镜辅助与开腹全胃D2根治术临床疗效的比较
2017-08-07李敏哲杜燕夫谢德红张峪东
李敏哲 沈 荐 杜燕夫 谢德红 渠 浩 张峪东
(首都医科大学附属北京朝阳医院普外科,北京 100020)
·临床论著·
进展期胃癌腹腔镜辅助与开腹全胃D2根治术临床疗效的比较
李敏哲 沈 荐 杜燕夫 谢德红 渠 浩 张峪东
(首都医科大学附属北京朝阳医院普外科,北京 100020)
目的 探讨腹腔镜辅助全胃D2根治术治疗进展期胃癌的应用价值。 方法 回顾性分析我科2012年2月~2015年7月83例进展期胃癌行全胃D2根治术的临床资料,其中腹腔镜辅助胃癌根治术40例(腔镜组),开腹胃癌根治术43例(开腹组)。比较2组围术期情况、术后病理和术后生存情况。 结果 腔镜组3例(7.5%)中转开腹。腔镜组手术时间明显长于开腹组[(266.0±36.3) min vs. (226.0±28.5) min,t=5.602,P=0.000],术中出血量明显少于开腹组[(156.2±55.7) ml vs. (261.6±87.2) ml,t=-6.609,P=0.000],术后肠功能恢复时间[(3.1±1.1) d vs. (3.7±1.5) d,t=-2.070,P=0.042]和住院时间[(14.5±3.9) d vs. (16.0±2.6) d,t=-2.135,P=0.036]明显短于开腹组。2组术中输血率差异无显著性[60.0% (24/40) vs. 65.1%(28/43),χ2=0.232,P=0.630]。2组术后并发症发生率差异无显著性[35.0%(14/40) vs. 44.2%(19/43),χ2=0.730,P=0.393]。2组所有病例均为R0切除,淋巴结清扫数目[(24.9±6.0)枚 vs.(26.3±5.1)枚,t=-1.163,P=0.248]、淋巴结转移率[85.0%(34/40) vs. 86.0%(37/43),χ2=0.018,P=0.892]、阳性淋巴结数目[(5.8±3.7)枚 vs.(6.2±3.1)枚,t=-0.452,P=0.653]、肿瘤TNM分期(χ2=0.673,P=0.714)均无显著性差异。2组生存率无显著性差异(log-rank检验,χ2=0.774,P=0.379)。 结论 与传统开腹手术相比,腹腔镜辅助全胃D2根治术治疗进展期胃癌安全可行,手术创伤小、术后恢复快,且胃周淋巴结清扫效果同开腹手术一致,术后总体生存率不低于开腹手术。
胃癌; 进展期; 全胃切除术; 腹腔镜; 淋巴结清扫
根据中国癌症统计2015年报道,国人胃癌的发病率及死亡率均仅次于肺癌,位居恶性肿瘤第2位[1]。手术切除目前仍是治疗进展期胃癌的主要方式[2]。近年来,随着腹腔镜技术的发展,很多消化道恶性肿瘤已能在腹腔镜下完成根治性手术切除,并与传统开腹手术疗效相同,如结直肠癌[3]。Ⅰ期胃癌行腹腔镜远端胃切除术已被确定为常规性治疗[2],但进展期胃癌以及全胃切除是否可行腹腔镜手术尚未明确。本研究回顾性比较我科2012年2月~2015年7月腹腔镜与开腹手术治疗进展期胃癌的临床资料,探讨腹腔镜辅助全胃D2根治术的临床疗效。
1 临床资料与方法
1.1 一般资料
病例选择标准:①年龄20~70岁;②病理确诊为胃腺癌;③术前经超声胃镜或全腹部增强CT检查确定肿瘤浸润深度为T2~T4a。排除标准:①急诊手术;②术前、术中发现远处脏器转移或腹腔广泛种植转移;③合并同时性或异时性其他脏器肿瘤;④危重症患者;⑤病例资料不完整者。共83例纳入本研究。首发症状:腹痛25例,腹胀21例,消化道出血15例,腹部包块14例,无症状体检胃镜发现8例。按患者意愿选择手术方式,腹腔镜辅助胃癌根治术40例(腔镜组),开腹胃癌根治术43例(开腹组),2组患者一般资料比较无统计学差异(P>0.05),有可比性,见表1。
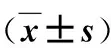
表1 2组一般资料比较
1.2 方法
术前均签署知情同意书。
腔镜组:按中华医学会外科学分会腹腔镜内镜外科学组制定的腹腔镜胃癌手术操作指南[4]进行手术操作,行D2淋巴结清扫。气管插管静脉全麻,平卧分腿位,采用五孔法置入trocar,术者位于患者左侧,助手位于患者右侧,扶镜手位于患者两腿间。首先,探查腹腔,确定肿瘤所在部位,有无肝脏、腹膜或腹盆腔转移等。确定无肿瘤转移后,将大网膜向头侧翻起置于胃前壁,沿横结肠上缘离断大网膜,进入小网膜囊,向右侧分离至结肠肝曲,于横结肠系膜前叶后方分离,切除横结肠系膜前叶。进入胃十二指肠和横结肠系膜之间的融合筋膜间隙,于根部离断胃网膜右静脉。沿胰头表面分离,显露胃十二指肠动脉,解剖胃网膜右动脉,于根部离断,清扫第6组淋巴结。进入网膜囊,显露胰尾,松解结肠脾曲,分离大网膜与脾的粘连,于根部离断胃网膜左动、静脉,清扫第4sb组淋巴结。将大网膜置于肝脏下方,将胃翻向头侧,切除胰腺被膜,紧贴胰腺上缘分离,暴露脾动脉近端,清扫第11p组淋巴结,向右侧分离显露腹腔干,分离胃左动、静脉,于根部离断,清扫第7、9组淋巴结。将胰腺向左下方牵拉,显露肝总动脉,沿其前方及上缘分离,清扫第8a组淋巴结。沿胃十二指肠动脉及肝总动脉分离显露胃右动脉及肝固有动脉,打开肝十二指肠韧带被膜,继续脉络化肝固有动脉前方及外侧,清扫第12a组淋巴结,于胃右动、静脉根部离断血管。沿胃壁小弯向贲门分离,清扫第3、1组淋巴结。裸化脾动脉,显露出脾门各分支血管,清扫第10、11d组淋巴结;于根部切断胃短血管,清扫第4sa组淋巴结,继续沿胃大弯向头侧游离至贲门,清扫第2组淋巴结,游离食管下段3 cm。腔内切割闭合器于幽门远端3 cm切断十二指肠,上腹部正中辅助小切口,取出标本,随意选择OrVil经口置入法[5]或反穿刺置入法[6]放置吻合器钉砧头,采用Roux-en-Y重建消化道,常规放置空肠营养管。
开腹组:取上腹部正中切口约20 cm,游离胃、清扫淋巴结、重建消化道方式均同腔镜组。
1.3 观察指标
围手术期情况:腔镜组手术中转开腹率、手术时间(以麻醉记录单为准)、术中出血量(以麻醉记录单为准)、术中输血率、术后肠功能恢复时间、术后住院时间(出院标准为能经口进软食、排便正常、拔除引流管、手术切口拆线,无发热、腹痛、腹胀);术后并发症情况;术后病理情况:R0切除率、淋巴结清扫数、淋巴结转移率、阳性淋巴结数、肿瘤TNM分期;术后随访情况:2组病例术后随访至2017年2月,了解患者生存情况。
1.4 统计学处理
2 结果
2组围术期情况比较:腔镜组3例(7.5%)中转开腹,1例为术中大出血,2例为腹腔干周围淋巴结清扫困难;腔镜组手术时间明显长于开腹组(P<0.05),术中出血量明显少于开腹组(P<0.05),术后肠功能恢复时间及住院时间明显短于开腹组(P<0.05);2组术中输血率差异无显著性(P>0.05),见表2。
2组术后并发症发生情况差异无显著性(P>0.05),见表3。所有并发症经对症支持治疗后好转,无再次开腹探查。
2组所有病例均为R0切除,2组淋巴结清扫数目、淋巴结转移率、阳性淋巴结数目、肿瘤TNM分期均无显著性差异(P>0.05),见表3。腔镜组术后随访6~56个月,中位随访时间30.5月,开腹组术后随访5~60个月,中位随访时间34个月,腔镜组术后死亡15例,开腹组术后死亡13例,2组生存曲线无显著性差异(χ2=0.774,P=0.379),见图1。
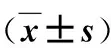
表2 2组围术期情况比较
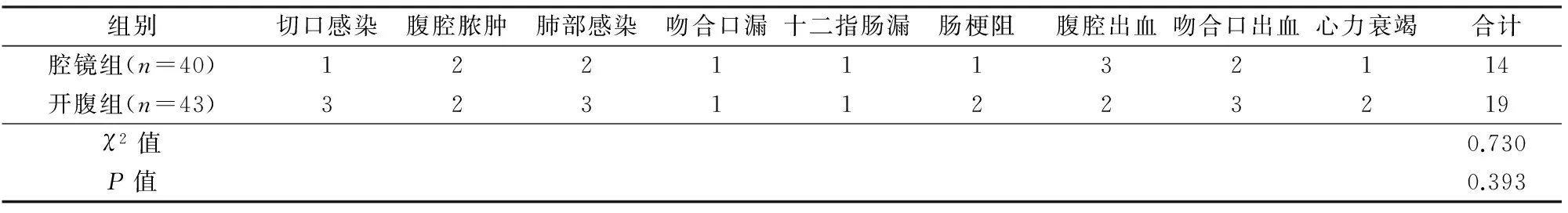
表3 2组术后并发症情况比较
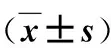
表4 2组术后病理情况比较
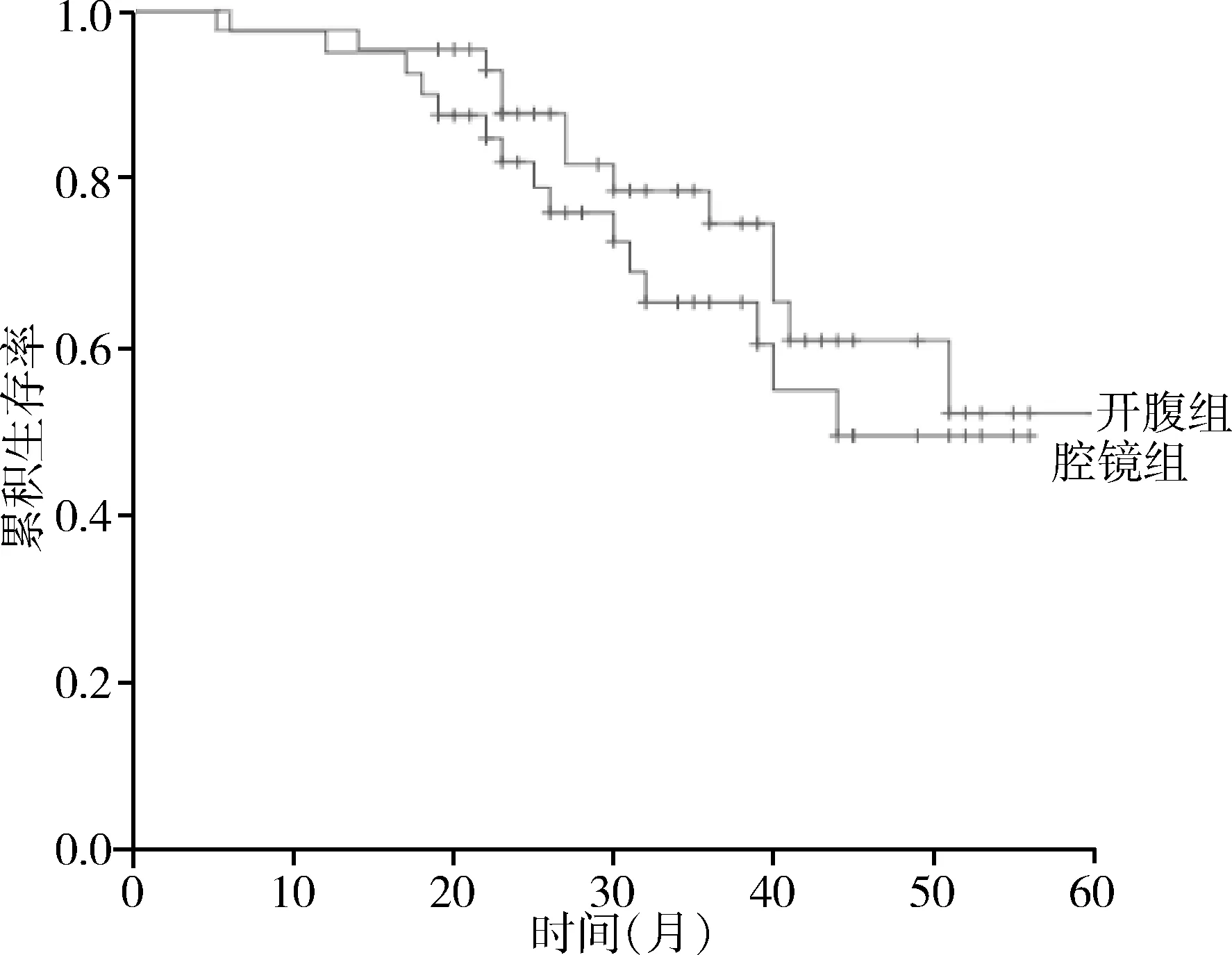
图1 2组生存曲线
3 讨论
最早关于腹腔镜胃手术的报道始于1994年[7],近年来,随着仪器设备和手术技术的不断发展,腹腔镜手术已逐渐应用于胃癌的临床实践[8]。
对于早期胃癌,很多研究表明腹腔镜手术短期疗效优于传统开腹手术,长期疗效与之相当[9~12]。但是这些研究多是关于远端胃大部切除的随机临床试验,通常腹腔镜远端胃大部切除出血更少,术后住院时间更短、并发症更低,且术后死亡率不高于开腹手术[12,13]。在现有的报道中,行腹腔镜全胃切除的病例普遍较低,这主要是由于技术上的原因,腹腔镜下全胃切除的操作难度远高于远端胃大部切除[14]。Hara等[15]研究显示对于早期胃癌,腹腔镜全胃切除手术时间明显长于开腹手术,但术中出血更少,2种术式术后并发症发生率及总住院费用没有差异。
对于进展期胃癌,腹腔镜下胃周淋巴结的清扫是手术的难点[16]。日本胃癌治疗指南[2]指出,远端胃大部切除D2淋巴结清扫范围为第1、3、4sb、4d、5、6、7、8a、9、11p、12a组淋巴结,全胃切除D2淋巴结清扫范围为第1~7、8a、9、10、11p、11d、12a组淋巴结。多项荟萃分析证实腹腔镜手术清扫的胃周淋巴结数目远少于开腹手术[13,17,18]。有研究[19,20]显示腹腔镜全胃D2根治术的短期疗效与开腹手术一致,但这些研究结果都是在特定的病例下得出的,比如限定了肿瘤浸润的深度、肿瘤大小、淋巴结转移的情况。到目前为止,还没有大规模的多中心随机对照临床试验来评估进展期胃癌腹腔镜全胃D2根治术的疗效。
本研究腔镜组术中出血量明显少于开腹组(t=-6.609,P=0.000),这得益于腹腔镜的放大效应,使手术视野更加清晰,沿正确解剖层面分离,可以避免血管的损伤。进展期胃癌术前新辅助化疗可以有效降低肿瘤分级分期,提高根治性手术切除率[21,22],目前,已是进展期胃癌综合性治疗的发展趋势,已在很多医疗中心开展。我们的经验是,新辅助化疗后,大多数病例肿瘤会显著缩小,有利于手术切除,但大量肿瘤细胞死亡后,局部纤维组织增生,手术创面出血倾向增高,故腹腔镜手术分离组织时要做到操作轻柔,止血彻底。本研究腔镜组1例新辅助化疗后术中出血较多,难以控制而中转开腹。腹腔镜手术中转开腹的原因还多见于胃周淋巴结融合成团,腹腔镜下清扫困难,腔镜组2例腹腔干和肝总动脉处遇到融合包绕血管的淋巴结团,清扫困难而中转开腹;2组清扫的淋巴结数目无明显差异(t=-1.163,P=0.248)。腔镜组手术时间虽明显长于开腹组(t=5.602,P=0.000),但手术创伤更小,故术后肠道功能恢复更快(t=-2.070,P=0.042),术后住院时间更短(t=-2.135,P=0.036)。2组术后并发症无明显差异(χ2=0.730,P=0.393),但高于其它文献报道[23],可能与我们选择的病例分期更晚,且均为全胃D2根治性切除有关。
综上所述,进展期胃癌行腹腔镜辅助全胃D2根治术,手术创伤小、术后恢复快,且胃周淋巴结清扫效果与开腹手术一致,短期随访两者生存率无明显差异,远期生存率尚待进一步随访观察。
1 Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016,66(2):115-132.
2 Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer, 2017,20(1):1-19.
3 Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med, 2015,372(14):1324-1332.
4 中华医学会外科学分会腹腔镜与内镜外科学组.腹腔镜胃癌手术操作指南(2007版).中华消化外科杂志,2007,6(6):476-480.
5 Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc,2009,23(11):2624-2630.
6 夏亚斌,窦 千,黄晓旭.反穿刺技术在腹腔镜胃癌根治术中的应用.中国微创外科杂志,2016,16(4):304-307.
7 Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted BillrothⅠ gastrectomy. Surg Laparosc Endosc, 1994,4(2):146-148.
8 Lee HJ, Yang HK. Laparoscopic gastrectomy for gastric cancer. Dig Surg,2013,30(2):132-141.
9 Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery, 2002,131 (1 Suppl):S306-S311.
10 Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc, 2005,19(9):1172-1176.
11 Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc,2009,23(8):1759-1763.
12 Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report-a phase Ⅲ multicenter, prospective, randomized trial (KLASS Trial). Ann Surg, 2010,251(3):417-420.
14 Lee SE, Ryu KW, Nam BH, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol, 2009,100(5):392-395.
15 Hara T, Fujiwara Y, Sugimura K, et al. Comparison of early clinical outcomes between laparoscopic total gastrectomy and open total gastrectomy for early-stage gastric cancer. Gan To Kagaku Ryoho, 2014,41(12):1476-1478.
16 Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the first-line advanced gastric cancer study. Eur J Cancer, 2013,49(17):3616-3624.
17 Kodera Y, Fujiwara M, Ohashi N, et al. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg,2010,211(5):677-686.
18 Zorcolo L, Rosman AS, Pisano M, et al. A meta-analysis of prospective randomized trials comparing minimally invasive and open distal gastrectomy for cancer. J Surg Oncol, 2011,104(5):544-551.
19 Li P, Huang CM, Zheng CH, et al. Laparoscopic spleen-preserving splenic hilar lymphadenectomy in 108 consecutive patients with upper gastric cancer. World J Gastroenterol,2014,20(32):11376-11383.
20 Huang CM, Chen QY, Lin JX, et al. Laparoscopic spleen-preserving splenic hilar lymphadenectomy performed by following the perigastric fascias and the intrafascial space for advanced upper-third gastric cancer. PLoS One, 2014,9(32):e90345.
21 Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastro esophageal cancer. N Engl J Med, 2006,55(1):11-20.
22 Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase Ⅲ trial. J Clin Oncol, 2011,29(13):1715-1721.
23 胡伟国.腹腔镜胃癌手术并发症及其对策.中华胃肠外科杂志, 2012,15(4):325-327.
(修回日期:2017-04-16)
(责任编辑:李贺琼)
Comparison of Laparoscopy-assisted Versus Open Radical Total Gastrectomy with D2 Lymph Node Dissection for Advanced Gastric Cancer
Li Minzhe, Shen Jian, Du Yanfu, et al.
Department of General Surgery, Beijing Chaoyang Hospital Affiliated to Capital Medical University, Beijing 100020, China
Shen Jian, E-mail: 410078@163.com
Objective To investigate the clinical value of laparoscopy-assisted radical total gastrectomy with D2 lymph node dissection for advanced gastric cancer. Methods Clinical data of 83 cases of radical total gastrectomy with D2 lymph node dissection for advanced gastric cancer from February 2012 to July 2015 in our hospital were analyzed retrospectively. Forty cases receiving laparoscopy-assisted radical total gastrectomy were defined as laparoscopy group, while 43 cases receiving open radical total gastrectomy were defined as open group. The condition during peri-operation period, postoperative pathology and survival were compared between the two groups. Results In the laparoscopy group, there were 3 patients (7.5%) converted to open surgery. The laparoscopy group had significant better results than the control group in the operation time [(266.0±36.3) min vs. (226.0±28.5) min,t=5.602,P=0.000], the bleeding during the operation [(156.2±55.7) ml vs. (261.6±87.2) ml,t=-6.609,P=0.000], postoperative exhaust time[(3.1±1.1) d vs. (3.7±1.5) d,t=-2.070,P=0.042], and postoperative hospital stay [(14.5±3.9) d vs. (16.0±2.6) d,t=-2.135,P=0.036]. There were no significant differences in blood transfusion during the operation (χ2=0.232,P=0.630) and the postoperative complications between the two groups (χ2=0.730,P=0.393). Analysis of postoperative pathology found all cases of R0 resection in both groups. There were no significant differences in the number of retrieved lymph nodes [(24.9±6.0) vs. (26.3±5.1),t=-1.163,P=0.248], lymph node metastasis rate (χ2=0.018,P=0.892), the number of positive lymph nodes [(5.8±3.7) vs. (6.2±3.1),t=-0.452,P=0.653], and TNM stages (χ2=0.673,P=0.714) between the two groups. The two groups had no significant difference in cumulative survival rate (χ2=0.774,P=0.379).Conclusion Laparoscopy-assisted radical total gastrectomy with D2 lymph node dissection for advanced gastric cancer is a safe and feasible procedure with minimal invasion and quicker recovery, and it is comparable with open total gastrectomy in lymph node dissection and overall survival.
Gastric cancer; Advanced stage; Total gastrectomy; Laparoscopy; Lymph node dissection
,E-mail:410078@163.com
A
1009-6604(2017)07-0589-05
10.3969/j.issn.1009-6604.2017.07.004
2017-03-10)
