The effect of lowering target oxygen saturation on retinopathy of prematurity in neonatal respiratory distress syndrome
2016-11-09YouhyunLeeSanglakLeeYuCheolKim
Youhyun Lee, Sanglak Lee, Yu Cheol Kim
The effect of lowering target oxygen saturation on retinopathy of prematurity in neonatal respiratory distress syndrome
Youhyun Lee1, Sanglak Lee2, Yu Cheol Kim1
1Department of Ophthalmology, Dongsan Medical Center, Keimyung University School of Medicine, Daegu 700712, Republic of Korea
2Department of Pediatrics, Dongsan Medical Center, Keimyung University School of Medicine, Daegu 700712, Republic of Korea
方法:回顾性分析2009-01/2012-12期间确诊为呼吸窘迫综合症的早产儿(N=252)的医疗记录。根据氧饱和度范围将患者分为高饱和度组(90%~97%;n=116)和低饱和度组(85%~93%,n=136)。并对早产儿视网膜病变的严重程度及其相关参数进行评估。
结果:早产儿视网膜病变的发生率,激光治疗的需求和进展性早产儿视网膜病变比例两组无显著差异,但低饱和度组存活率较高(P=0.005)。早产儿患者在恒温箱内多护理1d患视网膜病变几率减少1.041倍(OR=1.041;95%CI=1.003~2.275;P=0.002),而5min内Apgar评分每多1分患病可能性降低24.7%(OR=0.753; 95%CI=0.152~0.965;P=0.033)。
结论:85%~93%的氧饱和度可提高早产儿存活率,且不影响早产儿视网膜病变伴随呼吸窘迫综合症的发生。相关风险因素与恒温箱内护理的持续时间和5min内Apgar评分的情况有关。
•AIM: To determine the effect of lowered target oxygen saturation levels on and identify risk factors of retinopathy of prematurity (ROP) in neonatal respiratory distress syndrome (RDS).
•METHODS: Medical records of consecutive preterm infants (N=252) diagnosed with RDS between Jan. 2009 and Dec. 2012 were retrospectively reviewed. The patients were divided into higher-saturation (90-97%;n=116) and lower-saturation (85-93%;n=136) groups according to their target oxygen saturation ranges. ROP severity was assessed and relevant systemic parameters were evaluated.
•RESULTS: Incidence of ROP, need for laser ablation, and proportion of aggressive posterior ROP were not significantly different between the groups, but the lower-saturation group had a better survival rate (P=0.005). Patients who spent an additional day in incubator care had a 1.041 times greater chance of developing ROP (odds ratio=1.041; 95%CI=1.003-2.275;P=0.002), whereas a one-point increase in the 5min Apgar score reduced the possibility by 24.7% (odds ratio=0.753; 95%CI=0.152-0.965;P=0.033).
•CONCLUSION: Target oxygen saturation levels of 85% to 93% may improve survival without affecting ROP development in infants with RDS. The relevant risk factors are duration of incubator care and 5-min Apgar score.
Apgar score; neonatal respiratory distress syndrome; oxygen; retinopathy of prematurity; survival rate
INTRODUCTION
Retinopathy of prematurity (ROP) is thought to be the main cause of ocular morbidity in premature infants[1]. It was first described in the 1940s by Terry, who associated retrolental fibroplasia with premature birth[2]. Thereafter, researchers showed that excessive use of oxygen contributes to ROP[3-4]. Reduced arterial oxygen saturation could decrease the risk of ROP development and need for surgery[5-6], but it can induce hypoxic damage; therefore, the optimal range is controversial[7-8]. Recent studies have recommended target oxygen saturation levels as low as 85% and showed decreased ROP incidence[9-12]. However, most of these studies were limited to infants with a specific gestational age or birth weight and considered respiratory distress syndrome (RDS) only a comorbidity.
The suggested risk factors of ROP includelow gestational age, low birth weight, sepsis, intraventricular hemorrhage (IVH), and number of blood transfusions[13-16]. However, a recent study showed that neonatal RDS and IVH are the only predictive factors of ROP[17]. Neonatal RDS is caused by developmental insufficiency of surfactant production and structural immaturity of the lungs, and it usually requires long-term oxygen therapy. No research on ROP development restricted to infants with RDS has been reported.
The aims of this study were to determine whether lowered target oxygen saturation levels affect ROP development and identify its risk factors in neonatal RDS.
SUBJECTS AND METHODS
Subjects Medical records of consecutive preterm infants (N=252) diagnosed with RDS and admitted to the Dongsan Medical Center neonatal intensive care unit (NICU) between Jan. 2009 and Dec. 2012 were retrospectively reviewed. Ethical approval was obtained from the Institutional Review Board of Keimyung University School of Medicine, Dongsan Medical Center, and the study adhered to the tenets of the Declaration of Helsinki.
The patients were divided into two groups according to their target oxygen saturation ranges. The higher-saturation group included infants admitted between Jan. 2009 and Dec. 2010 and treated with the target range of 90% to 97%. Infants admitted between Jan. 2011 and Dec. 2012 and treated with the target range of 85% to 93% were classified into the lower-saturation group.
ROP was categorizedon the basis of the revised International Classification of Retinopathy of Prematurity[18]. Aggressive posterior ROP (AP-ROP) was diagnosed according to its posterior location, prominence of plus disease, and rapid progression of retinopathy. The first screening examination was executed at the postmenstrual age of 31 weeks and followed the guidelines proposed by the American Academy of Ophthalmology and Pediatrics and the Association for Pediatric Ophthalmology and Strabismus with some modifications[19]. The treatment was based on the early treatment for ROP study[20].
Data CollectionFundoscopic examinations were performed until the development of type Ⅰ ROP or recognition of full retinal vascularization using a binocular indirect ophthalmoscope with +20D lens. If the eyes showed asymmetric ROP severity, data from the worse eye were included in the analyses. In cases of zone I ROP and posterior zone II ROP, bevacizumab (0.625 mg, 0.025 ml solution) was injected through the pars plicata using a 30G needle at the end of laser treatment.
The following systemic parameters were evaluated: gestational age, birth weight, durations of total oxygen therapy, mechanical ventilation and continuous positive airway pressure, incubator care, and total parenteral nutrition; 1min and 5min Apgar scores, numbers of surfactant administrations, transfusions, and patent ductus arteriosus ligations, incidences of bronchopulmonary dysplasia, sepsis, necrotizing enterocolitis, and IVH, and survival rate.
Statistical AnalysisThe independent two-samplest-test, Chi-square test, or Mann-WhitneyU-test was used for intergroup comparisons. Multiple logistic regression analyses were performed to identify parameters associated with ROP development in neonatal RDS. Variables were further analyzed by forward conditional stepwise selection, and the risk ratio of ROP development was evaluated.P<0.05 were considered significant. All statistical analyses were performed in SPSS for Windows (version 19.0, IBM, Armonk, NY, USA).
RESULTS
The higher-saturation and lower-saturation groups comprised 116 and 136 infants, respectively (Table 1). They did not show significant differences in RDS severity and mean gestational age. The lower-saturation group had lower birth weight and longer durations of total oxygen therapy and mechanical ventilation, but these differences were also not significant. However, the mean duration of total parenteral nutrition was significantly longer (P=0.038) and survival rate was significantly better (P=0.005) in this group. None of the remaining parameters showed significant intergroup differences.
Twenty-nine of 64 infants who developed ROP were treated for type 1 disease (Table 2). Laser ablation was performed in 48.3% of the infants in the higher-saturation group and 42.4% of those in the lower-saturation group. Intravitreal bevacizumab was administered in 22.6% and 24.2% of the infants in the respective groups. The proportion of AP-ROP was not significantly different between the groups.
No significant differences in systemic parameters were noted between the infants treated by laser ablation only and those who underwent laser ablation with intravitreal bevacizumab injection (Table 3). However, an infant who had stage 3 with plus disease in zone I developed subtotal retinal detachment after laser ablation alone and was retreated by vitrectomy.
Multiple logistic regression analysesdid not reveal systemic parameters significantly related to ROP development (Table 4). However, by selecting variables using the forward conditional stepwise method, duration of incubator care and 5-min Apgar score were identified as risk factors (Table 5). Infants who spent an additional day in incubator care had 1.041 times greater chance of developing ROP, whereas a one-point increase in the 5min Apgar score resulted in 24.7% lower possibility of developing ROP. This trend was also observed in the case of type I ROP (Table 6).
DISCUSSION
ROP is a potentially preventable cause of blindness in children[21], and RDS is a common disease in the neonatal intensive care unit necessitating prolonged mechanical ventilation and systemic characteristics and oxygen therapy. One previous study showed that neonatal RDS is a predictive factor for ROP development. Supporting this finding, 29 of 40 neonates who underwent laser ablation from 2009 to 2012 were diagnosed with RDS in our clinics.We investigated ROP changes among preterm infants with RDS who were treated with the recently modified target oxygen saturation levels (85-93%): the lowered range was associated with a significantly better survival rate. The finding may be explained by the low percentage of comorbidities (IVH or pulmonary hemorrhage) in these infants compared with those who received the 90-97% target range[22]. However, our study showed no differences in ROP development and incidence of type I ROP between the target ranges, unlike the finding of the SUPPORT study that severe ROP is less frequent among survivors in the lower-saturation group[13]. The reasons for these results are that birth weight (>1000g mostly), target oxygen saturation range (85-93%vs. 85-89%), and definition of ROP severity (type I ROPvs. presence of threshold retinopathy, need for surgical intervention, or use of bevacizumab) were different from the previous study. We did not find significant differences in systemic parameters according to the treatment methods. One recent study showed that infants with shorter duration of total parenteral nutrition are prone to develop ROP because total parenteral nutrition affects serum levels of insulin-like growth factor-1 and insulin-like growth factor-binding protein 3[23]. Although the infants in the lower-saturation group had a longer duration of total parenteral nutrition, it was not a protective factor in our study. Further, neither gestational age nor birth weight was identified as a risk factor. The discrepancy from the previous reports is because the infants in our study had a higher gestational age and birth weight[9,15].
Table 1Intergroup comparison of demographic
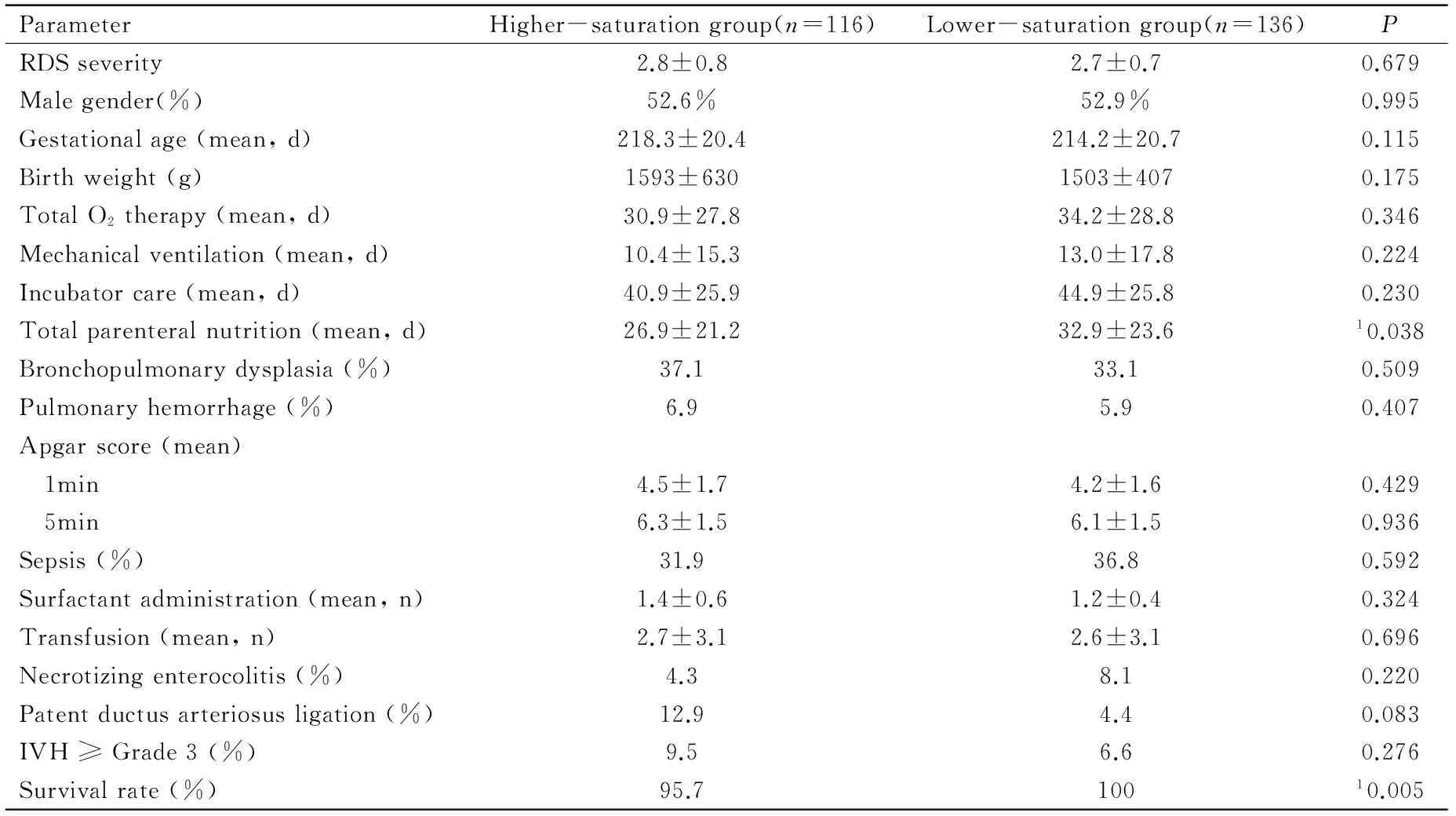
ParameterHigher-saturationgroup(n=116)Lower-saturationgroup(n=136)PRDSseverity2.8±0.82.7±0.70.679Malegender(%)52.6%52.9%0.995Gestationalage(mean,d)218.3±20.4214.2±20.70.115Birthweight(g)1593±6301503±4070.175TotalO2therapy(mean,d)30.9±27.834.2±28.80.346Mechanicalventilation(mean,d)10.4±15.313.0±17.80.224Incubatorcare(mean,d)40.9±25.944.9±25.80.230Totalparenteralnutrition(mean,d)26.9±21.232.9±23.610.038Bronchopulmonarydysplasia(%)37.133.10.509Pulmonaryhemorrhage(%)6.95.90.407Apgarscore(mean) 1min4.5±1.74.2±1.60.429 5min6.3±1.56.1±1.50.936Sepsis(%)31.936.80.592Surfactantadministration(mean,n)1.4±0.61.2±0.40.324Transfusion(mean,n)2.7±3.12.6±3.10.696Necrotizingenterocolitis(%)4.38.10.220Patentductusarteriosusligation(%)12.94.40.083IVH≥Grade3(%)9.56.60.276Survivalrate(%)95.710010.005
RDS:Respiratory distress syndrome; IVH: Intraventricular hemorrhage;1Significant difference by independent two-samplest-test.
Table 2Characteristics of ROP in the study groups

ParameterHigher-saturationgroup(n=31)Lower-saturationgroup(n=33)PLaserablation(%)48.342.40.601Stage0.4891(%)48.451.52(%)29.030.33(%)22.618.2Simultaneousintravitrealbevacizumabinjection(%)22.624.20.690AP-ROP(%)9.76.10.560
AP-ROP:Aggressive posterior retinopathy of prematurity.
Table 3Characteristics of the patients treated for ROP
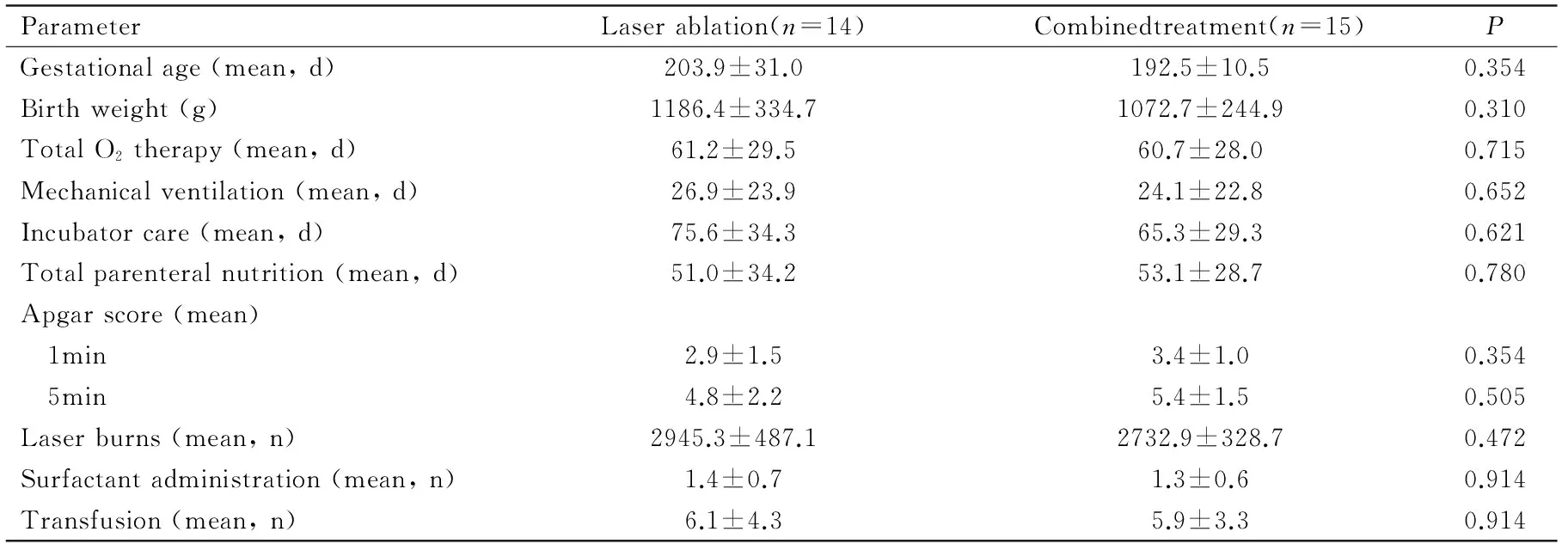
ParameterLaserablation(n=14)Combinedtreatment(n=15)PGestationalage(mean,d)203.9±31.0192.5±10.50.354Birthweight(g)1186.4±334.71072.7±244.90.310TotalO2therapy(mean,d)61.2±29.560.7±28.00.715Mechanicalventilation(mean,d)26.9±23.924.1±22.80.652Incubatorcare(mean,d)75.6±34.365.3±29.30.621Totalparenteralnutrition(mean,d)51.0±34.253.1±28.70.780Apgarscore(mean) 1min2.9±1.53.4±1.00.354 5min4.8±2.25.4±1.50.505Laserburns(mean,n)2945.3±487.12732.9±328.70.472Surfactantadministration(mean,n)1.4±0.71.3±0.60.914Transfusion(mean,n)6.1±4.35.9±3.30.914
ROP: Retinopathy of prematurity.
Table 4Results of multiple logistic regression analyses

RiskfactorPOR(95%CI)DurationoftotalO2therapy0.5551.009(0.792-2.231)Durationofmechanicalventilation0.7550.995(0.280-3.149)Durationoftotalparenteralnutrition0.9120.998(0.277-3.152)Durationofincubatorcare0.2431.023(0.872-2.891)Numberofsurfactantadministrations0.4370.731(0.382-2.152)Apgarscore(5min)0.0770.778(0.302-2.181)NumberofPDAligations0.6710.828(0.157-4.337)Gestationalage0.4140.989(0.287-3.097)Birthweight0.3620.999(0.275-3.176)
OR: Odds ratio;CI: Confidence interval; ROP: Retinopathy of prematurity; PDA: Patent ductus arteriosus.
Table 5Results of forward stepwise selection logistic regression analyses for development of ROP
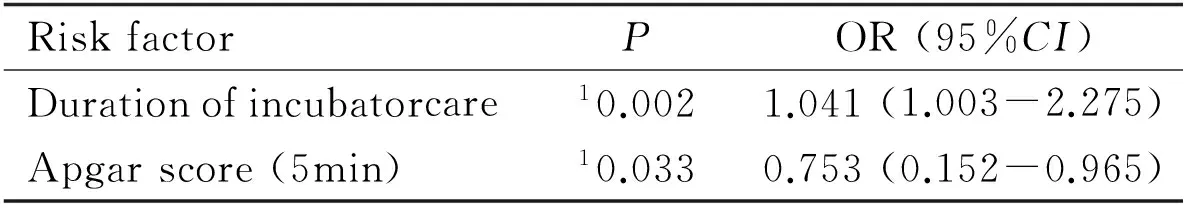
RiskfactorPOR(95%CI)Durationofincubatorcare10.0021.041(1.003-2.275)Apgarscore(5min)10.0330.753(0.152-0.965)
OR: Odds ratio; CI: Confidence interval; ROP: Retinopathy of prematurity;1Significant.
Table 6Results of forward stepwise selection logistic regression analyses for type 1 ROP development

RiskfactorPOR(95%CI)Durationofincubatorcare10.0011.276(1.031-3.121)Apgarscore(5min)10.0450.833(0.188-0.937)
OR: Odds ratio; CI: Confidence interval; ROP: Retinopathy of prematurity;1Significant.
Surfactant therapy, mechanical ventilation, and continuous positive airway pressure are considered the mainstay treatments of neonatal RDS. New protocols are continuously reported and show promising results, including decreased risk of acute pulmonary injury, neonatal mortality, and chronic lung disease[24]. All the infants in our study received surfactant treatment: although the lower-saturation group showed decreased mortality, there was no significant difference in surfactant administration between the groups, and number of surfactant treatments was not a risk factor of ROP.
A 1min Apgar score lower than 6 is related to a high risk of ROP development and a low 5min Apgar score indicates greater risk of mortality[25-26]. We found that the infants with high 5min Apgar scores have a reduced risk of developing ROP and type I ROP.
Incubator care is required for thermal stability of sick infants with low birth weight. Longer stays usually result from poor lung maturity and general condition. Our study showed that longer duration of incubator care is related to the development of ROP and type I ROP. An extra day in the incubator increases the likelihood of developing the condition by 1.041 times. Considering that durations of total oxygen therapy and mechanical ventilation are not risk factors of ROP, this result is likely multifactorial, including environmental effects that are not yet described.
Other controversial risk factors, such as blood transfusion and sepsis, did not affect ROP development.
A limitation of this study is the overlapping of target oxygen saturation levels (90-93%) between the groups. Further study with a larger sample is needed to eliminate this confounding factor.
In conclusion, target oxygen saturationlevels of 85% to 93% improve survival without affecting ROP development in neonatal RDS. In infants with RDS, duration of incubator care and 5min Apgar score are risk factors of ROP and type Ⅰ ROP. Duration of incubator care can be somewhat shortened by better control of nutrition, sepsis, temperature, and other modifiable factors in these infants.
1 Purohit DM, Ellison RC, Zierler S, Miettinen OS, Nadas AS. Risk factors for retrolental fibroplasia: experience with 3025 premature infants. National Collaborative Study on Patent Ductus Arteriosus in Premature Infants.Pediatrics1985;76(3):339-344
2 Terry TL. Retrolental fibroplasia in the premature infant: V. Further studies on fibroplastic overgrowth of the persistent tunica vasculosa lentis.TransAmOphthalmolSoc1944;42:383-396
3 Kinsey VE, Arnold HJ, Kalina RE, Stern L, Stahlman M, Odell G, Driscoll JM Jr, Elliott JH, Payne J, Patz A. PaO2levels and retrolental fibroplasia: a report of the cooperative study.Pediatrics1977;60(5):655-668
4 Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations.AmJOphthalmol1952;35(9):1248-1253
5 Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity.TransAmOphthalmolSoc2006;104:78-84
6 Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants ≤1250 g is associated with improved short- and long-term outcomes.JPerinatol2006;26(11):700-705
7 Castillo A, Sola A, Baquero H, Neira F, Alvis R, Deulofeut R, Critz A. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: is 85% to 93% an acceptable range?Pediatrics2008;121(5):882-889
8 SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network; Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, Schibler K, Newman NS, Ambalavanan N, Frantz ID 3rd, Piazza AJ, Sanchez PJ, Morris BH, Laroia N, Phelps DL, Poindexter BB, Cotten CM, Van Meurs KP, Duara S, Narendran V, Sood BG, O’Shea TM, Bell EF, Ehrenkranz RA, Watterberg KL, Higgins RD. Target ranges of oxygen saturation in extremely preterm infants.NEnglJMed2010;362(21):1959-1969
9 Tlucek PS, Corff KE, Bright BC, Bedwell SM, Sekar KC, Siatkowski RM. Effect of decreasing target oxygen saturation on retinopathy of prematurity.JAAPOS2010;14(5):406-411
10 Tarnow-Mordi WO, Darlow B, Doyle L. Target ranges of oxygen saturation in extremely preterm infants.NEnglJMed2010;363(13):1285-1286
11 Bateman D, Polin RA. A lower oxygen-saturation target decreases retinopathy of prematurity but increases mortality in premature infants.JPediatr2013;163(5):1528-1529
12 BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group; Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, Battin M, Bowler U, Broadbent R, Cairns P, Davis PG, Deshpande S, Donoghoe M, Doyle L, Fleck BW, Ghadge A, Hague W, Halliday HL, Hewson M, King A, Kirby A, Marlow N, Meyer M, Morley C, Simmer K, Tin W, Wardle SP, Brocklehurst P. Oxygen saturation and outcomes in preterm infants.NEnglJMed2013;368(22):2094-2104
13 Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity.PaediatrPerinatEpidemiol2004;18(2):130-134
14 Gupta VP, Dhaliwal U, Sharma R, Gupta P, Rohatgi J. Retinopathy of prematurity-risk factors.IndianJPediatr2004;71(10):887-892
15 Yau GS, Lee JW, Tam VT, Liu CC, Chu BC, Yuen CY. Incidence and risk factors for retinopathy of prematurity in extreme low birth weight Chinese infants.IntOphthalmol2015;35(3):365-373
16 Englert JA, Saunders RA, Purohit D, Hulsey TC, Ebeling M. The effect of anemia on retinopathy of prematurity in extremely low birth weight infants.JPerinatol2001;21(1):21-26
17 Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States.BrJOphthalmol2008;92(3):320-325
18 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited.ArchOphthalmol2005;123(7):991-999
19 Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity.Pediatrics2006;117(2):572-576
20 Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial.TransAmOphthalmolSoc2004;102:233-250
21 Clemett R, Darlow B. Results of screening low-birth-weight infants for retinopathy of prematurity.CurrOpinOphthalmol1999;10(3):155-163
22 Tomaszewska M, Stork E, Minich NM, Friedman H, Berlin S, Hack M. Pulmonary hemorrhage: clinical course and outcomes among very low-birth-weight infants.ArchPediatrAdolescMed1999;153(7):715-721
23 Can E, Bülbül A, Uslu S, Cömert S, Bolat F, Nuhoglu A. Effects of aggressive parenteral nutrition on growth and clinical outcome in preterm infants.PediatrInt2012;54(6):869-874
24 Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome.CochraneDatabaseSystRev2012;11:CD001456
25 Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore.AnnAcadMedSingapore2005;34(2):169-178
26 Phalen AG, Kirkby S, Dysart K. The 5-minute Apgar score: survival and short-term outcomes in extremely low-birth-weight infants.JPerinatNeonatalNurs2012;26(2):166-171
降低早产儿视网膜病变的氧饱和度对新生儿呼吸窘迫综合征的影响
Youhyun Lee1, Sanglak Lee2, Yu Cheol Kim1
700712韩国大邱启明大学医学院东山医院1眼科;2儿科)
Yu Cheol Kim. 韩国大邱启明大学医学院东山医院眼科. eyedr@dsmc.or.kr
目的:研究降低早产儿视网膜病变(ROP)的氧饱和度及其危险因素对呼吸窘迫综合症(RDS)的影响。
Apgar评分;新生儿呼吸窘迫综合症;氧;早产儿视网膜病变;存活率
Yu Cheol Kim. Department of Ophthalmology, Dongsan Medical Center, Keimyung University School of Medicine, Daegu 700712, Republic of Korea. eyedr@dsmc.or.kr
2015-02-03Accepted: 2016-09-07
10.3980/j.issn.1672-5123.2016.11.03
Lee YH, Lee SL, Kim YC. The effect of lowering target oxygen saturation on retinopathy of prematurity in neonatal respiratory distress syndrome.GuojiYankeZazhi(IntEyeSci) 2016;16(11):1992-1996
引用:Lee YH, Lee SL, Kim YC. 降低早产儿视网膜病变的氧饱和度对新生儿呼吸窘迫综合征的影响.国际眼科杂志2016;16(11):1992-1996
