具有单分子磁行为的二维Dy(Ⅱ)-配合物
2016-12-06杨玉亭屠长征姚立峰李俊莉陈广成飞翔曲靖师范学院化学化工学院曲靖655011
杨玉亭 屠长征 姚立峰 李俊莉 陈广 成飞翔(曲靖师范学院化学化工学院,曲靖655011)
具有单分子磁行为的二维Dy(Ⅱ)-配合物
杨玉亭*屠长征姚立峰李俊莉陈广成飞翔
(曲靖师范学院化学化工学院,曲靖655011)
在水热条件下,利用刚性配体Hapca(3-氨基吡嗪-2-羧酸)合成了一例基于双核镝结构基元的二维网格配合物[Dy2(apca)4(OH)2(H2O)2]n(1)。磁性研究表明:该配合物显示铁磁耦合,并具有典型的慢弛豫行为,其弛豫时间τ0=9.47×10-7s,各向异性能垒Ueff/kB=73.7 K,是一例少见的具有单分子磁行为的镧系二维网格配合物。
二维;镝;双核;单分子磁体
0 Introduction
Design and synthesis of SMMs continue to attract great attention,not only because of their interests in fundamental physics,but also for their potential application in high-density data storage or devices for future quantum computers[1-3].An SMM is a discrete moleculethatexhibitsthesuper-paramagnet-like property of slow relaxation of the magnetization at low temperature[1a].Assembling SMMs into coordination polymersofvariousdimensionalitiesincorporating either covalent or non-covalent interactions became an appealingnewgoalforchemistsandphysicists working in molecular magnetism,because the control of such SMM architectures may provide a unique opportunity to investigate new magnetic behaviors and also the frontier between SMMs and classical bulk magnets[4-8].To date,only limited 2D and 3D covalently bonded SMMs have been recorded,and most of them are focused on Mn-SMMs[5-6]and Co-SMMs[7].Following the increasing knowledge about lanthanide SMMs, the design,preparation and magnetic studies of 2D array of lanthanide SMMs seems more interesting.In 2008 and 2009,Murugesu et al.reported two possible 2D array of lanthanide SMMs[8b-8c],however,taking into the large span between SMM centers,we think the socalled quasi 2D array of lanthanide SMMs more suitable for the cases of discrete clusters.Veryrecently,Yue and co-works reported a dysprosium coordination polymer which features a 2D network structurewithdinuclearunitsandexhibitsthe frequency dependent peaks[9].However,the adjacent 2D networks were packed into 3D supermolecular framework by significant hydrogen bond interactions. Thereby,there is still no truly 2D array of lanthanide SMMs.
Herein,we report a two-dimensional Dy-based complex,[Dy2(apca)4(OH)2(H2O)2]n(1),where Hapca=3-aminopyrazine-2-carboxylic acid,synthesized by the reaction of Hapca with Dy2O3under hydrothermal reaction conditions.Based on the query on the CSD database,we find that the crystal data of 1 has been documented[10],however,the record that we can find is only the crystal data,and the authors only gave the structure description of it.As a potential magnetic material,the previous studies are obviously insufficient. Thus,for 1,there are still more investigations for us to explore,such as thermostability and magnetic studies.Notably,1 is stable in the solid state upon extended exposure to air,and has poor solubility in common organic solvents at room temperature,such as alcohol,acetonitrile,DMF,DMA and DMSO.
1 Experimental
1.1Materials and measurements
All the chemical regents were purchased commercially and were used as received without further purification.Elemental analyses for C,H,and N were performed on a Perkin-Elmer 240 analyzer.IR spectra were recorded as KBr pellets on a Nicolet Magna-FTIR 560 spectrometer in the 4 000~400 cm-1regions. The magnetic measurements were performed on the Quantum Design SQUID MPMS XL-7 instruments in a magnetic field of 1000 Oe in the temperature range of 2~300K.Thethermogravimetricanalyseswere investigated on a standard TG analyzer under a nitrogen flow at a heating rate of 5℃·min-1for all measurements.
1.2Preparation of complex 1
A mixture of Dy2O3(0.373 g,1.0 mmol),Hapca (0.278 g,2.0 mmol)in the molar ratio of 1∶2 was added into 8 mL of H2O/C2H5OH(5∶3,V/V).Consequently, the resulting solution was transferred and sealed in a 25 mL Teflon-lined stainless steel vessel,which was heated at 170℃for 60 h.After the reactor was slowly cooled to room temperature at a rate of 5℃·h-1,pure yellow block-shaped crystals were filtrated off,and dried in air.Yield:66%based on Dy.Elemental analysis Calcd.for C20H22Dy2N12O12(%):C 25.35,H 2.34,N 17.74;Found(%):C 25.71,H 2.17,N 17.21. IR(KBr,cm-1):3 469(s),3 402(vs),3 270(s),1 652(s), 1 602(vs),1 553(s),1 461(s),1 407(s),1 387(s),1 329 (m),1 233(m),1 179(s),922(m),831(s),706(m), 686(m),565(m),491(m).
1.3X-ray crystallography and structure determination
Accurate unit cell parameters of complex 1 were determined by a least-squares fit of 2θ values,and intensity data were measured on a Bruker SMART 1000 CCD diffractometer with Mo Kα radiation(λ= 0.071 073 nm)at 296 K.The intensity was corrected for Lorentz and polarization effects as well as for empirical absorption based on multi-scan technique. Empirical absorption corrections were applied using the SADABS program[11a]for the Bruker area detector. The structure was solved by direct method and refined by full-matrix least-squares fitting on F2by SHELX-97[11b-12].All non-hydrogen atoms were refined anisotropically,the hydrogen atoms of organic ligand were located by geometrically and those of water molecules were determined by different Fourier maps.Crystal data and structure refinement parameters for 1 are summarized in Table 1.Selected bond distances and angles are listed in Table 2.
CCDC:1438299.
2 Results and discussion
2.1Structural description of 1
Complex 1 crystallizes in the monoclinic space group P21/c.As shown in Fig.1a,the fundamental building unit is a dinuclear Dy(Ⅱ)structure.Both Dy1 and Dy2 sites are eight-coordinated by three oxygen atoms and two nitrogen atoms from three different apcaligands,oneoxygenatomfromterminalcoordinated water molecule and two oxygen atoms from the μ2-OH-groups.The polyhedron with donor atoms around the Dy1 is shown in Fig.1b,and Muetterties′approach can be used here to analyze the coordinate geometry of Dy(Ⅱ)ion[13].The data of δ and φ indicates the coordination polyhedron around the Dy(Ⅱ)ion can be attributed to a distorted trigonal dodecahedron.
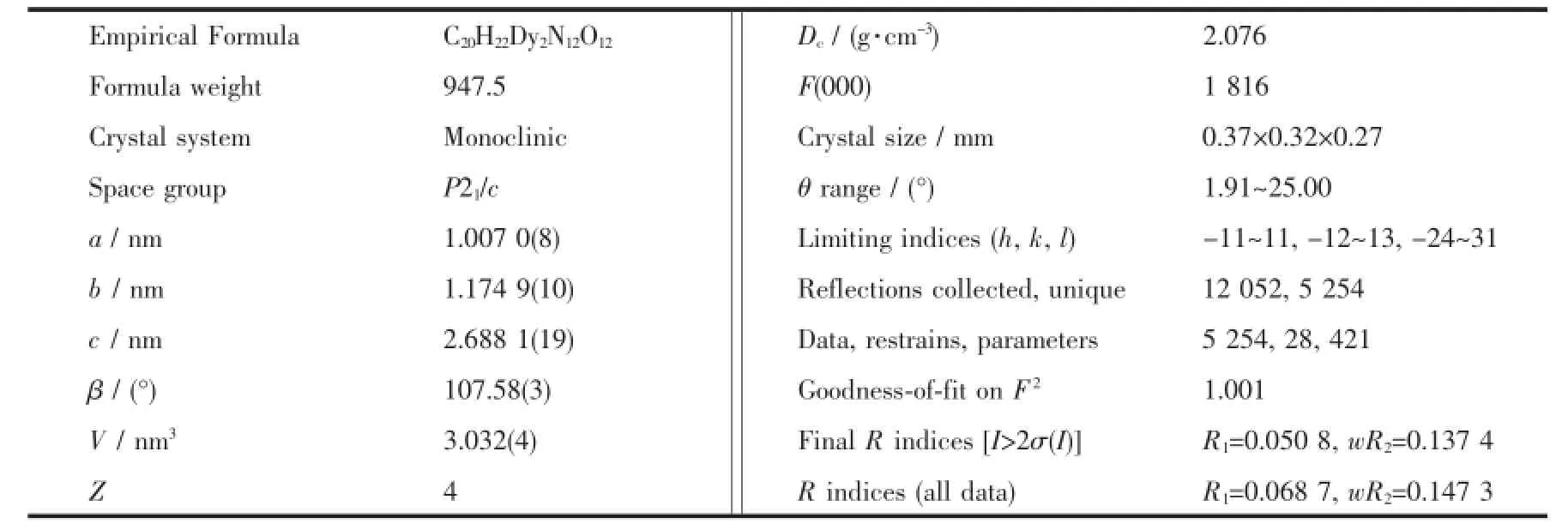
Table 1Crystallographic data for 1

Table 2Selected bond lengths(nm)and bond angles(°)for complex 1
Notably,this dinuclear Dy(Ⅱ)unit is highly rare as it is only bridged by two μ2-OH-bridges.The Dy-O-Dy angles and Dy…Dy distance is ca.110°and ca. 0.38 nm,respectively.Further,through the carboxyl oxygen atoms of apca anions these identical dinuclear fragments are combined together,generating a two-dimensional network(Fig.2).By contrast to the two reported quasi 2D array of lanthanide SMMs,the neighboring Dy2units in this 2D net are bridged by one apca carboxyl group with the span of ca.0.6 nm, which means that from the viewpoint of structure the present one is possibly a truly 2D array of lanthanide SMM.

Fig.1(a)Perspective view of the local coordination environment in 1;(b)Distorted trigonal dodecahedra coordination of the Dy1

Fig.2View of 2D network of complex 1
2.2Thermogravimetric analysis and PXRD
To examine the thermal stability of complex 1, thermogravimetric analyses(TGA)was carried out in the temperature range of 30~900℃(Fig.3a).From the first plateau we can conclude that complex 1 can be stable up to 280℃.Upon further heating,the structure begins to collapse,following the ligand molecules being released.The remaining residue is presumed to be Dy2O3(Calcd.39.37%;Found 38.61%).One strong exothermic peak at 321℃can be observed in the DSC curve,which corresponds to the breakdown of the framework,being in good agreement with the TGA results.

Fig.3(a)TGA curve of complex 1;(b)Experimental and simulated PXRD patterns of 1
The powder X-ray diffraction(PXRD)analysis hasalsobeencarriedoutforcomplex1.The measured PXRD pattern of 1 is in good agreement with the pattern simulated from the respective singlecrystal data,confirming the bulk purity of the samples (Fig.3b).
2.3Magnetic property
Temperature-dependentmagneticsusceptibility for 1 was measured in an applied magnetic field of 1 000 Oe in the temperature range of 2~300 K(Fig. 4).The χMT value at 300 K is 27.99 cm3·K·mol-1, which is in good agreement with the expected value of 28.34 cm3·K·mol-1for two noninteracting Dy(Ⅱ)ions(Dy(Ⅱ):S=5/2,L=5,6H15/2,g=4/3;C=14.17 cm3· K·mol-1)[14].Upon cooling,the χMT curve decreases slowly,reaching a minimum of 26.44 cm3·K·mol-1at about28K,whichismainlyascribedtothe progressive depopulation of excited Stark sublevels. Then increases sharply to a maximum value of 41.64 cm3·K·mol-1at 2 K,which obviously suggests the presenceofintramolecularstrongferromagnetic interactions between Dy(Ⅱ)spin carries as observed in other dinuclear systems[8c].

Fig.4Temperature dependence of χMT at 1 000 Oe for complex 1
The best Curie-Weiss law ftting of χM-1vs T from 300 to 2 K gives the Curie constant C=27.75 cm3·K· mol-1and the Weiss constant θ=-0.53 K.The negative Weiss constant as well as the decrease of χMT value withdecreasingtemperaturemaypresumablybe ascribed to the crystal field effect,the large magnetic anisotropy and the progressive thermal depopulation of the excited Stark sublevels of the Dy(Ⅱ)ions.
The M vs H/T(Fig.4,inset)data at different temperatures below 10 K show a rapid increase of the magnetization at low magnetic fields,which eventually reaches the value of 10.47Nβ at 2 K under 60 kOe without clear saturation.This is expected for materials having ferromagnetically coupled spins.The lack of a clear saturation on the M vs H/T data further confirms the effects arising from low-lying excited states and/or anisotropy.The value is lower than the expected saturation value of 20Nβ(10Nβ for each Dy(Ⅱ)ions) likely due to anisotropy and important crystal-field effects[14b]at the Dy(Ⅱ)ion that eliminates the 16-fold degeneracy of the6H15/2ground state[15].Indeed,The maximum of magnetization is in good agreement with the expected value(2×5.23Nβ)for two isolated Dy(Ⅱ)ions with a value of 5.23Nβ per Dy(Ⅱ)ion assuming the presence of considerable ligand-field effects[16]. The high-field variation and the non-superposition on a single master curve of the M vs H/T data suggest the presence of a significant magnetic anisotropy and/ or low-lying excited states in these systems.Moreover, the material of 1 has no clear hysteresis effect at 2 K withsweepratesusedinatraditionalSQUID magnetometer(Fig.5).

Fig.5M vs H data of complex 1 at 2.0 K showing the absence of hysteresis effect

Fig.6Frequency dependence of the in-phase(χ′,a)and out-of-phase(χ″,b)ac susceptibility from 2.0 to 3.4 K at an interval of 0.2 K and from 3.5 to 15 K at an interval of 0.5 K under zero-dc field for 1

Fig.7Temperature dependence under zero-dc field of the in-phase(χ′,a)and the out-of-phase(χ″,b) ac susceptibility component at different ac frequency for 1
The frequency and temperature dependencies of the alternating current(ac)susceptibility measurements were carried out for complex 1 under zero-dc field to investigate the dynamics of the magnetization (Fig.6 and 7),which show the strong frequency and temperature dependences.Both of them reveal the presence of slow relaxation of the magnetization, which are the typical features associated with SMM behavior.
The relaxation time at different temperatures was extracted by fitting the χ″acvs frequency curves[17](Fig. 7b).The magnetization relaxation rate(1/τ)follows the Arrhenius equation.Plotting the relaxation time versus the reciprocal temperature afforded the Arrhenius plot (Fig.8).This is the characteristic behavior for a thermally activated Orbach process.The theoretical equation for this process is given as:

Where Ueffis the effective anistropy energy barrier,kBis the Boltzmann constant,and T is the temperature. The red line in Fig.8a shows the result of a leastsquares fit of the ac susceptibility relaxation data to Eq.(1).The best fitting results gave the relaxation time τ0=9.47×10-7s and the energy barrier Ueff/kB=73.7 K. This value of energy barrier is relative higher than some other reported values for polynuclear SMMs[8b,18], exceeds that observed in the two reported quasi 2D array of lanthanon SMMs,and the relaxation time is consistent with the expected value covering 10-6~10-11s for a typical SMM[19].
Fromfrequencydependencesoftheac susceptibilities measurements,Cole-Cole plots(Fig. 8b)in the form of χ″vs χ′with nearly semi-circle shape have also been obtained.These data have been fitted to the generalized Debye model[20],giving the small distribution coefficient α value of 0.02~0.14(α= 0 for a Debye model between 6.0 and 13.5 K), indicating the narrow distribution of relaxation times at these temperatures.All these magnetic features support the SMM nature of this remarkable 2D complex.

Fig.8(a)Plot of natural logarithm of rate(1/τ)vs inverse of temperature(1/T)for complex 1 in the temperature range of 7.5~11.2 K;(b)Cole-Cole plots measured from 6.0 to 13.5 K at an interval of 0.5 K and zero-dc field
3 Conclusions
Inconclusion,basedon3-aminopyrazine-2-carboxylic acid,a 2D complex which consisted of dinuclearDy(Ⅱ)unitshasbeenpreparedand structurallyandmagneticallycharacterized.Ac susceptibilitymeasurementsrevealfrequencydependent signals,indicative of slow relaxation of magnetization and a relatively large energy barrier (73.7 K).It is confirmed that using highly anisotropic lanthanide building blocks is an effective approach for creating novel SMMs.And more detailed studies are currently carried out to probe further the properties of magnetization in lanthanide clusters with different topologies.
References:
[1](a)Sessoli R,Gatteschi D,Caneschi A,et al.Nature,1993, 365:141-143 (b)Woodruff D N,Winpenny R E P,Layfield R A.Chem. Rev.,2013,113:5110-5148
[2](a)Gavey E L,Hareri M A,Reqier J,et al.J.Mater.Chem.C, 2015,3:7738-7747 (b)Song Y M,Luo F,Luo M B,et al.Chem.Commun.,2012, 48:1006-1008 (c)Chen L,Wang J,Wei J M,et al.J.Am.Chem.Soc.,2014, 136:12213-12216 (d)Aulakh D,Pyser J B,Zhang X,et al.J.Am.Chem.Soc., 2015,137:9254-9257
[3](a)Xue S F,Zhao L,Guo Y N,et al.Chem.Commun.,2012, 48:8946-8948 (b)Blagg R J,Ungur L,Tuna F,et al.Nat.Chem.,2013,5: 673-678 (c)Zou H H,Wang R,Chen Z L.Dalton Trans.,2014,43: 2581-2587 (d)Gorczyński A,Kubicki M,Pinkowicz D,et al.Dalton Trans.,2014,44:16833-16839 (e)Cao W,Zhang Y H,Wang H L,et al.RSC Adv.,2015,5: 17732-17737
[4](a)Wernsdorfer W,Aliaga-Alcalde N,Hendrickson D N,et al. Nature,2002,416:406-409 (b)Long J,Habib F,Lin P H,et al.J.Am.Chem.Soc.,2011, 133:5319-5328 (c)Ren M,Bao S S,Hoshino N,et al.Chem.Eur.J.,2013, 19:9619-9628
[5]Jeon I,Clérac R.Dalton Trans.,2012,41:9569-9586
[6](a)Roubeau O,Clérac R.Eur.J.Inorg.Chem.,2008:4325-4342 (b)Miyasaka H,Nakata K,Lecren L,et al.J.Am.Chem. Soc.,2006,128:3770-3783 (c)Miyasaka H,Nakata K,Sugiuara K I,et al.Angew.Chem. Int.Ed.,2004,43:707-711
[7](a)Galloway K W,Schmidtmann M,Sanchez-Benitez J,et al. Dalton Trans.,2010,39:4727-4729 (b)Burzurí E,Campo J,Falvello L R,et al.Chem.Eur.J., 2011,17:2818-2822
[8](a)Chen M,Saudo E C,Jiménez E,et al.Inorg.Chem.,2014, 53:6708-6714 (b)Savard D,Lin P H,Burchell T J,et al.Inorg.Chem., 2009,48:11748-11754 (c)Lin P H,Burchell T J,Clérac R,et al.Angew.Chem.Int. Ed.,2008,47:8848-8851(d)Yi X H,Calvez G,Daiguebonne C,et al.Inorg.Chem., 2015,54:52135219
[9]Yue Y M,Yan P F,Sun J W,et al.Inorg.Chem.Commun., 2015,54:5-8
[10]Deng Z P,Kang W,Huo L H,et al.Dalton Trans.,2010,39: 6276-6284
[11](a)SheldrickGM.SADABS,University of Göttingen,Germany, 1996. (b)Sheldrick G M.SHELXL-97,Programs for X-ray Crystal Structure Refinement,University of Göttingen,Göttingen, Germany,1997.
[12]Sheldrick G M.SHELXS-97,Programs for X-ray Crystal StructureSolution,UniversityofGöttingen,Göttingen, Germany,1997.
[13](a)Muetterties E L,Guggenberger L J.J.Am.Chem.Soc., 1974,96:1748-1756 (b)Casanova D,Llunell M,Alemany P,et al.Chem.Eur.J., 2005,11:1479-1494 (c)Liu Q Y,Li Y L,Xiong W L,et al.CrystEngComm, 2014,16:585-590
[14](a)Xu G F,Wang Q L,Gamez P,et al.Chem.Commun., 2010,46:1506-1508 (b)Ma Y,Xu G F,Yang X,et al.Chem.Commun.,2010, 46:8264-8266
[15]Gao Y J,Xu G F,Zhao L,et al.Inorg.Chem.,2009,48: 11495-11497
[16]Zheng Y Z,Lan Y,Anson C E,et al.Inorg.Chem.,2008, 47:10813-10815
[17]Poneti G,Bernot K,Bogani L,et al.Chem.Commun.,2007: 1807-1809
[18]Guo Y N,Xu G F,Gamez P,et al.J.Am.Chem.Soc.,2010, 132:8538-8539
[19]Bernot K,Bogani L,Caneschi A,et al.J.Am.Chem.Soc., 2006,128:7947-7956
[20]Aubin S M J,Sun Z,Pardi L,et al.Inorg.Chem.,1999,38: 5329-5340
Single Molecule Magnet Behavior in a Two-Dimensional Array of Dysprosium(Ⅱ)Complex
YANG Yu-Ting*TU Chang-ZhengYAO Li-FengLI Jun-LiCHEN GuangCHENG Fei-Xiang
(College of Chemistry and Chemical Engineering,Qujing Normal University,Qujing,Yunnan 655011,China)
A two-dimensional(2D)network[Dy2(apca)4(OH)2(H2O)2]n(1),which consisted of dinuclear Dy2units, was gained by utilization of the rigid ligand 3-aminopyrazine-2-carboxylic acid(Hapca)through hydrothermal synthetic approach.Complex 1 displays ferromagnetic coupling and slow magnetic relaxation with τ0=9.47×10-7s and Ueff/kB=73.7 K,presenting the rare 2D array of lanthanide single molecule magnet(SMM).CCDC:1438299.
two-dimensional;dysprosium;dinuclear;single molecule magnet
O614.342
A
1001-4861(2016)08-1311-08
10.11862/CJIC.2016.185
2016-02-27。收修改稿日期:2016-06-22。
上海师范大学上海市稀土功能材料重点实验室和云南省科技厅(No.201401CB00299)资助项目。*通信联系人。E-mail:18288438002@163.com
