海洋中Fe(Ⅱ)的行为及微生物参与下的过程研究概述
2017-02-09曲胜路杨茹君耿倩倩
曲胜路, 杨茹君, 苏 函, 刘 媛, 耿倩倩
海洋中Fe(Ⅱ)的行为及微生物参与下的过程研究概述
曲胜路1, 2, 杨茹君1, 苏 函1, 刘 媛1, 耿倩倩1
(1. 中国海洋大学 化学化工学院, 山东 青岛 266100; 2. 青岛海洋科学与技术国家实验室 海洋地质过程与环境功能实验室, 山东 青岛 266071)
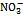
Fe(Ⅱ); 微生物活动; N移除
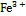
1 海水中Fe(Ⅱ)的分布

海水中Fe(Ⅱ)的分布及含量受输入、迁移转化、迁出等过程的影响。从整体上看, Fe(Ⅱ)在垂直分布上主要集中在表层、OMZ区和近底层水中, 在水平分布上则呈现出近岸高, 远岸低的特点。
2 海洋中Fe(Ⅱ)的化学行为
海洋中Fe(Ⅱ)的循环过程包括Fe(Ⅱ)的输入和输出, 其中Fe(Ⅱ)的输入分为外源输入和海洋再生输入, 外源输入方式包括大气沉降[23]、沉积物释放[24]、地下水输入[25]、冰川水释放[26]等; 海洋再生方式包括光催化还原[7]、生物细胞裂解释放[27]、微生物还原[28]等。海水中Fe(Ⅱ)的输出则主要通过生物摄取[29]、化学氧化[30]、微生物氧化[31]、颗粒物吸附[32]等方式从海洋中移除。海水中Fe(Ⅱ)的化学反应主要包括: 光催化Fe(Ⅲ)还原产生Fe(Ⅱ)、有机质与Fe(Ⅱ)的配合, Fe(Ⅱ)的化学氧化移除等。
2.1 光还原过程产生Fe(Ⅱ)
在海洋透光层, 光催化还原是海水中Fe(Ⅱ)的主要来源。有机质吸收光能, 释放电子, 并将电子转移给水合Fe(Ⅲ)氧化物, Fe(Ⅲ)还原为Fe(Ⅱ), 氧化物表面Fe(Ⅱ)-O的不稳定, 发生解离将Fe(Ⅱ)释放到水中(式1)。通常Fe(Ⅲ)氧化物的不定形程度越高, 表面有机络合程度越大, 还原反应效率就越高[9, 33-34]。
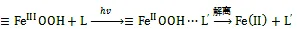
L(ligand)为与Fe(Ⅱ)络合的有机配体, L′为有机配体发生光催化反应后的氧化产物。
海水中大部分的溶解有机态Fe(Ⅲ)需要被还原为Fe(Ⅱ)才能被生物吸收利用, 有机态DFe(Ⅲ)的光还原机制为光诱导的有机配体向Fe(Ⅲ)的直接电子转移的反应过程(ligand-to-metal charge transfer, LMCT)(式2)[35-36]。
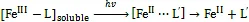
该还原反应速率受配体种类、光照条件等因素影响较大[35, 37]。Kuma等[38]测得日本春季藻华期间的Funka湾表层水Fe(Ⅱ)含量为20~40 nmol/L, 光照模拟实验结果表明, 在羟基羧酸存在下, Fe(Ⅲ)能够被还原为Fe(Ⅱ), 而在腐殖酸存在下, Fe(Ⅲ)没有被光还原; Rijkenberg等[39]的研究表明, 脱镁叶绿素、铁色素、肌醇六磷酸等配体能够有效增强Fe(Ⅲ)的光还原效率, 使Fe(Ⅱ)的浓度增加; 而去铁敏B存在下却抑制Fe(Ⅲ)的还原, 另外原卟啉IX能够与Fe(Ⅱ)发生强络合, 降低光产物Fe(Ⅱ)的浓度。此外研究发现, 波长在275~520 nm的光才能催化Fe(Ⅲ)的还原反应发生, 短波长的光催化效率较高[6, 40]。
2.2 有机质与Fe(Ⅱ)的配合
Fe(Ⅱ)能够与海水中的有机质配合, 较稳定地存在于海水中。Millero等[30]测定了有机质含量不同的Biscayne Bay和Gulf Stream 样品中Fe(Ⅱ)的氧化速率, 结果表明有机质含量较高的Biscayne Bay水中Fe(Ⅱ)的氧化半衰期为2.4~6.2 min, 是低含量有机质Gulf Stream水的2~5倍, 有机质成分分析表明, 分子质量≤500 g/mol的有机质能有效阻止Fe(Ⅱ)的氧化; 而Roy等[41]对亚北极太平洋西部表层水的研究再次验证了有机质与Fe(Ⅱ)的络合作用能够延长Fe(Ⅱ)的稳定时间。
湿沉降是海水中Fe(Ⅱ)配体的重要来源。Kieber等[23]研究发现雨水来源的Fe(Ⅱ)的氧化速率远低于其他来源。这主要是由于雨水中的有机质与Fe(Ⅱ)发生络合, 抑制了Fe(Ⅱ)的氧化, 而紫外光消解可以破坏配合效应[42]。Willey等[43]比较了雨水、河水等来源的有机配体与Fe(Ⅱ)的络合能力, 发现雨水中疏水可萃取溶解有机质能够与Fe(Ⅱ)发生络合, 使氧化半衰期由理论值的几分钟延长至4 h, 其络合能力与菲咯嗪(ferrozine, FZ)近似, 而河水来源的有机配体则对Fe(Ⅱ)的络合作用不明显。
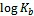
2.3 海水中Fe(Ⅱ)的化学氧化
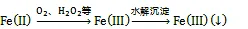
海水中Fe(Ⅱ)的氧化剂除了O2外, 还包括一些氧化中间体(如H2O2、O2–、OH·)。这些中间体主要由Fe(Ⅱ)的化学氧化、光催化反应产生。King等[47]的研究表明, O2和H2O2对水体中Fe(Ⅱ)的氧化存在竞争关系, 当水体中H2O2的浓度高于一定值时(10–7mol/L),则可作为水体中Fe(Ⅱ)的主要氧化剂[48]。
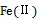
目前, 国际上主要通过流动注射化学发光法(flow injection- chemiluminescence, FI-CL)现场测定Fe(Ⅱ), 并在采样和分析过程中采取尽量缩短测定时间、保持海水温度、氛围二级分样、加入Fe(Ⅱ)固定剂等措施来降低Fe(Ⅱ)的氧化损失[8, 50-52]。由于发光信号与Fe(Ⅱ)浓度之间呈一元二次函数关系, 即y=kx2+bx+c, 因此在计算的过程中, 一般采用作标准曲线的方法求海水中Fe(Ⅱ)浓度。首先采样后立即测定未知海水发光信号y′, 然后将同时采集的海水静置10 h后作为空白海水, 并在空白海水中添加Fe(Ⅱ)标准, 测定发光信号y与Fe(Ⅱ)浓度之间的函数式, 将y′代入方程计算得x′值即为未知海水中Fe(Ⅱ)的浓度[50](图1)。之前Moffett等[51]采用不同的作图方法, 在采样后立即加Fe(Ⅱ)作标准加入曲线, 得到一元二次函数方程, 但该曲线在x轴上的截距大小是海水中Fe(Ⅱ)与试剂中干扰物质浓度之和, 因此需减去试剂中干扰物质的浓度, 才是海水中实际的Fe(Ⅱ)浓度。
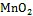
3 生物过程对Fe(Ⅱ)的生成及迁移转化的影响
海洋浮游植物在生长和消亡的过程中吸收、释放Fe(Ⅱ)造成其在海洋生物圈中的循环, 海洋中Fe(Ⅱ)参与的生物过程主要包括浮游生物对Fe(Ⅱ)的摄取、微生物还原产生Fe(Ⅱ)以及Fe(Ⅱ)的生物氧化等过程。与Fe(Ⅲ)的吸收相比, Fe(Ⅱ)可以直接穿透生物细胞膜或被细胞膜上配体蛋白夺取至细胞内[55-56], 不需要进行与有机质的络合或还原过程[57-58], 是海洋生物所需Fe的重要来源。在Fe(Ⅱ)含量较低的海域, 一些藻类、酵母、细菌也能够将Fe(Ⅲ)化合物还原为Fe(Ⅱ)后再进行吸收。
3.1 异化Fe(Ⅲ)还原过程
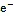
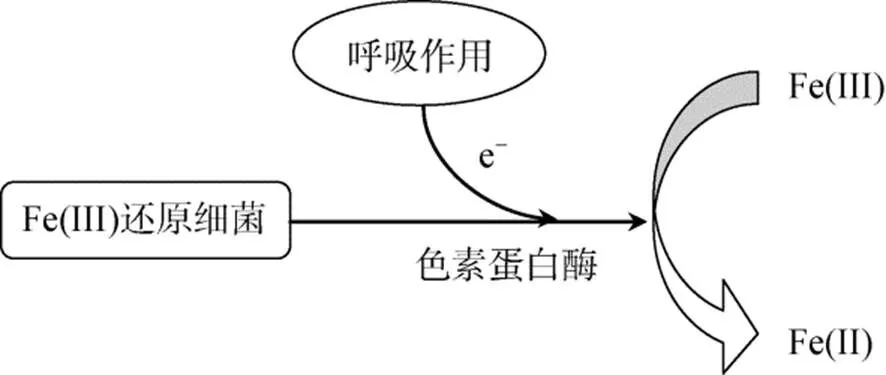
图2 细菌异化还原Fe(Ⅲ)机制
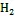
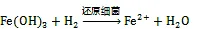
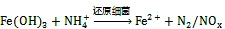

目前研究最多的是sp.和sp.等还原菌, 它们主要通过一系列电子转移蛋白的协同作用, 将细胞质内电子逐步由细胞内膜、细胞质向细胞外膜转移。进入细胞质的溶解态Fe(Ⅲ)被还原酶所携带的电子被还原, 不溶态Fe(Ⅲ)氧化物则在胞外得到细胞外膜上的电子发生还原[65]。White等[66]还对细胞外膜中转移蛋白结构及其对电子转移速率的影响进行了研究, 结果表明细菌细胞膜上存在三种色素蛋白组合体。由于组合体的存在, 电子向胞外转移的速率增加至单个转移蛋白的103倍。这种高效的电子转移机制为环境中Fe(Ⅲ)的快速还原提供了重要保证。
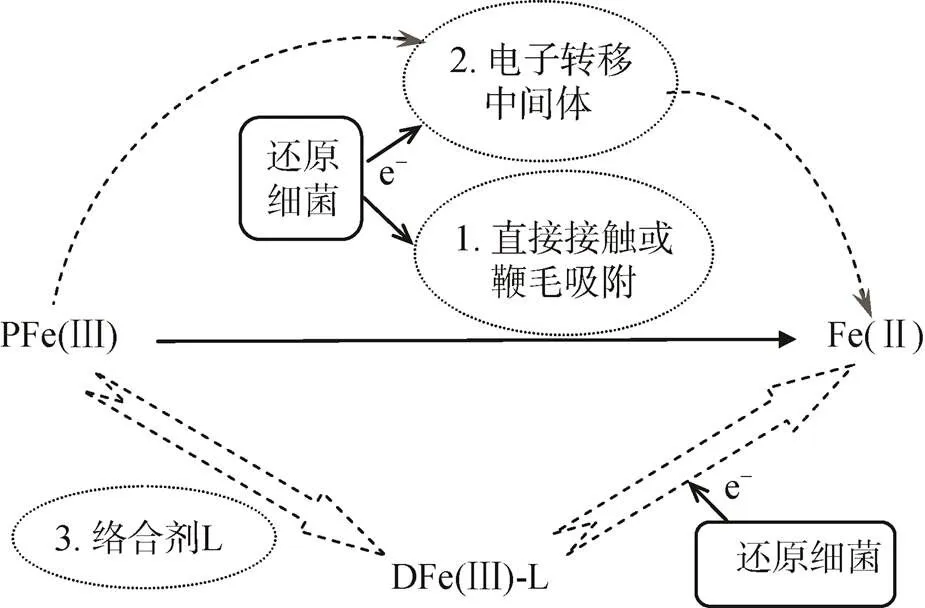
图3 微生物还原Fe(Ⅲ)的三种电子转移途径
3.2 铁氨氧化过程产生Fe(Ⅱ)
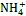
3.3 Fe(Ⅱ)的微生物氧化
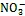
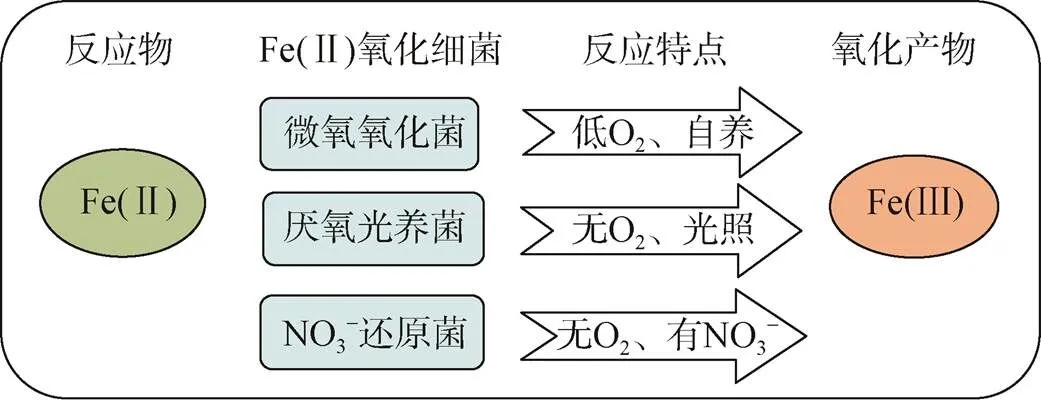
图4 三类海洋细菌催化的Fe(Ⅱ)氧化反应
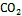
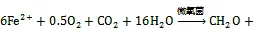
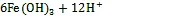
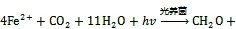
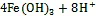
参与厌氧光氧化Fe(Ⅱ)反应的细菌种类包括绿硫细菌、紫色非硫细菌和紫色硫细菌等[78], 由位于细菌细胞膜上的专一性氧化酶催化氧化反应的发生[79]。其他研究表明细菌的厌氧光合作用比有氧光合作用出现时间更早, 而地球早期地层中铁矿带(banded iron formations, BIFs)的形成也与光养细菌的厌氧Fe(Ⅱ)氧化过程紧密相关[31, 80]。
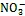
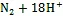
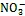
细菌氧化Fe(Ⅱ)产生的Fe(Ⅲ)会吸附在细胞表面形成结壳, 严重阻碍细胞生命活动。研究发现一些Fe(Ⅱ)氧化细菌可通过产生Fe(Ⅲ)有机配体[85]、降低pH[86]等方式来避免Fe(Ⅲ)的结壳, 因此具有更强的环境适应能力。
4 Fe(Ⅱ)的氧化过程与N移除之间的关系
Fe(Ⅱ)主要通过参与透光层中浮游植物硝酸还原酶的合成来影响N的吸收。而海洋缺氧沉积物和OMZ作为海洋脱氮的主要区域, 每年通过反硝化或氨氧化过程移除的氮可达海洋总移除量的70%[87-89], 且沉积物中分布着丰富的铁矿和还原微生物, 厌氧间隙水和OMZ水体中DFe(Ⅱ)含量普遍较高[18, 90], 因此推测海洋缺氧环境中存在着微生物活跃Fe-N迁移转化活动。
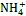
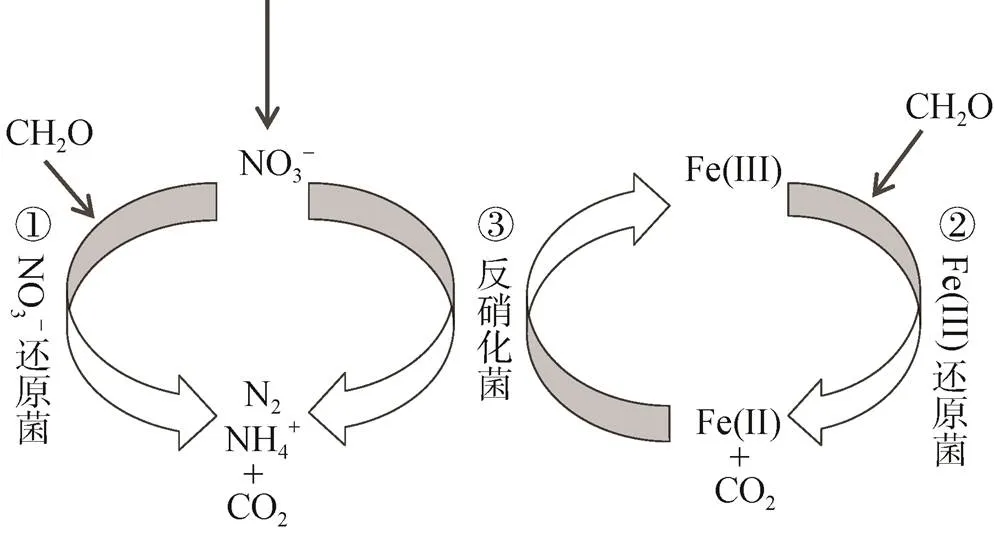
图5 沉积物中的Fe-N循环[91]
5 结论与展望
[1] Marsh H V, Evans H J, Matrone G. Investigations of the role of iron in chlorophyll metabolism I. effect of iron deficiency on chlorophyll and heme content and on the activities of certain enzymes in leaves[J]. Plant Physiology, 1963, 68: 632-638.
[2] Neilands J B. Iron absorption and transport in microorganisms[J]. Annual Review of Nutrition, 1981, 1: 27-46.
[3] Valentine R C, Mortenson L E, Mower H F, et al. Ferredoxin requirement for reduction of hydroxylamine by[J]. The Journal of Biological Chemistry, 1963, 238:856-858.
[4] Venzlaff H, Enning D, Srinivasan J, et al. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria[J]. Corrosion Science, 2013, 66: 88-96.
[5] Boyd P W, Law C S, Wong C S, et al. The decline and fate of an iron-induced subarctic phytoplankton bloom[J]. Nature, 2004, 428: 549-553.
[6] Rich H W, Morel F M M. Availability of well-defined iron colloids to the marine diatom[J]. Limnology and Oceanography, 1990, 35(3): 652-662.
[7] Johnson K S, Coale K H, Elrod V A, et al. Iron photochemistry in seawater from the equatorial Pacific[J]. Marine Chemistry, 1994, 46: 319-334.
[8] Hopkinson B M, Barbeau K A. Organic and redox speciation of iron in the eastern tropical North Pacific suboxic zone[J]. Marine Chemistry, 2007, 106(1-2): 2- 17.
[9] Sulzberger B, Suter D, Siffert C, et al. Dissolution of Fe(Ⅲ)(hydr)oxides in natural waters; laboratory assessment on the kinetics controlled by surface coordination[J]. Marine Chemistry, 1989, 28: 127-144.
[10] Croot P L, Bowie A R, Frew R D, et al. Retention of dissolved iron and Fe(II)in an iron induced Southern Ocean phytoplankton bloom[J]. Geophysical Researche Letters, 2001, 18(28): 3425-3428.
[11] Lin S, Huang K M, Chen S K. Sulfate reduction and iron sulfide mineral formation in the southern East China Sea continental slope sediment[J]. Deep-Sea Research I, 2002, 49: 1837-1852.
[12] Lam P J, Ohnemus D C, Marcus M A. The speciation of marine particulate iron adjacent to active and passive continental margins[J]. Geochimica et Cosmochimica Acta, 2012, 80: 108-124.
[13] Rozan T F, Taillefert M, Trouwborst R E, et al. Iron- sulfur-phosphorus cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms[J]. Limnology and Oceanography, 2002, 47(5): 1346-1354.
[14] de Jong J T M, den Das J, Bathmann U, et al. Dissolved iron at subnanomolar levels in the Southern Ocean as determined by ship-board analysis[J]. Analytica Chimica Acta, 1998, 377: 113-124.
[15] Bruland K W, Orians K J, Cowen J P. Reactive trace metals in the stratified central North Pacific[J]. Geochimica et Cosmochimica Acta, 1994, 58(15): 3171- 3182.
[16] Landing W M, Bruland K W. The contrasting biogeochemistry of iron and manganese in the Pacific Ocean[J]. Geochimica et Cosmochimica Acta, 1986, 51: 29-43.
[17] O’Sullivan D W, Hanson A K. Measurement of Fe(Ⅱ) in surface water of the equatorial Pacific[J]. Limnology and Oceanography, 1991, 36(8): 1727-1741.
[18] Bennett W W, Teasdale P R, Welsha D T, et al. Optimization of colorimetric DET technique for the in situ, two-dimensional measurement of iron(Ⅱ) distributions in sediment porewaters[J]. Talanta, 2012, 88: 490-495.
[19] Breitbarth E, Gelting J, Walve J, et al. Dissolved iron(Ⅱ) in the Baltic Sea surface water and implications for cyanobacterial bloom development[J]. Biogeosciences, 2009, 6: 2397-2420.
[20] Boye M, Aldrich A P, van den Berg C M G, et al. Horizontal gradient of the chemical speciation of iron in surface waters of the northeast Atlantic Ocean[J]. Marine Chemistry, 2003, 80: 129-143.
[21] Hansard S P, Landing W M, Measures C I, et al. Dissolved iron(Ⅱ) in the Pacific Ocean: Measurements from the PO2and P16N CLIVAR/CO2repeat hydrography expeditions[J]. Deep-Sea Research I, 2009, 56: 1117-1129.
[22] Hong H, Kester D R. Redox state of iron in the offshore waters of Peru[J]. Limnology and Oceanography, 1986, 31(3): 512-524.
[23] Kieber R J, Williams K, Willey J D, et al. Iron speciation in coastal rainwater concentration and deposition to seawater[J]. Marine Chemistry, 2001, 73: 83-95.
[24] Lohan M C, Bruland K W. Elevated Fe(Ⅱ) and dissolved Fe in hypoxic shelf waters off Oregon and Washington: an enhanced source of iron to coastal upwelling regimes[J]. Environmental Science and Technology, 2008, 42(17): 6462-6468.
[25] Charette M A, Sholkovitz E R. Oxidative precipitation of groundwater-derived ferrous iron in the subterranean estuary of a coastal bay[J]. Geophysical Research Letters, 2002, 29(10): 85.
[26] Sedwick P N, Ditullio G R. Regulation of algal blooms in Antarctic shelf waters by the release of iron from melting sea ice[J]. Geophysical Research Letters, 1997, 24(20): 2515-2518.
[27] Cooper S R, Mcardle J V, Raymond K N. Siderophore electrochemistry: Relation to intracellular iron release mechanism[J]. Proceedings of the National Academy of Sciences, 1978, 75(8): 3551-3554.
[28] Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese[J]. Applied and Environmental Microbiology, 1998, 54(6): 1472-1480.
[29] Morel F M M, Kustka A B, Shaked Y. The role of unchelated Fe in the iron nutrition of phytoplankton[J]. Limnology and Oceanography, 2008, 53(1): 400-404.
[30] Millero. F J, Sotolongo S, Izaguirre M. The oxidation kinetics of Fe(Ⅱ) in seawater[J]. Geochimica et Cosmochimica Acta, 1987, 51: 793-801.
[31] Widdel F, Schnell S, Heising S, et al. Ferrous iron oxidation by anoxygenic phototrophic bacteria[J]. Nature, 1993, 362(29): 834-836.
[32] Sharma S K, Greetham J M R, Schippers J C. Adsorption of iron(Ⅱ) onto filter media[J]. Journal of Water Supply: Research and Technology-AQUA, 1999, 48(3): 84-91.
[33] Cunningham K M, Goldberg M C, Weiner E R. Mechanisms for aqueous photolysis of adsorbed benzoate, oxalate, and succinate on iron oxyhydroxide (goethite) surfaces[J]. Environmental Science and Technology, 1988, 22(9): 1090-1097.
[34] Waite T D, Morel F M M. Photoreductive dissolution of colloidal iron oxide: effect of citrate[J]. Journal of Colloid and Interface Science, 1984, 102: 121-137.
[35] Kuma K, Nakabayashi S, Matsunaga K. Photoreduction of Fe(Ⅲ) by hydroxycarboxylic acids in seawater[J]. Water Research, 1995, 29(6): 1559-1569.
[36] Voelker B M, Morel F M M, Sulzberger B. Iron redox cycling in surface waters: effects of humic substances and light[J]. Environmental Science and Technology, 1997, 31: 1004-1011.
[37] Faust B C, Zepp R G. Photochemistry of aqueous iron(Ⅲ)-polycarboxylate complexes: roles in the chemistry of atmospheric and surface waters[J]. Environmental Science and Technology, 1993, 27: 2517- 2522.
[38] Kuma K, Nakabayashi S, Suzuki Y, et al. Photo-reduction of Fe(Ⅲ) by dissolved organic substances and existence of Fe(Ⅱ) in seawater during spring blooms[J]. Marine Chemistry, 1992, 37: 15-27.
[39] Rijkenberg M J A, Loes J A G, Carolus V E, et al. Enhancement and inhibition of iron photoreduction by individual ligands in open ocean seawater[J]. Geochimica et Cosmochimica Acta, 2006, 70: 2790-2805.
[40] Rijkenberg M J A, Fischer A C, Kroon K J, et al. The influence of UV irradiation on the photoreduction of iron in the Southern Ocean[J]. Marine Chemistry, 2005, 93: 119-129.
[41] Roy E G, Wells M L, King D W. Persistence of iron(Ⅱ) in surface waters of the western subarctic Pacific[J]. Limnology and Oceanography, 2008, 53(1):89-98.
[42] Kieber R J, Skrabal S A, Smith B J, et al. Organic complexation of Fe(Ⅱ) and its impact on the redox cycling of iron in rain[J]. Environmental Science and Technology, 2005, 39: 1576-1583.
[43] Willey J D, Kieber R J, Seaton P J, et al. Rainwater as a source of Fe(Ⅱ)-stabilizing ligands to seawater[J]. Limnology and Oceanography, 2008, 53(4): 1678-1684.
[44] Iwai H, Fukushima M, Yamamoto M. Binding characteristics and dissociation kinetics for iron(Ⅱ) complexes with seawater extractable organic matter and humic substances in a compost[J]. Analytical Sciences, 2012, 28: 819-821.
[45] Statham P J, Jacobson Y, van den Berg C M G. The measurement of organically complexed FeIIin natural waters using competitive ligand reverse titration[J]. Analytica Chimica Acta, 2012, 743: 111-116.
[46] Statham P J, German C R, Connelly D P. Iron(Ⅱ) distribution and oxidation kinetics in hydrothermal plumes at the Kairei and Edmond vent sites, Indian Ocean[J]. Earth and Planetary Science Letters, 2005, 236(3-4): 588-596.
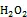
[48] Moffett J W, Zika R G. Reaction kinetics of hydrogen peroxide with copper and iron in seawater[J]. Environmental Science and Technology, 1987, 21(8): 804- 810.

[50] Kondo Y, Moffett J W. Dissolved Fe(Ⅱ) in the Arabian Sea oxygen minimum zone and western tropical Indian Ocean during the inter-monsoon period[J]. Deep Sea Research I, 2013, 73: 73-83.
[51] Moffett J W, Goepfert T J, Naqvi S W A. Reduced iron associated with secondary nitrite maxima in the Arabian Sea[J]. Deep Sea Research I, 2007, 54: 1341-1349.
[53] Williams A G B, Scherer M M. Spectroscopic evidence for Fe(Ⅱ)-Fe(Ⅲ) electron transfer at the iron oxide-water interface[J]. Environmental Science and Technology, 2004, 38: 4782-4790.
[54] Canfield D E, Thamdrup B, Hansen J W. The anaerobic degradation of organic matter in Danish coastal sediments: iron reduction, manganese reduction, and sulfate reduction[J]. Geochimica et Cosmochimica Acta, 1993, 57: 3867-3883.
[55] Shaked Y, Kustka A B, Morel F M M. A general kinetic model for iron acquisition by eukaryotic phytoplankton[J]. Limnology and Oceanography, 2005, 50(3): 872-882.
[56] Sunda W G. Bioavailability and bioaccumulation of iron in the sea[A].edited by: Turner D R. and Hunter K H, in: The biogeochemistry of iron in seawater[M]. IUPAC Series on Analytical and Physical Chemistry of Environmental Systems, 2001, 41-84, .
[57] Kranzler C, Lis H, Finkel O M, et al. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria[J]. The ISME Journal, 2014, 8: 409- 417.
[58] Kranzler C, Lis H, Shaked Y, et al. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium[J]. Environmental Microbiology, 2011, 13(11): 2990-2999.
[59] Vargas M, Kashefi K, Blunt-Harris E L, et al. Microbiological evidence for Fe(Ⅲ) reduction on early Earth[J]. Nature, 1998, 395: 65-67.
[60] 刘洪艳, 王红玉, 谢丽霞, 等.海洋沉积物中一株铁还原细菌分离及Fe(Ⅲ)还原性质[J].海洋科学, 2016, 40(3): 65-70. Liu Hongyan, Wang Hongyu, Xie Lixia, et al. Isolation and characterization of Fe(Ⅲ)-reducing bacteriumsp. KB52 from marine sediment[J]. Marine Sciences, 2016, 40(3): 65-70.
[61] Bridge T A M, Johnson D B. Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria[J]. Applied and Environmental Microbiology, 1998, 64(6): 2181-2186.
[62] Kashefi K, Lovley D R. Reduction of Fe(Ⅲ), Mn(IV), and toxic metals at 100℃ by[J]. Applied and Environmental Microbiology, 2000, 66(3): 1050-1056.
[63] 司友斌, 王娟.异化铁还原对土壤中重金属形态转化及其有效性影响[J]. 环境科学, 2015, 36(9): 3533- 3542. Si Youbin, Wang Juan. Influence of dissimilatory iron reduction on the speciation and bioavailability of heavy metals in soil[J]. Environmental Science, 2015, 36(9): 3533-3542.
[64] Lovley D. Dissimilatory Fe(Ⅲ)-and Mn(IV)-reducing prokaryotes[J]. Prokaryotes, 2006, 2: 635-658.
[65] Weber K A, Achenbach L A, Coates J D. Microbes pumping iron: anaerobic microbial iron oxidation and reduction[J]. Nature Reviews Microbiology, 2006, 4: 752-764.
[66] White G F, Shi Z, Shi L, et al. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(Ⅲ) minerals[J]. Proceedings of the National Academy of Sciences, 2013, 110(16): 6346-6351.
[67] Gorby Y A, Yanina S, Mclean J S, et al. Electrically conductive bacterial nanowires produced bystrain MR-1 and other microorganisms[J]. Proceedings of the National Academy of Sciences, 2006, 103(30): 11358-11363.
[68] Lovley D R, Coates J D, Blunt-Harris E L, et al. Humic substances as electron acceptors for microbial respiration[J]. Nature, 1996, 382: 445-448.
[69] Kotloski N J, Gralnick J A. Flavin electron shuttles dominate extracellular electron transfer by[J]. mBio, 2013, 4(1): 1-4.
[70] 陈洁. 奥奈达希瓦氏菌MR-1的Fe(Ⅲ)还原特性及其影响因素研究[D]. 合肥: 安徽农业大学, 2011.Chen Jie. Dissimilatory Fe(Ⅲ) reduction byMR-1 and the influence factors[D]. Hefei: Anhui Agricultural University, 2011.
[71] Urrutia M M, Roden E E, Zachara J M. Influence of aqueous and solid-phase Fe(Ⅱ) complexants on microbial reduction of crystalline iron(Ⅲ) oxides[J]. Environmental Science and Technology, 1999, 33(22): 4022- 4028.
[72] 冯雅丽, 周良, 祝学远, 等.异化还原铁氧化物三种方式[J]. 北京科技大学学报, 2006, 28(6): 524-529. Feng Yali, Zhou Liang, Zhu Xueyuan, et al. Three paths to reduce ferric oxides taken by[J]. Journal of University of Science and Technology Beijing, 2006, 28(6): 524-529.
[73] Yang W H, Weber K A, Silver W L. Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction[J]. Nature Geoscience, 2012, 5: 538-541.
[74] van de Vossenberg J, Rattray J E, Geerts W, et al. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production[J]. Environmental Microbiology, 2008, 10(11): 3120-3129.
[75] Li X, Hou L, Liu M, et al. Evidence of nitrogen loss from anaerobic ammonium oxidation coupled with ferric iron reduction in an intertidal wetland[J]. Environmental Science and Technology, 2015, 49: 11560- 11568.
[76] Chan C S, Emerson D, Luther G W. The role of microaerophilic Fe-oxidizing micro-organisms in producing banded iron formations[J]. Geobiology, 2016, 14: 509- 528.
[77] Alt J C. Hydrothermal oxide and nontronite deposits on seamounts in the Eastern Pacific[J]. Marine Geology, 1988, 81: 227-239.
[78] Kappler A, Newman D K. Formation of Fe(Ⅲ)-minerals by Fe(Ⅱ)-oxidizing photoautotrophic bacteria[J]. Geochimica et Cosmochimica Acta, 2004, 68(6): 1217- 1226.
[79] Croal L R, Jiao Y, Newman D K. The foxoperon fromstrain SW2 promotes phototrophic Fe(Ⅱ) oxidation inSB1003[J]. Journal of Bacteriology, 2007, 189(5): 1774-1782.
[80] Jiao Y, Newman D K. The piooperon is essential for phototrophic Fe(Ⅱ) oxidation inTIE-1[J]. Journal of Bacteriology, 2007, 189(5): 1765-1773.
[81] Carlson H K, Clark I C, Blazewicz S J, et al. Fe(Ⅱ) oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions[J]. Journal of Bacteriology, 2013, 195(14): 3260-3268.
[82] Chakraborty A, Roden E E, Schieber J, et al. Enhanced growth ofsp. strain 2AN during nitrate- dependent Fe(Ⅱ) oxidation in batch and continuous- flow systems[J]. Applied and Environmental Microbiology, 2011, 77(24): 8548-8556.
[83] Muehe E M, Gerhardt S, Schink B, et al. Ecophysiology and the energetic benefit of mixotrophic Fe(Ⅱ) oxidation by various strains of nitrate-reducing bacteria[J]. FEMS Microbiol Ecology, 2009, 70: 335-343.
[84] Straub K L, Buchholz-Cleven B E E. Enumeration and detection of anaerobic ferrous iron oxidizing, nitrate-reducing bacteria from diverse European sediments[J]. Applied and Environmental Microbiology, 1998, 64(12): 4846-4856.
[85] Croal L R, Johnson C M, Beard B L, et al. Iron isotope fractionation by Fe(Ⅱ)-oxidizing photoautotrophic bacteria[J]. Geochimica et Cosmochimica Acta, 2004, 68(6): 1227-1242.
[86] Hegler F, Schmidt C, Schwarz H, et al. Does a low-pH microenvironment around phototrophic Fe(II)-oxidizing bacteria prevent cell encrustation by Fe(III)minerals?[J]. FEMS Microbiology Ecology, 2010, 74: 592-600.
[87] Hulth S, Aller R C, Canfield D E, et al. Nitrogen removal in marine environments: recent findings and future research challenges[J]. Marine Chemistry, 2005, 94(1-4): 125-145.
[88] Dalsgaard T, Canfield D E, Petersen J, et al. N2production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica[J]. Nature, 2003, 422: 606-608.
[90] Vedamati J, Goepfert T, Moffett J W. Iron speciation in the eastern tropical South Pacific oxygen minimum zone off Peru[J]. Limnology and Oceanography, 2014, 59(6): 1945-1957.
[91] Weber K A, Urrutia M M, Churchill P F, et al. Anaerobic redox cycling of iron by freshwater sediment microorganisms[J]. Environmental Microbiology, 2006, 8(1): 100-113.
[93] Jorgensen C J, Jacobsen O S, Elberling B, et al. Microbial oxidation of pyrite coupled to nitrate reduction in anoxic groundwater sediment[J]. Environmental Science and Technology, 2009, 43: 4851-4857.
[94] Clément J C, Shrestha J, Ehrenfeld J G, et al. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils[J]. Soil Biology and Biochemistry, 2005, 37(12): 2323-2328.
[95] Myers C R, Myers J M. Cloning and sequence of, a gene encoding a tetraheme cytochromerequired for reduction of iron(Ⅲ), fumarate, and nitrate byMR-1[J]. Journal of Bacteriology, 1997, 179(4): 1143-1152.
[96] Oshiki M, Ishii S, Yoshida K, et al. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria[J]. Applied and Environmental Microbiology, 2013, 79(13): 4087-4093.
[97] Kopf S H, Henny C, Newman D K. Ligand-enhanced abiotic iron oxidation and the effects of chemical versus biological iron cycling in anoxic environments[J]. Environmental Science and Technology, 2013, 47: 2602-2611.
[98] Ottow J C G. Selection, Characterization and iron- reducing capacity of nitrate reductaseless (nit) mutants of iron-reducing bacteria[J]. Journal of Basic Microbiology, 2010, 10(1): 55-62.
[99] Dichristina T J. Effects of nitrate and nitrite on dissimilatory iron reduction by200[J]. Journal of Bacteriology, 1992, 174(6): 1891-1896.
(本文编辑: 康亦兼)
A review of the behavior and microbial activity of Fe(Ⅱ) in seawater
QU Sheng-lu1, 2, YANG Ru-jun1, SU Han1, LIU Yuan1, GENG Qian-qian1
(1. College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China; 2. Laboratory for Marine Geology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China)
Fe(Ⅱ); microbialactivities; nitrogen removal
Apr. 28, 2017
P734.2
A
1000-3096(2017)10-0139-10
10.11759/hykx20170428001
2017-04-28;
2017-10-25
青岛海洋科学与技术国家实验室海洋地质过程与环境功能实验室开放基金资助项目(MGQNLM-KF201701)
[Supported by the Laboratory for Marine Geology, Qingdao National Laboratory for Marine Science and Technology, grant No. MGQNLM-KF201701]
曲胜路(1993-), 女, 河北衡水人, 硕士研究生, 主要从事海洋痕量元素生物地球化学研究, 电话: 17864277355, E-mail: 1175198588@qq.com; 杨茹君, 通信作者, 博士, 副教授, 电话: 0532-66781815, E-mail: yangrj@ouc.edu.cn
