结肠癌转移相关因子-1和间质-上皮细胞转化因子检测在肺腺癌患者并发恶性胸腔积液中的诊断价值
2016-11-03尤青海孙耕耘
胡 婕 尤青海 孙耕耘
·论著·
结肠癌转移相关因子-1和间质-上皮细胞转化因子检测在肺腺癌患者并发恶性胸腔积液中的诊断价值
胡婕尤青海孙耕耘
目的探讨结肠癌转移相关因子-1(MACC1)、间质-上皮细胞转化因子(c-MET)联合癌胚抗原(CEA)在肺腺癌患者并发恶性胸腔积液中的诊断价值。方法将91例胸腔积液患者(良性38例,肺腺癌53例)纳入研究,采用 ELISA 法测定血清和胸腔积液中MACC1、c-MET浓度,放射免疫法测定CEA浓度。结果肺腺癌患者恶性胸腔积液中MACC1、c-MET和CEA浓度明显高于良性患者胸腔积液中的含量(P<0.05);两组患者血清中MACC1和c-MET浓度无统计学差异(P>0.05)。胸腔积液中MACC1和c-MET含量呈正相关(r=0.728,P<0.01)。根据ROC曲线,以MACC1浓度90.98 pg/ml为临界值, 对肺腺癌患者并发恶性胸腔积液的诊断灵敏度为62.26%,特异度为84.21%;c-MET浓度757.67 ng/ml为临界值,肺腺癌患者恶性胸腔积液的诊断灵敏度为52.83%,特异度为84.21%。MACC1联合c-MET检测灵敏度为75.47%,特异度为92.11%;MACC1、c-MET联合CEA诊断的灵敏度为98.10%,特异度为100%。 结论MACC1和c-MET在肺腺癌患者并发恶性胸腔积液的诊断中具有一定价值,联合CEA检测可明显提高诊断的灵敏度和特异度。
肺腺癌;结肠癌转移相关因子-1;间质-上皮细胞转化因子;癌胚抗原;恶性胸腔积液
恶性胸腔积液(malignant pleural effusions, MPE)因恶性肿瘤侵犯胸膜引起,其中以肺腺癌转移后胸腔积液形成最常见,MPE的产生严重影响患者的生存质量,且提示预后不良[1]。目前临床对于肺腺癌并发MPE的诊断仅有癌胚抗原(carcino embryonie antigen, CEA)是其有效的标志物,因而有必要探讨在肺腺癌并发MPE诊断中有价值的新型标志物。结肠癌转移相关因子-1(metastasis-associated in colon cancer-1, MACC1)是近几年发现的一个与结肠癌转移有着密切关系的因子,通过对间质-上皮转化因子(mesenchymal-epithelial transition factor, c-MET)介导的信号通路的异常调控作用,发挥促进肿瘤的发生、侵袭和转移的作用[2]。本研究通过检测血清和胸腔积液中MACC1、c-MET含量,以探讨两者在肺腺癌并发MPE中的诊断价值。
对象与方法
一、研究对象
选取安徽医科大学第一附属医院2014年11月至2015年7月呼吸内科住院患者,恶性组53例,男31例,女22例,年龄40~90(62.83±13.61)岁,均为肺腺癌并发MPE(胸水脱落细胞学查见腺癌细胞);良性组共38例,男30例,女8例,年龄16~84(49.82±22.23)岁,其中肺结核19例,肺炎13例,心力衰竭2例,肝硬化1例,病因不明漏出液3例。
二、研究方法
1. 标本采集: 均在患者治疗前采集,抽取晨起空腹静脉血5 ml,留取首次胸腔穿刺积液10 ml,以离心半径8 cm在常温下3 000 r/min 离心10 min,吸取上清液0.5 ml,分装后放置-20 ℃低温冷冻保存,期间避免反复冻融。
2. 标本检测 :血清及胸水CEA含量由安徽医科大学第一附属医院检验科采用放射免疫法测定。MACC1和c-MET含量应用ELISA试剂盒(批号:MACC1试剂盒为E-14431,c-MET试剂盒为E-12829,由合肥欣乐生物有限公司提供),操作严格按试剂盒说明书进行。
三、统计学方法
用SPSS20.0统计软件进行分析,采用Kolmogorov-Smirnov test确定所得数据是否符合正态分布,所有数据用表示。两组变量的比较采用t检验,相关性分析用Pearson相关分析,判断敏感度及特异度采用受试者工作曲线(ROC曲线),以P<0.05为差异有统计学意义。
结 果
一、胸腔积液和血清中MACC1、c-MET和CEA含量测定结果比较
肺腺癌组MACC1、c-MET、CEA浓度均明显高于良性组(P<0.05)。两组血清CEA浓度差异有统计学意义(P<0.01),恶性组明显高于良性组。血清中MACC1和c-MET浓度肺腺癌组高于良性组,但差异无统计学意义(P>0.05)。根据Pearson相关分析得知胸腔积液中MACC1和c-MET含量呈正相关(r=0.728,P<0.01),见表1。在恶性组胸腔积液CEA低于诊断临界值的4例样本中,有3例的MACC1高于诊断临界值。


项 目良性(n=38)恶性(n=53)P值t值胸水 MACC172.53±20.93105.49±47.64<0.053.993 c⁃MET603.44±156.93797.03±270.84<0.054.294 CEA1.46±1.08539.68±275.00<0.0512.05血清 MACC1111.86±33.86127.99±83.700.337-0.97 c⁃MET795.48±184.65842.78±388.860.295-1.06 CEA2.14±1.26115.33±79.190.002-3.39
注:MACC1:结肠癌转移相关因子-1;c-Met:间质-上皮细胞转化因子; CEA:癌胚抗原
二、不同因素与肺腺癌并发恶性胸腔积液中MACC1、c-MET和CEA浓度变化的相关性
不同性别、年龄(以60岁分界)、是否吸烟、有无淋巴结和远处转移的肺腺癌并发恶性胸腔积液中MACC1、c-MET及CEA含量差异无统计学意义(P>0.05),见表2。

表2 53例恶性胸腔积液MACC1、c-MET的浓度与其临床特征的关系±s)
注:MACC1:结肠癌转移相关因子-1;c-Met:间质-上皮细胞转化因子
三、MACC1、c-MET和CEA的诊断价值
应用ROC曲线分析实验所得数据,约登指数最大值对应的切点为诊断的最佳临界点。胸腔积液MACC1浓度90.98 pg/ml为最佳临界点(灵敏度62.26%,特异度84.21%,曲线下面积AUC为0.787);胸腔积液c-MET浓度757.67 ng/ml为最佳临界值(灵敏度为52.83%,特异度为84.21%,AUC为0.728);CEA浓度1.95 ng/ml为最佳临界值(灵敏度为92.45%,特异度为92.11%,AUC为0.953)。见图1,表3。联合检测:MACC1和c-MET联合灵敏度为75.47%,特异度为92.11%;MACC1和c-MET联合CEA灵敏度为98.10%,并联特异度为97.37%,见表4。

表3 MACC1、c-MET及CEA单项对肺腺癌并发恶性胸腔积液的诊断效能(%)
注:MACC1:结肠癌转移相关因子-1;c-MET:间质-上皮细胞转化因子;CEA:癌胚抗原

表4 MACC1、c-MET及CEA联合对肺腺癌并发恶性胸腔积液的诊断效能(%)
注:MACC1:结肠癌转移相关因子-1;c-MET:间质-上皮细胞转化因子;CEA:癌胚抗原
讨 论
MACC1是位于人染色体7p21.1上的MACC1基因编码的蛋白质,由Stein等[3]在2009年首次报道,并发现其是HGF(肝细胞生长因子)/c-MET信号通路的重要调节因子,对结肠癌的转移和生长起到重要调控作用。MACC1在多种实体肿瘤组织中有高表达,如肝癌,乳腺癌,直肠癌,肺癌等[4-7]。Chundong等[8]通过检测197例肺癌手术患者的癌组织中的MACCl表达水平,随访发现复发组MACC1表达水平明显高于未复发组(82.50%vs. 65.50%),提示MACC1对肺癌转移起到调控作用。临床研究发现MACC1mRNA和蛋白在非小细胞肺癌组织中高表达并提示预后不良,MACC1mRNA在非小细胞肺癌外周血中亦有高表达,在发生淋巴结转移患者中水平更高[9-10]。故MACC1水平的检测在肺癌的诊断中可能有一定帮助。
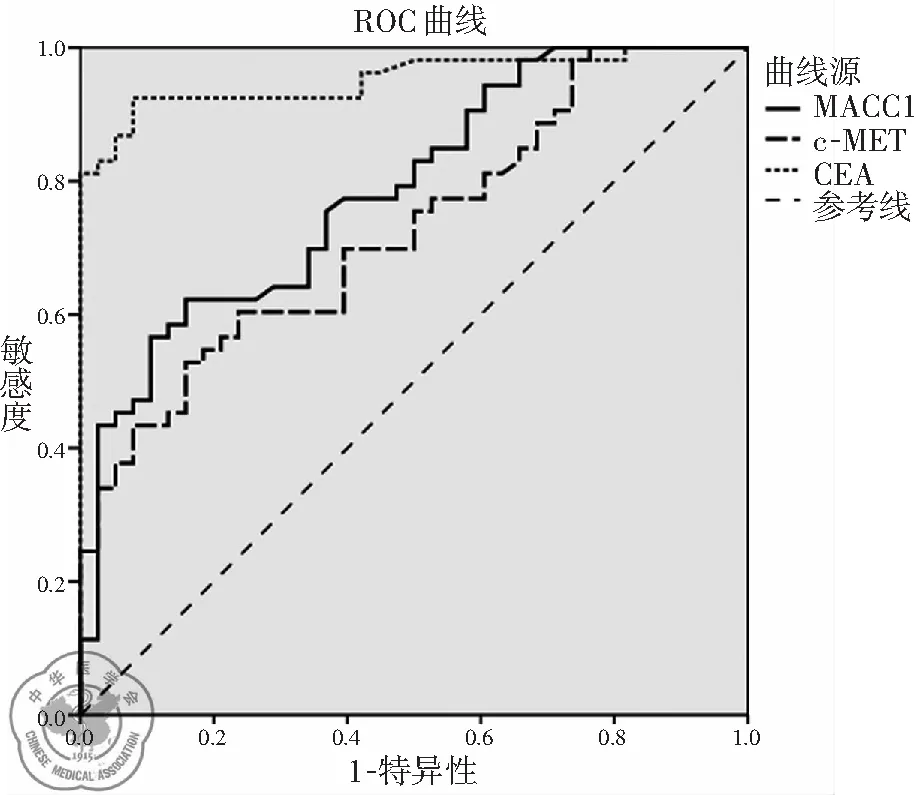
图1胸腔积液MACC1、c-MET和CEA的ROC曲线
c-MET是由原癌基因c-met编码的HGF的细胞膜特异性受体,主要在各种上皮细胞中表达[11]。HGF/c-MET信号通路主要调节细胞的增殖、分化、收缩、运动及分裂等多种生物学行为。该通路在癌细胞中的调控机制不同于正常细胞。研究发现c-MET介导的异常信号转导在多种肿瘤包括肺癌中起着重要作用,HGF依赖的c-MET信号通路活化胞质内多种信号通路,如PI3K /AKT、Ras-Rac/Rho、MAPK及Stat3信号通路等,从而介导肿瘤发生、侵袭和转移、血管新生、上皮-间质转化等过程[12-13]。有研究分析发现,c-MET在非小细胞肺癌组织中约61%呈高表达,其中约67%为肺腺癌[14]。也有多项研究发现,抑制c-MET表达后肺癌细胞的增殖和侵袭能力下降,从而证实c-MET与肺癌细胞侵袭生长紧密相关[15-16]。目前c-MET已被认为是非小细胞肺癌治疗的有效靶点,并有其针对性的靶向药物出现[17-18]。
MACC1在转录水平上增强c-MET的表达,两者结合后通过扩增或变异的方式激活下游传导通路,而不依赖与HGF的结合,因此MACC1作为诱导转移的HGF/c-MET通路的重要调节因子也发挥促进肿瘤细胞增殖、侵袭和转移的作用[19]。本研究结果显示胸腔积液MACC1和c-MET含量呈正相关,亦提示MACC1和c-MET间的紧密联系。最近由Kokostynska等[20]通过对345例患者胸腔积液中c-MET的含量分析发现,非小细胞肺癌组MPE的c-MET较良性组明显高表达,其灵敏度为75.2%,特异度为89.9%,与本研究结果较为相似。目前尚未见有文献报道MACC1在肺癌患者外周血及恶性胸腔积液中的表达水平。本研究结果提示肺腺癌并发恶性胸腔积液中MACC1的表达水平明显高于良性胸腔积液,其灵敏度为62.26%,特异度为84.21%,在肺腺癌并发恶性胸腔积液的诊断中有一定价值。
CEA是目前广泛应用于临床的肿瘤标志物,它是一种外分泌型的糖蛋白,最早发现于人的结肠癌组织中[21-23]。研究证实,CEA主要来源于肿瘤组织,在正常组织和良性病变组织中仅有少量合成和分泌[24-26]。在各型肺癌中,其对肺腺癌的诊断价值最高。本研究结果与既往研究结果一致,CEA在肺腺癌并发恶性胸腔积液中具有较高诊断价值。
本研究结果提示胸腔积液中的MACC1及c-MET在肺腺癌并发恶性胸腔积液中均有一定诊断价值,两者在肺腺癌并发恶性胸腔积液患者血清中浓度较良性组高,但其差异无统计学意义,可能与MACC1和c-MET的组织起源和表达强度有关,也可能与样本量、误差及偏倚等因素有关,需要扩大样本量等进一步研究。根据统计分析MACC1联合c-MET,MACC1、c-MET联合CEA可提高诊断的灵敏度和特异度。本研究同时发现在恶性组胸腔积液CEA低于诊断临界值的4例样本中,有3例的MACC1高于诊断临界值,故对于MACC1能否在CEA发生漏诊时作为补充诊断的假设尚有待扩大样本量进一步探讨。
综上所述,MACC1和c-MET对肺腺癌并发恶性胸腔积液的诊断具有一定价值,两者联合CEA可明显提高诊断的灵敏度和特异度。本实验因时间和地点限制具有其局限性:①MPE病理类型只收集了肺腺癌;②样本量较小;③无随访,未能分析肺腺癌患者治疗前后MACC1及c-MET水平的变化及与患者总生存期有无相关性。故对于MACC1及c-MET能否作为肺腺癌患者并发恶性胸腔积液的诊断指标应用于临床,还有待进一步研究。
1张燕, 孙耕耘. 恶性胸腔积液的临床诊断及治疗进展[J/CD]. 中华肺部疾病杂志: 电子版, 2013, 6(1): 81-84.
2William WN Jr, Lin HY, Lee JJ, et al. Revisiting stage ⅢB and Ⅳ non-small cell lung cancer: analysis of the surveillance, epidemiology and end results data[J]. Chest, 2009, 136(3): 701-709.
3Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling,predicts colon cancer metastasis[J]. Nat Med, 2009, 15(1): 59-67.
4Qiu J, Huang P, Liu Q, et al. Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma[J]. J Transl Med, 2011, 9: 166.
5Huang Y, Zhang H, Cai J, et al. Overexpression of MACC1 and its significance in human breast cancer progression[J]. Cell Biosci, 2013, 3(1): 16.
6Kawamura M, Saigusa S, Toiyama Y, et al. Correlation of MACC1 and MET expression in rectal cancer after neoadjuvant chemoradiotherapy[J]. Anticancer Res, 2012, 32(4): 1527-1531.
7Hu X, Fu X, Wen S, et al. Prognostic value of MACC1 and c-met expressions in non-small cell lung cancer[J]. Chin J Lung Cancer, 2012, 15(7): 399-403.
8Chundong G, Uramoto H, Onitsuka T, et al. Molecular diagnosis of MACCl status in lung adenocarcinoma by immunohistochemical analysis[J]. Anticancer Res, 2011, 31(4): 1141-1145.
9Wang Z, Li Z, Wu C, et al. MACC1 overexpression predicts a poor prognosis for non-small cell lung cancer[J]. Med Oncol, 2014, 31(1): 790.
10Wang Z, Cai M, Weng Y, et al. Circulating MACC1 as a novel diagnostic and prognostic biomarker for non small cell lung cancer[J]. J Cancer Res Clin Oncol, 2015, 141(8): 1353-1361.
11Ma PC, Maulik G, Christensen J, et al. C-Met: Structure, functions and potential for therapeutic inhibition[J]. Cancer Metastasis Rev, 2003, 22(4): 309-325.
12Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges[J]. Trends Mol Med, 2010, 16(1): 37-45.
13Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SUl1274 and small interfering RNA in non-small cell lung cancer[J]. Cancer Res, 2005, 65(4): 1479-1488.
14Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met proto-oncogene[J]. Cancer Cell, 2003, 3(4): 347-361.
15Wang ZX, Lu BB, Yang JS, et al. Adenovirus-mediated siRNA targeting c-Met inhibits proliferation an dinvasion of small-cell lung cancer(SCLC) cells[J]. J Surg Res, 2011, 171(1): 127-135.
16Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer [J]. J Clin Oncol, 2013, 31(32): 4105-4114.
17Landi L, Minuti G, D′Incecco A, et al. Targeting c-MET in the battle against advanced nonsmall-cell lung cancer[J]. Curr Opin Oncol, 2013, 25(2): 130-136.
18Liu PJ, Chen CD, Wang CL, et al. In-depth proteomic analysis of six types of exudative pleural effusions for non small cell lung cancer biomarker discovery[J]. Mol Cell Proteomics, 2015, 4(4): 917-932.
19ArIt F, Stein U. CoIon cancer metastasis: MACC1 and Met as metastatic pacemakers[J]. Int J Biochem Cell BioI, 2009, 41(12): 2356-2359.
20Kokoszynska K, Krynski J, Rychlewski L, et al. Unexpected domain composition of MACC1 links MET signaling and apoptosis[J]. Acta Biochim Pol, 2009, 56(2): 317-323.
21Ji L, Yan T, Li Y, et al. Preparation of Au-polydopamine functionalized carbon encapsulated Fe3O4magnetic nanocomposites and their application for ultrasensitive detection of carcino-embryonic antigen[J]. Sci Rep, 2016, 6: 21017.
22Han J, Jiang L, Li F, et al. Ultrasensitive non-enzymatic immunosensor for carcino-embryonic antigen based on palladium hybrid vanadium pentoxide/multiwalled carbon nanotubes[J]. Biosens Bioelectron, 2016, 77: 1104-1111.
23Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: Results of the randomized “CEAwatch” trial[J]. Eur J Surg Oncol, 2015, 41(9): 1188-1196.
24Wang YR, Yan JX, Wang LN. The diagnostic value of serum carcinoembryonic antigen, alpha fetoprotein and carbohydrateantigen 19-9 for colorectal cancer[J]. J Cancer Res Ther, 2014, 10 Suppl: 307-309.
25Zhou ZM, Feng Z, Zhou J, et al. Capillary electrophoresis-chemiluminescence detection for carcino-embryonic antigen based on aptamer/graphene oxide structure[J]. Biosens Bioelectron, 2015, 64: 493-498.
26Lai H, Jin Q, Lin Y, et al. Combined use of lysyl oxidase, carcino-embryonic antigen, and carbohydrate antigens improves the sensitivity of biomarkers in predicting lymph node metastasis and peritoneal metastasis in gastric cancer[J]. Tumour Biol, 2014, 35(10): 10547-10554.
(本文编辑:张大春)
胡婕,尤青海,孙耕耘. 结肠癌转移相关因子-1和间质-上皮细胞转化因子检测在肺腺癌患者并发恶性胸腔积液中的诊断价值[J/CD]. 中华肺部疾病杂志: 电子版, 2016, 9(2): 120-124.
Diagnostic value of combined measurement of metastasis-associated in colon cancer 1 and mesenchymal-epithelial transition factor in lung adenocarcinoma patients with malignant pleural effusion
HuJie,YouQinghai,SunGengyun.
DepartmentofRespiratoryMedicine,theFirstAffiliatedHospitalofAnhuiMedicalUniversity,Hefei230022,ChinaCorrespondingauthor:SunGengyun,Email:sungengyun@tom.com
ObjectiveTo investigate the diagnostic value of combined measurement of metastasis-associated in colon cancer 1(MACC1), carcino embryonie antigen(CEA) and mesenchymal-epithelial transition factor(c-MET) in lung adenocarcinoma patients with malignant pleural effusion. MethodsNinety-one patients with pleural effusions were observed, including 53 cases of malignant pleural effusions from lung adenocarcimoma and 38 cases of benign effusions. MACC1 and c-MET levels in plasma and pleural effusion were detected by ELISA. CEA was detected by radioimmunoassay. ResultsThe expressions of MACC1, c-MET and CEA in the group of patients with malignant pleural effusion(MPE) from lung adenocarcinoma were significantly higher than those in the benign group(P<0.05). There was no significant difference of the expression of MACC1 and c-MET in plasma for the two groups(P>0.05). The expression of MACC1 was correlated significantly positive with the expression c-MET in pleural effusion(r:0.728,P<0.01).A receiver operating characteristic (ROC) curve analysis was carried out to assess the value of MACC1, c-MET and CEA in MPE from lung adenocarcinoma patients. The cut-of values for MACC1, c-MET and CEA were defined at 90.98 pg/ml, 757.67 ng/ml and 1.95 ng/ml, respectively. And the sensitivities of MACC1, c-MET and CEA were 62.26%, 52.83% and 92.45%, the specificities were 84.21%, 84.21% and 92.11%, respectively. The sensitivity and specificity of combined detection of MACC1 and c-MET increased to 75.47% and 92.11%, respectively. Moreover, for MACC1, c-MET and CEA, the sensitivity and specificity combined detection increased to 98.10% and 100%, respectively. ConclusionsMACC1 and c-MET can be used as the reference indexes for the differentiation of benign pleural effusions and malignant pleural effusions from lung adenocarcinoma patients. Combined examination with CEA can significantly improve the diagnostic value.
Lung adenocarcinoma;Metastasis-associated in colon cancer 1;Mesenchymal-epithelial transition factor;Carcino embryonie antigen;Malignant pleural effusion
10.3877/cma.j.issn.1674-6902.2016.02.002
国家临床重点专科建设项目基金(2012·649)
230022 合肥,安徽医科大学第一附属医院呼吸内科
孙耕耘,Email: sungengyun@tom.com
R563,R743
A
2015-10-08)
