精浆和精子对母兔子宫免疫及孕向发育的影响
2017-05-02张安国刘瑞风刘新峰秦清明王平玉郝志明
张安国, 刘瑞风, 刘新峰, 秦清明, 王平玉, 郝志明
实验研究
精浆和精子对母兔子宫免疫及孕向发育的影响
张安国, 刘瑞风, 刘新峰, 秦清明, 王平玉, 郝志明
(天津农学院 动物科学系,天津300384)
在结扎子宫颈的情况下,分别在兔同一子宫的两侧子宫角剖腹注入生理盐水(对照)、精浆或精子以研究精浆和精子对子宫免疫和孕向发育的影响。试验共用母兔18只,每组6只。在注入后72 h时处死试验用兔,取子宫进行一般测量,并切片染色,用图象采集分析系统进行研究。结果表明:1) 注入子宫的精浆和精子,都能明显降低黏膜中的肥大细胞数量和增加子宫角中冲洗液内的细胞数(P0.01)。精浆与精子相比,精浆降低黏膜中肥大细胞数量的作用更大(P0.05);而精子提高子宫角冲洗液内细胞数的作用更强(P0.01)。2)无论精浆还是精子,都能显著(P0.05)或极显著(P0.01)促进子宫(子宫角指数、子宫角长度和子宫角直径)、子宫壁(子宫壁厚度、黏膜层厚度、黏膜上皮层厚度和肌层厚度)、黏膜腺体(腺体数和腺上皮的厚度)以及黏膜血管的孕向发育。精浆与精子相比,就子宫、子宫壁及黏膜血管的孕向发育来说,精浆的作用更明显(P0.05或P0.01);而对子宫黏膜腺体孕向发育的作用,精浆和精子间没有明显差异(P>0.05)。
免疫;孕向发育;精浆;精子;子宫;兔
近年来的研究表明,精液与子宫内膜的接触能够促进胚胎的生长,能够提高母畜的受胎率和产仔数。Carp H J 等研究发现,不与结扎输精管的公鼠交配,胚胎移植受体鼠的胚泡着床率会下降,胚胎的发育生长会减缓[1]。在家畜,胚胎移植受体母畜若未与输精管结扎的公畜交配,移植的胚胎虽能正常附植,但胎儿生长和胎盘发育缓慢[2]。另外,Mah J 等在进行猪的配种时发现,自然本交的母猪,若再用输精管结扎的公猪交配来补充精浆能够提高母猪的受胎率和产仔数[3]。2012年,刘瑞凤等在兔子上进行对比试验证明,注入精液的兔子宫,其长度、直径、子宫指数明显增加,子宫壁、黏膜层、黏膜上皮层以及腺上皮的厚度显著增加,黏膜中的腺体和血管明显增多;子宫冲洗液中的细胞数和中性粒细胞数显著增加,黏膜中的肥大细胞数明显减少[5]。在上述研究的基础上,本试验就精子和精浆对兔子宫免疫及孕向发育的影响进行探究,以期揭示接触精子和精浆的子宫所发生的形态学和免疫学特性的变化及其差异,为提高人工授精和胚胎移植的成功率奠定理论基础。
1 材料和方法
1.1 试验动物
健康的新西兰白兔20只,空怀,体重2.0~2.5 kg,其中母兔18只,公兔2只。试验动物购买后,饲养观察两周后进行试验。
1.2 试验方法
试验方法同我们先前的方法[5]。
2 结果与分析
2.1 对子宫免疫的影响
如表1所示,子宫角分别注入精浆、精子或生理盐水后72 h,每平方毫米子宫黏膜内的肥大细胞平均数精浆组和精子组都极显著少于对照组(P0.01);精浆组和精子组比较,精浆组也明显少于精子组(P0.05)。而子宫冲洗液中的细胞数精浆组和精子组极显著高于对照组(P0.01),注射后72 h时,精浆组和精子组分别为32.62±1.18×109/L 和53.40±1.43×109/L,而对照组则只有8.87 ± 0.36×109/L;精浆组与精子组比较,精子组也明显高于精浆组(P0.01)。
2.2 对子宫孕向发育的影响
2.2.1 对子宫大小和子宫角指数的影响
上述的数据帧构成,附带车辆身份认证信息、帧头和帧尾、校验位等,每帧总共设定为300bit。在实际应用中,与每辆车发送帧相关的汽车,通常为附近的接收车辆,设定附近车辆为30辆,根据该车辆密度控制发射功率,以IEEE802.11p为基础,假设每车发送10帧/秒,可以通过统计和计算,计算出数据帧所使用的总数据量。每秒为:
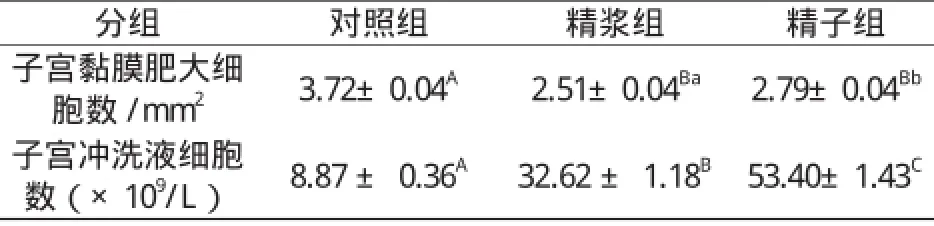
表1 子宫注入精浆和精子对子宫黏膜肥大细胞数及子宫冲洗液细胞数的影响Table 1 The effect of seminal plasma and sperm on the number of the mast cells in uterine mucosa and the cells in uterine flushing fluid
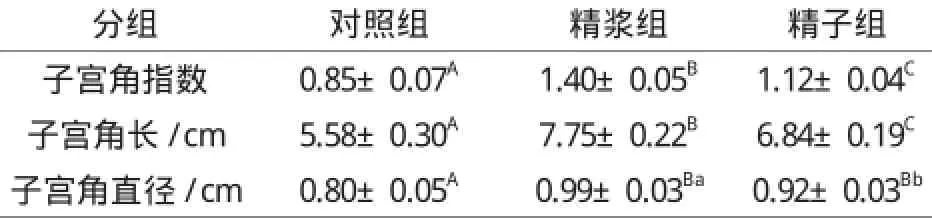
表2 子宫注入精浆和精子对子宫角指数、长度及直径的影响Table 2 The effect of seminal plasma and sperm on uterine horn index, length and diameter
由表2可看出,子宫注入72 h后,精浆组和精子组子宫角指数、子宫角长以及子宫角直径均极显著大于对照组(P0.01);精浆组与精子组比较,无论是子宫角指数还是子宫角长和直径,精浆组都显著大于精子组(P0.05或P0.01),表明精浆对子宫角指数、子宫角长和子宫角直径的孕向发育的影响更大。
2.2.2 对子宫壁、黏膜层、黏膜上皮层和肌层厚度的影响
与子宫角长和子宫角直径的增长相类似,子宫角注入精浆和精子72 h后,子宫壁、黏膜层、黏膜上皮层和肌层的厚度都较对照组有极显著的增加(P0.01);与注入精子相比,子宫注入精浆后,子宫壁和肌层的增生显著增加(P0.05),黏膜层和黏膜上皮层的增生极显著增加(P0.01)。详见表3。
2.2.3 对黏膜腺体和血管的影响
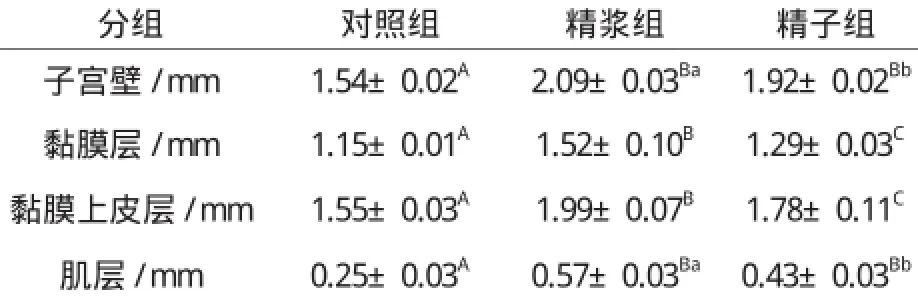
表3 子宫注入精浆和精子对子宫壁、黏膜层、黏膜上皮层和肌层厚度的影响Table 3 The effect of seminal plasma and sperm on thickness of uterus wall, mucosa, mucosal epithelium and muscle layer
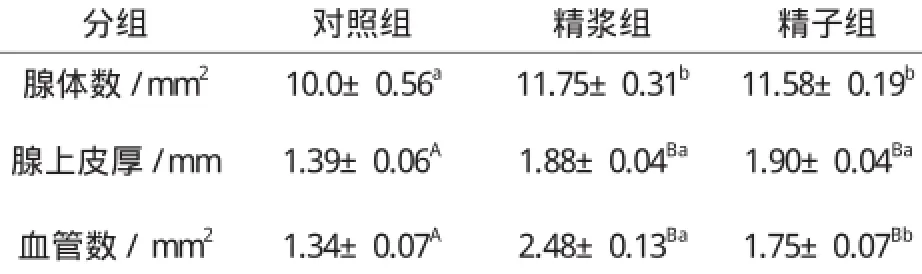
表4 子宫注入精浆和精子对子宫黏膜腺体数、腺上皮厚及血管数的影响Table 4 The effect of seminal plasma and sperm on number of gland, thickness of glandular epithelium and the number of blood vessel
就精浆和精子对子宫腺体和血管的影响而言,从表4看,精浆组和精子组子宫黏膜中的腺体数、腺上皮厚以及血管数均明显多于或厚于对照组(P0.05或P0.01);与注入精子比较,子宫注入精浆后,每平方毫米子宫黏膜中的腺体数和血管数以及腺上皮的厚度都有所增加,但只有血管数才具有统计学意义上的差异。每平方毫米子宫黏膜中的血管数精浆组为2.48±0.13,精子组则为1.75±0.07。
3 讨论
3.1 对子宫免疫的影响
有研究表明,在整个妊娠期小鼠和田鼠子宫内肥大细胞的数量都呈先减少后增加的变化,据认为这种变化与胚泡的附植有关[4]。胚泡植入子宫壁时肥大细胞数量减少,可使子宫局部免疫水平暂时低下,从而使具有父方抗原特征的胚泡不被排斥,可能是胚泡不被母体排斥的免疫学机制之一。我们先前的研究发现兔的精液可明显降低母兔子宫壁肥大细胞数量,生理盐水组子宫壁肥大细胞数量为3.72±0.04个,而精液组只有2.85±0.05个[5]。从本研究表1看,精浆和精子比较,显然精浆的这种降低肥大细胞数量的作用更大。
3.2 精液对子宫孕向发育的影响
精液注入兔子宫腔72 h后,可以显著增加子宫的大小;可明显增加子宫壁、黏膜层和黏膜上皮层的厚度;可明显增加黏膜中腺管和血管的数量,可显著增加腺上皮的厚度[4]。精浆和精子在其中伴有的作用,从本研究表2、表3和表4来看,在子宫角指数、子宫角长度、子宫壁厚度、黏膜层厚度、黏膜上皮层厚度、肌层厚度以及单位面积黏膜内的血管数方面,注入子宫的精浆对子宫孕向发育的作用明显优于精子,表明精浆才是精液中促进子宫壁及其黏膜血管孕向发育的主要成分。而对于子宫黏膜腺体数量和腺上皮层的厚度,精浆和精子的作用差异不明显,表明精液对子宫黏膜腺体孕向发育的作用是精子和精浆共同作用的结果。
[ 1 ]Carp H J, Serr D M, Mashiach S, et al. Influence of insemination on the implantation of transferred rat blastocysts[J]. Gynecol Obstet Invest,1984,18:194-198.
[ 2 ]Bromfield J J, Roberts C T, Robertson S A.Seminal plasma programs uterine receptivity and pregnancy outcome[J].Biol Reprod,2004,17:94.
[ 3 ]Mah J, Tilton J E, Williams G L et al.The Effect of repeated mating at short intervals on reproductive performance of gilts[J]. Anim Sci,1985,60:1052-1054.
[ 4 ]Deng-ZP.Quantitative studies on mast cells and histamine in the endometrium of cow and sheep[J].China Vet Sci,1994,1:50.
[ 5 ]刘瑞凤, 刘新峰, 郝志明.精液对母兔子宫免疫及孕向发育的影响[J].黑龙江动物繁殖,2012,2:1-4.
[ 6 ]Lovell J W,Getty R. Fate of semen in the uterus of the sow: histologic study of endometrium during the 27 hours after natural service. American Journal of Veterinary Research,1968,29:609-625.
[ 7 ]Rozeboom K J, Tredsson M H, Crabo B G. Characterization of uterine leukocyte infiltra -tion in gilts after artificial insemination[J]. Reprod Fertil,1998,114:195-199.
[ 8 ]Alghamdi A S, Foster D N, Troedsson M H T.Equine seminal plasma reduces sperm binding to polymorphonuclear neutrophils (PMNS) and improves the fertility of fresh semen inseminated into inflamed uteri[J]. Reprodution,2004,127:593-600.
[ 9 ]Rozeboom, K J, Tredsson M H, Hodson H H, et al. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine[J]. Anim Sci,2000,78:443-448.
The Effect of Seminal Plasma and Sperm on Immunity and Development of Uterus to Pregnancy in Female Rabbits
ZHANG An-guo,LIU Rui-feng,LIU Xin-feng,QIN Qing-ming,WANG Ping-yu,HAO Zhi-ming
(Department of Animal Science, Tianjin Agricultural College, Tianjin,300384,China)
Under the ligation of the cervix, both side of the same uterus in rabbit were injected with saline, seminal plasma and sperm. The effect of seminal plasma and sperm on the immunity and development of uterus to pregnancy were studied. 18 rabbits were used in the experiment, 6 in each group. The rabbits were killed 72h after injection. Uterus from the rabbits were used to section staining, and the organization pictures were analysised by image gathing system. The results are as follows: 1. The seminal plasma and sperm injected into uterus can reduce the number of mast cell (MC) in mucous membrane and increase the number of the cells in uterine flushing fluid (P0.01). The number of MC in seminal plasma group was also significantly less than sperm group (P0.05), but the number of cells in uterine flushing fluid in sperm group was significantly more than in seminal plasma group (P0.01). 2. Whether seminal plasma or sperm, can significantly promote the development of uterine (index of uterine horn, uterine horn length and diameter), its wall (uterine wall thickness, mucosal thickness, mucosal epithelium thickness, muscle thickness), mucous glands ( the number of gland, glandular epithelium thickness) and blood vessels (the number of blood vessel in unit area) to pregrancy(P0.05 or P0.01). Comparison between seminal plasma and sperm, the effect of seminal plasma on the development of uterine, uterine wall, mucous blood vessels was greater(P0.05 or P0.01), and there is no significant difference between the effect of seminal plasma and sperm on development of the mucoue glands (P>0.05).
Immunity; Development of Uterus to Pregnancy; Seminal Plasma;Sperm; Uterus; Rabbits
S829.1;S814.3
A
1005-2739(2017)02-0003-04
2016-12-09
张安国(1960-),男,陕西三原人,副教授,主要从事动物免疫学研究。
郝志明(1963-),男,陕西绥德人,硕士,教授,主要从事动物生殖技术研究。
Email:haozhimingg@163.com
