特戊酸和含氮配体构筑的Ln(Ⅱ)配合物的结构、热稳定性和荧光性质
2016-12-06方洁芳熊艳菊程靖祥黄树婷方乐欣陆珣乐善堂华南师范大学化学与环境学院广州510006
方洁芳 熊艳菊 程靖祥 黄树婷 方乐欣 陆珣 乐善堂(华南师范大学化学与环境学院,广州510006)
特戊酸和含氮配体构筑的Ln(Ⅱ)配合物的结构、热稳定性和荧光性质
方洁芳熊艳菊程靖祥黄树婷方乐欣陆珣乐善堂*
(华南师范大学化学与环境学院,广州510006)
在水热条件下2个配体特戊酸(pivH)和1-H-咪唑[4,5-f][1,10]-菲咯啉(IP)和稀土金属反应得到了6个稀土配合物[Ln(piv)3(IP)2],(Ln=Nd(1),Eu(2),Gd(3),Tb(4),Dy(5),Ho(6))。结果显示,2个混合配体和不同的稀土金属形成了6个相似的零维结构,进而通过N-H…O氢键和π-π堆积作用,形成一维链状结构。6个配合物均用元素分析、粉末衍射(PXRD)、红外光谱(FTIR)进行了表征,且对配合物2和4的荧光性质及1和2的热稳定性进行了详细的分析。
稀土配合物;特戊酸;荧光性质;热重分析
0 Introduction
The versatile lanthanide complexes with novel structures and optical properties have recently drawn considerable focus as a result of their promising applications in many fields[1-3].Especially,lanthanide(Ⅱ)coordination polymers have unique properties such asline-likeemission,highcolorpurity,long luminescence lifetime and high quantum yields[4]. However,in general,the spin/parity-forbidden nature of the f-f transitions of lanthanide(Ⅱ)ion results in abominably low molar extinction coefficients and weakluminescence intensity[5-8].Nevertheless,this drawback can be overcome when organic ligands acting as the sensitizer are introduced into the systems.As a result, chromophoric antenna ligands,specially,π-conjugated organic chromophores have been of intense interest owning to their broad and intense adsorption band in ultraviolet[5].So conjugated and rigid multidentate 1-H-imidazo[4,5-f][1,10]-phenanthroline(IP)is chosen as a ligand.Whats more,if the coordination sites of the lanthanide ions can be filled up,the radiationless deactivation processes maybe diminish[9].In this aspect, pivalic acid(pivH),as a sterically hindered carboxylate ligand[10],together with lanthanide(Ⅱ)ions are applied to prepare low-dimensional structures.
By this strategy,six new monometallic Ln(Ⅱ)complexes,([Ln(piv)3(IP)2],(Ln=Nd(1),Eu(2),Gd(3), Tb(4),Dy(5),Ho(6))were obtained under hydrothermal condition.Block crystals of complexes 1~6 were synthesized with a mixture of pivH,IP and Ln2O3in a 1∶1∶1 molar ratio in 10 mL water at 433 K for 3 days.Only when Lanthanide oxides were employed can the crystals be obtained.If the lanthanide nitrates were used under the various conditions,such as temperature, reaction time,pH value and solvent,none of targeted products can be afforded.
1 Experimental
1.1Materials and methods
Allchemicalsemployedwerecommercially availableandusedasreceivedwithoutfurther purificaion.Elemental(C,H,N)analyseswere performed on a Perkin-Elmer 2400 element analyzer. The FTIR spectra were recorded from KBr pellets in the range of 4 000~400 cm-1on a Nicolet 6700 spectrometer. Thermogravimetric analyses were performed on Perkin-Elmer TGA 7 analyzer with a heating rate of 10℃·min-1in flowing air atmosphere.Luminescence spectroscopy wasrecordedonanEdinburghF900FLS-900 spectrophotometer analyzer with a xenon arc lamp as the light source.In the measurement of emission and excitation spectra,the pass width is 5.0 nm.Powder X-ray diffraction(PXRD)patterns were recorded on a X-pert diffractometer or Rigaku D/M-2200T automated diffractometer for Cu Kα radiation(λ=0.154 056 nm), with operating voltage of 40 kV,current of 15 mA,a scan speed of 4°·min-1and a step size of 0.02°in 2θ range of 5°~50°.
1.2Syntheses of complexes 1~6
A mixture of pivH(0.166 1 g,0.5 mmol),IP (0.127 g,0.5 mmol),Nd2O3for 1(0.168 2 g,0.5 mmol),Eu2O3for 2(0.176 0 g,0.5 mmol),Gd2O3for 3 (0.181 2 g,0.5 mmol),Tb4O7for 4(0.186 9 g,0.25 mmol),Dy2O3for 5(0.118 65 g,0.5 mmol),Ho2O3for 6(0.188 9 g,0.5 mmol)and H2O(10 mL)was sealed in a 23 mL Teflon-lined stainless-steel autoclave,then heated to 160℃for 72 h.After cooling to room temperature at a rate of 3℃·h-1,colorless block crystals(complex 1 is purple)were collected manually, washed with distilled water for several times and dried in air.For 1,55%yield based on Nd.Anal.Calcd.for C41H43NdN8O6(%):C,55.40;H,4.84;N,12.61.Found (%):C,55.40;H,4.85;N,12.60.For 2,78%yield based on Eu.Anal.Calcd.for C41H43EuN8O6(%):C, 54.92;H,4.80;N,12.50%.Found(%):C,54.95;H, 4.77;N,12.50%.For 3,73%yield based on Gd. Anal.Calcd.for C41H43GdN8O6(%):C,54.60;H,4.77; N,12.43.Found(%):C,54.68;H,4.75;N,12.37.For 4,71%yieldbasedonTb.Anal.Calcd.for C41H43TbN8O6(%):C,55.50;H,4.76;N,12.41.Found (%):C,55.53;H,4.74;N,12.40.For 5,77%yield based on Dy.Anal.Calcd.for C41H43DyN8O6(%):C, 54.28;H,4.74;N,12.36.Found(%):C,54.29;H, 4.75;N,12.34.For 6,66%yield based on Ho.Anal. Calcd.for C41H43HoN8O6(%):C,54.14;H,4.73;N, 12.32.Found(%):C,54.15;H,4.71;N,12.33.
1.3X-raydatacollectionandstructurerefinement
Data collections were performed at 296 K on a BrukerApexⅡSmartCCDdiffractometerwith graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)forcomplexes 1~6.Multi-scanabsorption correctionswereappliedbyusingtheprogram SADABS[11].Structural solutions and full-matrix leastsquares refinements based on F2were performed with the SHELXL-97 and SHELXS-97[12]program packages, respectively.Anisotropicthermalparameterswere used to refine all non-H atoms.The hydrogen atomsfor C-H and N-H were placed in idealized positions. Details of the crystal parameters,data collections,and refinements for complexes 1~6 are summarized in Table 1.Selected bond lengths and angles are listed in Table 2.Hydrogen bond lengths and angles for complex 4 are listed in Table 3.
CCDC:1476957,1;1476958,2;1476959,3; 1476960,4;1476961,5;1476962,6.
2 Results and discussion
2.1Description of the structures of complexes 1~6 To the best of our knowledge,the lanthanide supramolecular complexes based on both piv-and IP ligands have never been reported.The IR spectra ofallcomplexesexhibitsimilarities(Suppourting information).The absorption peaks around 3 450 cm-1was assigned to the N-H stretching vibrations.There are strong absorption peaks around 1 618~1 561 cm-1(antisymmetric stretching vibrations)and 1 399~1 376 cm-1(symmetric stretching vibrations).Closer inspection shows that there are no absorption peaks around 1 700 cm-1indicating that carboxyl groups are of completely deprotonation[13].
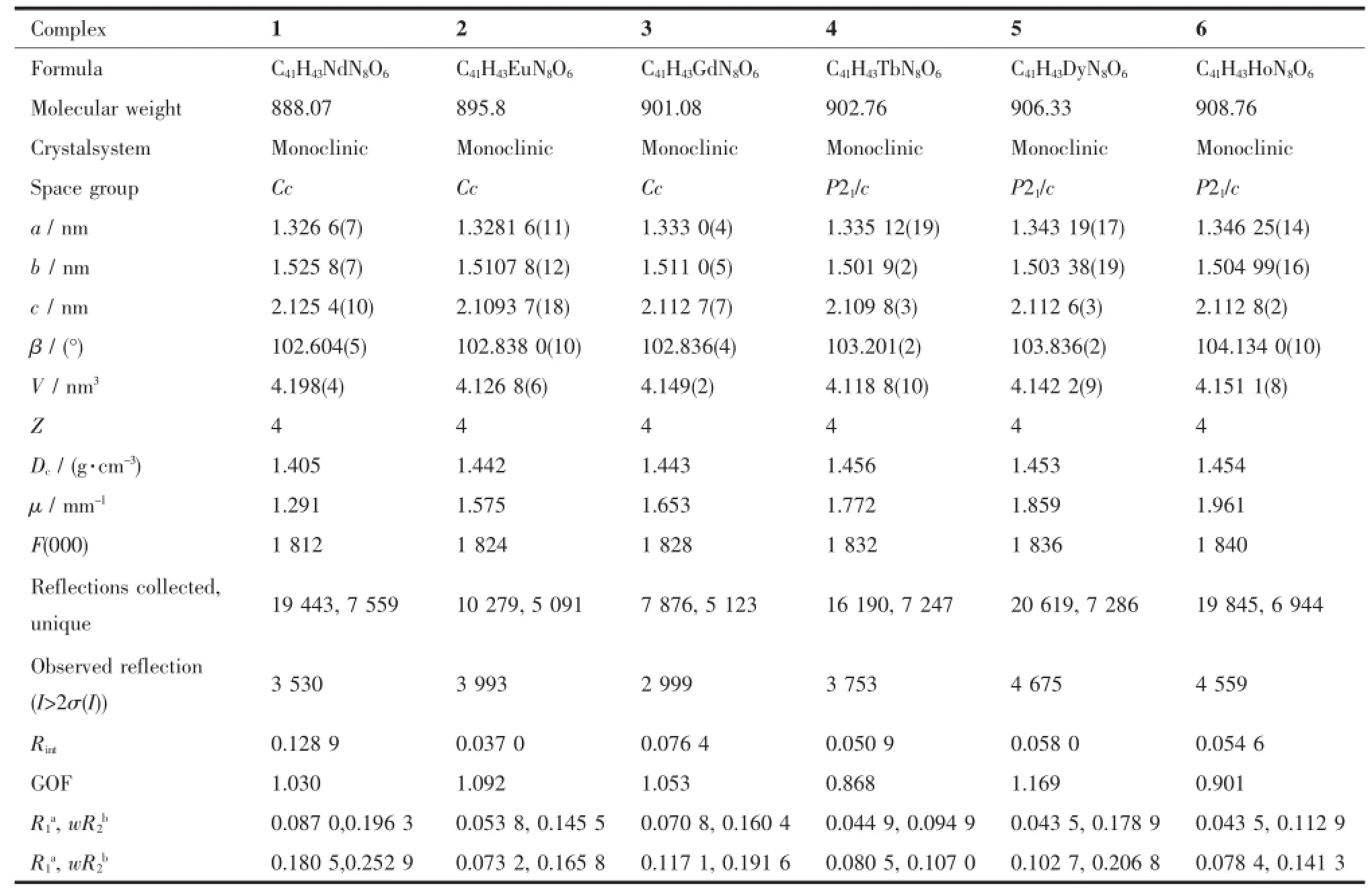
Table 1Crystallographic and structural refinement parameters for complexes 1~6
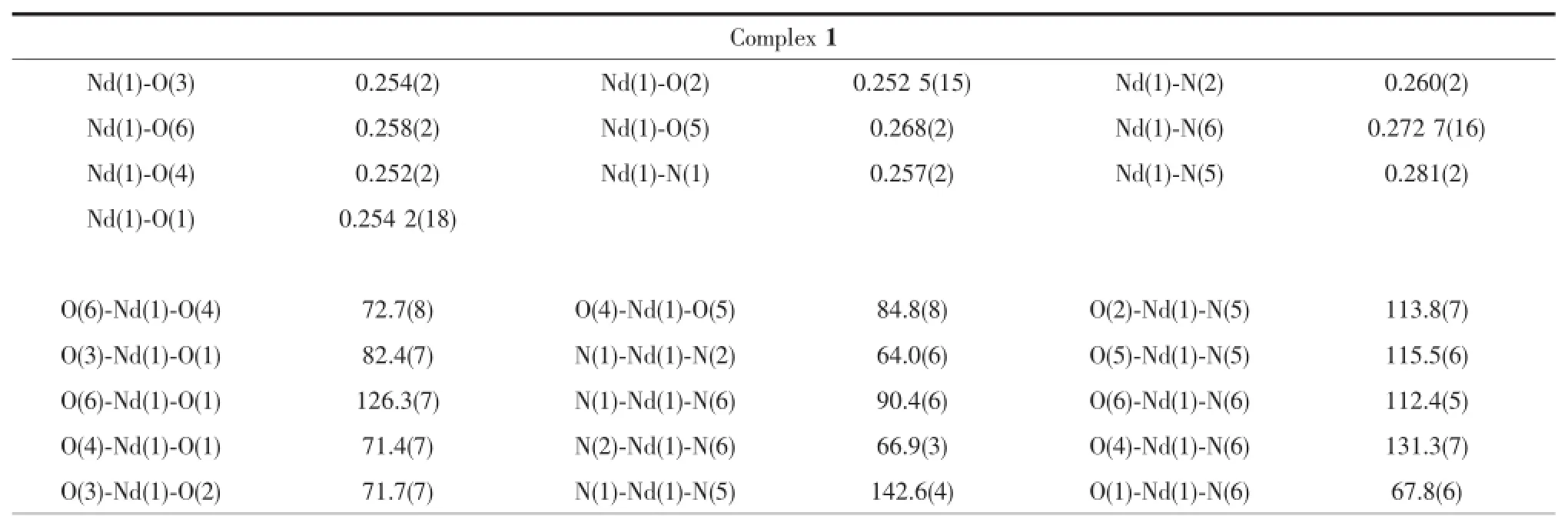
Table 2Selected bond lengths(nm)and angles(°)for complexes 1~6

Continued Table 2

Continued Table 2

Table 3Hydrogen bonding data for complex 4
X-raysingle-crystaldiffractionrevealsthat complexes 1~6 crystallize in the monoclinic system with similar cell parameters.All complexes present thesamemolecularstructuresalthoughthe coordination number is a little different.Complexes 1~4 are ten-coordinated while 5~6 are nine-coordinated.Itmaybeassignedtothelanthanide contraction effect[14-15].Herein only the structure of 4 is described in detail.The asymmetric unit of complex 4 consists of one crystallographically independent Tb(Ⅱ)ion,three completely deprotonated piv-ligands,two IP ligands.As shown in Fig.1a,the center Tb(Ⅱ)is tencoordinated by six oxygen atoms from three pivligands,four nitrogen atoms from two IP ligands, forming a somewhat distorted two-capped quadrangularprism coordination geometry.The bond lengths of Tb-O span the range of 0.242 1(5)~0.273 7(7)nm and the bond lengths of Tb-N are in the range of 0.256 3(5)~0.261 7(5)nm.In addition,in the structure of 4,the N-Tb-N bond angle are in the range of 62.65(17)°~143.73(16)°,and the bond angle of O-Tb-O and O-Tb-N are in the range of 48.2(2)°~169.7(2)°and 67.36(16)°~157.19(18)°,respectively.Closer inspection shows that there are two types of hydrogen bonds between phenanthroline nitrogens atom and carboxylic oxygen atoms(N1-H…O40.2753(7)nm,N4-H…O1 0.2766(9) nm).Themononuclear molecules are linked by these hydrogen bonds in-AA-mode to afford a 1D boundless chain(Fig.1b).In addition,the π-π stacking interactions also exist between the two neighboring IP ligands with the average center to-center distance of ca.0.3579nm,whichfurtherconsolidatethestructure.

Fig.1Structure of complex 4:(a)Coordination polyhedron geometry of the Tb(Ⅱ)ion;(b)1D structure connected by hydrogen bonds
2.2PXRD results and thermal analyses
Powder X-ray diffraction(PXRD)experiments were performed on complexes 1~6 at room temperature to estimate if the crystalline samples are of phase purity.All major peaks in the measured patterns match quite well with those of the simulated ones, indicating that the bulk as-prepared products are pure (Fig.2).

Fig.2Powder X-ray diffraction patterns of complexes 1~6
The isomorphous structures result in a similar thermal decomposition process,so complexes 1 and 2 werechosenasrepresentativeexamplesfor thermogravimetric analyses in order to estimate the stability of the complexes.TGA experiments were carried out on these new crystalline materials in the temperature range of 30~750℃with a heating rate of 10℃·min-1in dry air atmosphere,as illustrated in Fig.3.Complex 1 shows no obvious weight loss process under 355℃(358℃for 2),exhibiting a high thermal stability as there are no coordinated water molecules or solvent molecules in the coordination structure.With the temperature increasing,complexes began to experience stepwise destructive changes,ligands and result in the collapse of whole structure.

Fig.3TGA curves for complexes 1~2
2.3Photoluminescent properties
As shown in Fig.4,the solid-state luminescent properties of complexes of 2 and 4 were investigated in the range of 400~750 nm at ambient temperature. TheEu-basedcomplexof2emitsintensered fluorescence upon excitation at 350 nm,exhibiting characteristic bands at 579(5D0→7F0),593(5D0→7F1), 618(5D0→7F2),652(5D0→7F3)and 694 nm(5D0→7F4). The5D0→7F0and5D0→7F3transition are relative weak, which are regarded as both strictly forbidden in magnetic-dipole(MD)andelectricdipole(ED) schemes[5,14-18].It can be noted that5D0→7F1and5D0→7F2are the more intense transitions.The5D0→7F1is a MD-allowed transition and insensitive to the local environment around Eu(Ⅱ)ions.However,thetransition is an ED transition,of which the intensity decreases as the site symmetry of Eu(Ⅱ)increases[13-14]. The luminescent intensity ratio ofapproximate 1.3,which suggests that Eu(Ⅱ)ions in 2 have a relatively high centrosymmetric coordination environment matching well with the results of the single-crystal X-ray analysis.On complexation of IP and piv-with Tb(Ⅱ),complex 4 generates pure green luminescence.The emission peaks occur at 490,545, 585and622nmwhenexcitedat394nm, corresponding to5D4→7F6,5D4→7F5,5D4→7F4,5D4→7F3, respectively.

Fig.4Solid state emissions spectra of complexes 2 and 4 at ambient temperature
3 Conclusions
In summary,six Ln(Ⅱ)-monometallic-based coordination complexes have been successfully prepared by using Ln2O3and mixed ligands IP and piv-.Ligand piv-plays a sterically hindered role and may offers a suitable acidic environments to construct the complexes. Thermogravimetricanalysesrevealthatcomplexes exhibit high thermal stability.Furthermore,complexes 2 and 4 exhibit excellent characteristic lanthanidecentered luminescent propertities.
Supportinginformationisavailableathttp://www.wjhxxb.cn
References:
[1]An J,Geib S J,Rosi N L.J.Am.Chem.Soc.,2009,131(24): 8376-8377
[2]Dohner E R,Hoke E T,Karunadasa H I.J.Am.Chem.Soc., 2014,136(5):1718-1721
[3]Struzhkin V V,Militzer B,Mao W L,et al.Chem.Rev., 2007,131(10):4133-4151
[4]Shi W J,Hou L,Zhao W,et al.Inorg.Chem.Commun.,2011, 14(12):1915-1919
[5]Zhang S,Yang Y,Xia Z Q,et al.Inorg.Chem.,2014,53(20): 10952-10963
[6]Feng X,Feng Y Q,Liu L.Dalton Trans.,2013,42(21):7741 -7754
[7]ZHANG Qing(张青),LIU Chu-Bo(刘春波).CHE Guang-Bo (车广波),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(10):2188-2194
[8]RAN Xing-Rui(冉行蕊),XIE Wei-Ping(谢卫萍),XIONG Yan-Ju(熊艳菊),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(11):2662-2668
[9]Fomina I G,Dobrokhotova Z V,Kazak V O,et al.Eur.J. Inorg.Chem.,2012,2012(22):3595-3610
[10]Liu S J,Zhao J P,Song W C,et al.Inorg.Chem.,2013,52 (4):2103-2109
[11]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[12](a)Sheldrick G M.SHELXL-97,Program for X-ray Structure Determination,UniversityofGöttingen,Göttingen,Germany,1997. (b)Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Refinement,University of Göttingen,Göttingen, Germany,1997.
[13]Liu Q Y,Wang W F,Tang J K.Inorg.Chem.,2012,51(4): 2381-2392
[14]Jiang Z Q,Jiang G Y,Hou D C,et al.CrystEngComm, 2013,15(2):315-323
[15]Gao J Y,Xiong X H,Chen C J,et al.Inorg.Chem. Commun.,2013,31(5):5-12
[16]Xie W P,Wang N,Long Y,et al.Inorg.Chem.Commun., 2014,40(2):151-156
[17]Liu J X,Hu Y F,Zhu T.CrystEngComm,2012,14(20):6983 -6989
[18]LU Wen-Guan(卢文贯),LIU Hong-Wen(刘宏文).Chinese J. Inorg.Chem.(无机化学学报),2010,26(8):1450-1456
Lanthanide(Ⅱ)Coordiantion Complexes with Pivalates and Chelating N-Donor Ligands: Syntheses,Structures,Thermal Stabilities and Luminescence Properties
FANG Jie-FangXIONG Yan-JuCHENG Jing-XiangHUANG Shu-Ting FANG Le-XinLU XunYUE Shan-Tang*
(School of Chemistry and Environment,South China Normal University,Guangzhou 510006,China)
A series of novel monometallic coordination complexes[Ln(piv)3(IP)2],(Ln=Nd(1),Eu(2),Gd(3),Tb(4), Dy(5),Ho(6),IP=1-H-imidazo[4,5-f][1,10]-phenanthroline,pivH=pivalic acid)have been successfully synthesized under hydrothermal condition.Complexes 1~6 have been determined by single-crystal X-ray diffraction analyses, elemental analyses,IR spectroscopy and powder X-ray diffraction.The result reveals that all the six new complexes present similar 0-dimensional structures,which are further extended into the one-dimensional(1D) architectures through hydrogen bonds and π-π stacking interactions.Meanwhile,the thermogravimetric analyses of complexes 1 and 2 and photoluminescent properties of complexes 2 and 4 have also been investigated.CCDC: 1476957,1;1476958,2;1476959,3;1476960,4;1476961,5;1476962,6.
Ln(Ⅱ)complex;pivalic acid;luminescent;thermogravimetric analysis
O614.33+5;O614.33+8;O614.33+9;O614.341
A
1001-4861(2016)08-1449-08
10.11862/CJIC.2016.186
2016-03-31。收修改稿日期:2016-06-23。
国家自然科学基金(No.20971047,21271076,21471060)、广东省教育厅重点项目(No.cxzd1020)和广州市科技计划项目(No.2013J4100049)资助。
*通信联系人。E-mail:yuesht@scnu.edu.cn,Fax:+86-20-39310187,Tel:+86-20-39310187
