脲基嘧啶酮四氢键二茂铁二聚体:电子相互作用的调控
2016-09-13王素敏赵绒娜王奇观郭浩李金华张文慧
王素敏 赵绒娜 王奇观郭浩 李金华 张文慧
(西安工业大学材料与化工学院,陕西省光电功能材料与器件重点实验室,西安710032)
脲基嘧啶酮四氢键二茂铁二聚体:电子相互作用的调控
王素敏赵绒娜王奇观*郭浩李金华张文慧
(西安工业大学材料与化工学院,陕西省光电功能材料与器件重点实验室,西安710032)
以脲基嘧啶酮四重氢键为桥联单元,组装了二茂铁同体二聚体(1∙1)。电化学实验显示1∙1中两个等同的二茂铁基团的氧化还原电位差值(ΔE)达260mV,说明1∙1中两个二茂铁基团间通过四氢键发生了显著的电子相互作用。在1∙1/CHCl3中逐步加入0.5、1和2等摩尔的CF3COOH时,由于脲基嘧啶酮四氢键的逐步解离,二茂铁间的电子相互作用强度逐渐减弱,其ΔE从260m V逐步减小至150、100和0m V,此时再加入三乙胺又可以使电子相互作用逐步恢复至初始状态,说明通过加入质子酸、碱可有效调控四氢键体系中发色团间通过氢键的电子相互作用。
脲基嘧啶酮;四氢键;电子相互作用;调控;二茂铁
Inaddition to pursuing largeextentofelectronic communication and understanding the mechanism,control of the electronic communicationbetween two redox centersisalsoattractive in view of the potentialapplication as intelligentswitchesonmolecular w iresandmolecularmachines10-14.A few reportshavebeen found on thecontrolofelectronic communicationby anexternalstimuli such as protoation.Alvarez and Kaifer15prepared a dinuclear ferrocene com pound containing a―CH2―N(R)―CH2―bridge, which exhibits amoderate degree of electronic communication between the ferrocene subunits,and it can be disrupted by protonation or N-methylationof thebridge′s tertiary nitrogen atom. Sasaki etal.16utilized a2,5-dimercapto-1,3,4-triadiazolateasa bridge to conduct the electronic communication of dinuclear ruthenium(II)whichcanbequenchedbyprotonationofthebridgeunit.
After itwas realized thathydrogen bonds can alsomediate the electronic coupling between redox sets behaving asσandπ bonds17,the electronic communication through hydrogen-bonded bridges had also been em phasized18-20.In 2006,Kaifer et al.21reported large extentof electronic communication occurred between two identical ferrocene centersw ith separation distance more than 0.1 nm linked by AADD(A:hydrogen bond acceptor, D:hydrogen bond donor)quadruple hydrogen-bonded motif sim ilar to thewell-known ureidopyrim idinone22,23.Compared w ith covalentbonds,hydrogen bondsaremore sensitive to theexternal stimuli,such as solvent,pH,and temperature,so the control of electronic communication via hydrogen bonding ismore desired. However,itwas scarcely reported up to now.In thispaper,ureidopyrim idinonemodified ferrocene derivative 1,in w hich the ferrocenegroupwasdirectly linked to the6-position carbon atom of pyrimidinone heterocycle,was synthesized.Resulted from the great binding strength and self-com plementary characteristic of 2-ureido-4[1H]-pyrim idinoneAADD hydrogen-bondedmodule developed by Meijer and co-workers22,23,stable hydrogen-bonded dimer1∙1(Fig.1)was formed in apolar solvents such asCHCl3and CH2Cl2.Electrochemical resultsproved that in CHCl3the2-ureido-4[1H]-pyrim idinone-bridged ferrocene dimer 1∙1 exhibits a remarkable level of electronic communication across the hydrogen-bonded interface.More importantly,the level of electronic communication of ferrocene dimer 1∙1 can be reversibly controlled by the stepw ise addition of CF3COOH/triethylam ine (Et3N),through the protonation/deprotonation of pyrimidinone unit in compound 1.
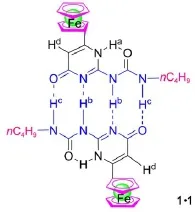
Fig.1 2-Ureido-4[1H]-pyrim idinone quadruplehydrogenbonded ferrocenedimer 1∙1
2 Experimental
2.1Apparatus,materials and m easurem en ts
1HNMRspectrawere recordedonaBrukerAvancedpx 400MHz instruments(Germany),using tetramethylsilane(TMS)as internal standard.UV-Visspectrawereobtained using a Shimadzu 1901 spectrophotometer(Japan).Massspectrawereobtained on Bruker APEX IIspectrometers(Germany).Elementalanalyseswereperformed ona Carlo Erba1106elementalanalyzer(Italy).Electrochem ical measurements were carried out on CHI 660C electrochemicalworkstation in CHCl3containing0.1mol∙L-1ammonium perchlorate as the supporting electrolyte,w ith Ptslide auxiliary electrodeand Ptslideworkingelectrode,saturated calomelelectrode (SCE)referenceelectrode.
NaH(52%m ineral oil dispersion),acetylferrocene(95%), diethyl carbonate(99%),guanidinium carbonate(99%),ammonium perchlorate(99%)were purchased from Aldrich and used as received.Tetrahydrofuran(THF),CHCl3(99%),triethylam ine (Et3N,99%),and pyridine(99%)were purchased from Aladdin. Anhydrous THFwasobtained by distillation from sodium/benzophenone.Pyridine,Et3N,and CHCl3were heated under reflux w ith CaH2for 3 h,and then distilled.The other reagents and solvents w ith analytical grade were obtained from Sinopharm Chem icalReagentCo.,Ltd.and used as received.
2.2Syn thes is
2.2.1Ethyl3-ferroceny l-3-oxoprop ionate(2)
A suspension of NaH(52%on dispersion oil,1.85g,40.0mmol)and diethyl carbonate(3.6m L,29.7mmol)in dry THF (100mL)was refluxed underan argon atmosphere.A solution of acetylferrocene(2.74g,12.0mmol)in 10m L of dry THFwas then added dropwiseover2 h and themixturewas refluxed overnight. The dense suspension was carefully poured into am ixture of saturated aqueous of NH4Cl,5%aqueous HCl,and ice w ith volume ratio of them being 1:1:1,and extracted with CHCl3. The organic layerwaswashed w ith saturated aqueous NH4Cl,then dried by MgSO4for12 h,and then concentrated to dryness.The crude was purified by performing column chromatography on silicagel(CHCl3/petroleum,5:1(V/V))to afford the product in 54%yield as red brown oil.1H NMR(CDCl3,400MHz)δH:4.79 (br.s,2H,Fc),4.56(br.s,2H,Fc),4.29(br.s,5H,Fc),4.25-4.18(q,2H,J=8.0Hz,―COOC H2―),3.74(s,2H,―COC H2COO-), 1.32-1.28(t,3H,J=8.0Hz,―C H3);13C NMR(CDCl3,400MHz)δC:195.99(―C OOCH2CH3),167.53(―C OCH2COO-), 78.27(―C H of Fc),72.95(―C H of Fc),70.09(―C HofFc),69.67 (―C H of Fc),61.32(―CO C H2COO-),46.87(―COO C H2CH3), 14.20(―COOCH2C H3);MS(EI)m/z:301[M+H]+;Anal.,Calcd. for C15H16FeO3:C,60.03%;H,5.37%.Found:C,59.99%;H, 5.52%.
2.2.26-Ferrocenylisocytosine(3)
A suspensionofguanidinium carbonate(0.60g,3.33mmol)and ethyl3-ferrocenyl-3-oxopropionate(1.65g,5.5mmol)in absolute ethanol(60m L)was refluxed overnight.A fter cooling to room temperature,the resultantclear,brown red solutionwasevaporated under vacuum and then 50m L of CHCl3wasadded.The organic layerwaswashedwithbrine,then driedw ith Na2SO4for12h,and concentrated to dryness.The crude was purified by performing column chromatography on silicagel(CH2Cl2/CH3OH,10:1(V/V)) to afford the productin56%yield as red brown powder.1HNMR (DMSO-d6,400MHz)δH:10.53(s,1H,―C=C―O H),6.44(s, 2H,―NH2),5.79(s,1H,―C=C H―C=N),4.82(br.s,2H,Fc), 4.35(br.s,2H,Fc),4.08(br.s,5H,Fc);13CNMR(d6-DMSO,400MHz)δC:166.28(―C=N―C―NH2),155.20(FC―C―C=C―OH),96.16(FC―C―C=C―OH),81.75(FC―C―C=C―OH), 69.87(―C H of Fc),69.51(―C H of Fc),67.60(―C H of Fc);MS (EI)m/z:295[M]-;Anal.,Calcd.forC14H13FeN3O:C,56.98%;H, 4.44%;N,14.24%.Found:C,56.80%;H,4.41%;N,14.29%.
2.2.32-(4-Buty l)ureido-6-fe rrocenyl-4[1H]-pyrim idinone(1)
Amixtureof 6-ferrocenylisocytosine(3)(0.602 g,2.04mmol) and butylisocyanate(0.449 g,4.53mmol)in dry pyridine(30mL) washeated under reflux for12 h.A fter evaporating the solvent, the resultant residuewas thoroughlywashedwith coldmethanol, and then subjected to the purification by chromatography on silica gel(CH2Cl2/MeOH,100:1(V/V))to afford the productasa yellow solid in 68%yield.1H NMR(CDCl3,400MHz)δH:13.62(s,1H, H of intramolecular hydrogen bond),12.03(s,1H,H of intermolecular hydrogen bond),10.25(s,1H,H of intermolecular hydrogen bond),6.06(s,1H,H of 5-position of pyrimidinone), 4.72(br.s,2H,Fc),4.50(br.s,2H,Fc),4.23(br.s,5H,Fc),3.34(t,2H,J=8.0Hz,―C H2CH2CH2CH3),1.45(m,2H,J=8.0Hz,―CH2C H2CH2CH3),1.26(m,2H,J=8.0Hz,―CH2CH2C H2CH3), 0.88(t,3H,J=8.0Hz,)―C H2CH2CH2C H3);13C NMR(CDCl3, 400MHz)δ:173.03(―NH C ONH-),156.81(―C=N),154.58 (Fc―C=C―C=O),150.80(Fc―C=C―C=O),101.64(Fc―C=C―C=O),74.23(―C H of Fc),71.28(―C H of Fc),70.39 (―C H of Fc),66.75(―C H of Fc),39.93(―C H2CH2CH2CH3), 29.90(―CH2C H2CH2CH3),20.26(―CH2CH2C H2CH3),13.86(―CH2CH2CH2C H3);MS(ESI)m/z:395[M+H]+;Anal.,Calcd. for C19H22FeN4O2,C,57.88%;H,5.62%;N,14.21%.Found:C, 57.77%;H,5.69%;N,14.29%.
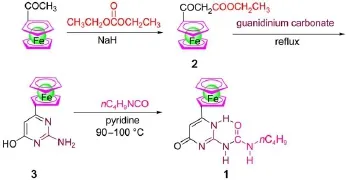
Fig.2 Synthetic route to com pound 1
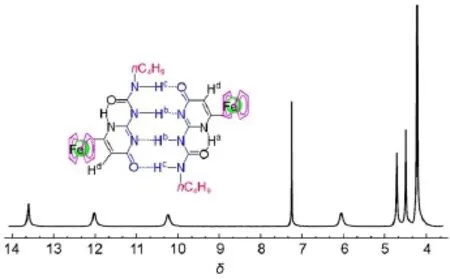
Fig.3 Partial1H NMR spectrum of compound 1 in CDC l3
3 Results and discussion
The preparationsof keymonomer for ferrocene dimer 1∙1 are summarized in Fig.2.Briefly,treatmentof acetylferrocenew ith diethy l carbonate in the presence of NaH affordedβ-ketoester 2 as an intermediate,which was then reacted with guanidinium carbonate in refluxing absoluteethanol.After separation,the crude product3was reactedw ith n-butylisocyanate in pyridine to give compound 1 in good chem icalyield.1
H NMR spectroscopy(Fig.3)clearly revealed that quadrup le AADD hydrogen-bonded linked homodimer1∙1 was formed in CDCl3because three hydrogen-bonded NH am ide peaks(13.62, 12.03,and 10.25)were observed at low field,which are in accordancew ith the reported resonance position for the4[1H]-pyrimidinone tautomer24,25.Moreover,diluting compound 1 CDCl3solution to 1×10-6mol∙L-1did not lead to dissociation ofproton resonance signals in the range from 10to 14.Thisshows that the binding constant can be as low as105L∙mol-1,which is consistent w ith the results from the reported similar compounds22,24.
The1H NMR titration experimentwasprogressed to comprehend the structural transformation of compound 1 in CDCl3upon addition of protonic acid such as CF3COOH.Asshown in Fig.4, the stepw ise addition of CF3COOH to a solution of 1 in CDCl3resulted in three importantchangesin1HNMR spectra.Firstly,the gradual disappearance of the original signals for Ha,Hb,and Hcoccurred,accompanied by a concomitant reappearanceof signals for Hcat the high field.This showed that the dimer 1∙1 was gradually dissociated due to protonation upon stepw ise addition of CF3COOH.When themolar ratio of CF3COOH to compound 1was increased to 2:1(Fig.4(e)),the signalsassociatedwith the formation of intermolecular hydrogen bonds(Hband Hc)were almostvanished becauseof the dissociation of dimer 1∙1.Sec-ondly,with the addition of CF3COOH the singlet peak at 6.06ascribed to Hdof pyrim idinone ring w as dim inished and two new peakswereappeared at6.34and 6.12(Fig.4).It isbecause some of the 4[1H]-pyrimidinone tautomer of 1 were converted to the pyrim idin-4-ol tautomeric form in the protonation process.As2 equimolar CF3COOH wasadded to the solution of compound 1 in CDCl3,the integralareaof the two peaksat6.34and 6.12 isequal, which indicated thatequalamountof pyrimidinoneand pyrimidin-4-ol tautomer were present.Thirdly,the resonance signals for protons of the ferrocenemoiety at4-5were gradually downfield shifted because charge transfer from the ferrocene to the protonated pyrimidine ring occurred upon titration with CF3COOH. From above results,itcanbeseen thatadditionofCF3COOH leads to theprotonation-induceddissociationofdimer1∙1,accompanied by the50%transformation from 4[1H]-pyrim idinone topyrim idin-4-oltautomer(Fig.5),which caused thechangesof them icroenvironmentof ferroceneprotons.Inaddition,2equimolarCF3COOH cancause the fulldissociationofdimer1∙1.Theadditionequimolar amountof the Et3N in above solution can lead to the complete recovery of the initial spectrum(Supporting Information).This showed thegood reversibility ofdisassociationofdimer1∙1.
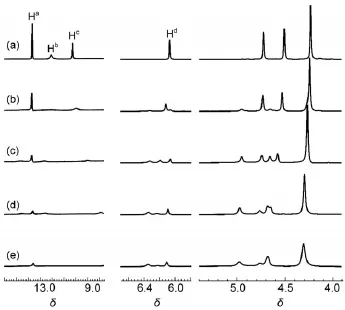
Fig.4Partial1H NMR spectra of 1 in CDCl(5×10-3mol∙L-1)3upon addition of(a)0,(b)0.5,(c)1,(d)1.5, (e)2 equim olar CF3COOH
TheUV-Vis titration spectraof compound 1 in CHCl3werealso performed to demonstrate the reversible protonation/deprotonation process.Asshown in the Fig.6a,theUV-Visabsorption spectrum of compound 1 showed an intenseπ-π*absorptionband at293and aw eak low energy band attributable to the d-d transition arising from the ferroceneunitat456nm.Upon titrationwith CF3COOH (Fig.6a to Fig.6e),both absorption bandswere red-shifted because charge transfer from the ferrocene to the protonated pyrim idine heterocycleoccurred.The changewascompletedwith theaddition of 2 equimolaracid which further proved that the reactionmolar ratio of CF3COOH to compound 1 was 1:2.A fterwardsadding equimolaramountof theEt3N could alsomake the initialUV-Vis spectrum recover,which indicates thattheprotonation/deprotonation of compound 1 iswell reversible,sim ilar to the case of1H NMR experiment(Supporting Information).
The redox behavior of the ferrocene dimer1∙1 was exam ined in chloroform.The cyclic voltammogram(CV)ofhomodimer1∙1(Fig.7(a))welldisplays two separated oxidation wavesat0.27 and 0.53V(vs SCE).In general,functionalmolecules possessing multiple redox sites such as ferrocene groups can exhibit just a single redox peak even if the redox active sitesare electronically isolated.However,in the caseelectronic coupling existsacross the redox sites,theymay be influenced by each other,which routinely makes the single redox potential separated into several different redox peaks.The value between the separated oxidation potentials (ΔE)can be considered asa guide to themagnitude of the electronic communication26.Thus in homodimer1∙1 the separation of the oxidation waves indicates the existence of a remarkable level of electronic communication between the two ferrocene groups, w ith a large value ofΔE(260mV)in CHCl3.W ith respect to the crystal structure of self-complementary quadruple hydrogenbinded unit of 2-ureido-4[1H]-pyrimidinonemotif22,24,the ferrocenegroup in dimer 1∙1 should have the side-by-side arrangementwith edge-to-edge separation of 1 nm.In such a configuration,the two ferrocene groupswere stabilized mainly by the directionality and rigidity of thehydrogen-bonded unitused here, which inhibit theelectronic communication between the ferrocene groups via the through spacemechanism.Therefore,the electronic communicationbetween the ferrocenegroups inhomodimer1∙1 should occur across 2-ureido-4[1H]-pyrim idinone hydrogenbonded interface.
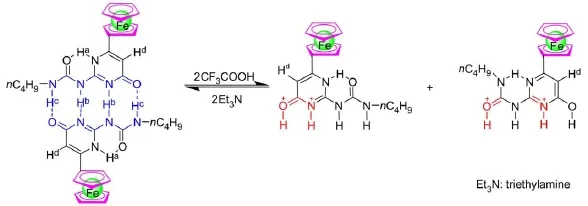
Fig.5Structural transformation of com pound 1 upon addition of CF3COOH/Et3N in CDCl3
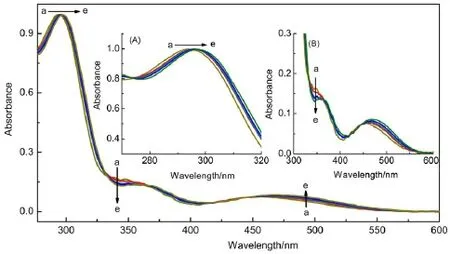
Fig.6Absorp tion spectral changes of com pound 1(4×10-5mol∙L-1)in CHC l upon addition of CFCOOH33From curve a to curve e,the concentration of CF3COOH increases from 0to 2×10-5,4×10-5,6×10-5,and 8×10-5m ol∙L-1,respectively. Insetsshow thepartialmagnified figureof theabsorption spectraof compound 1.
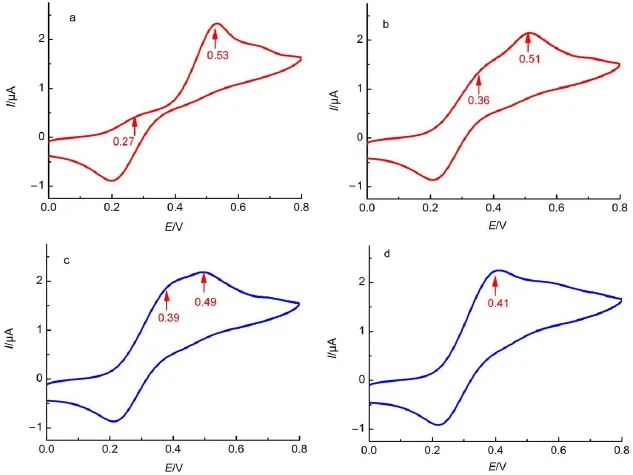
Fig.7 Cyclic voltammogram curvesof 1(4×10-4mol∙L-1)in CHC l upon addition of(a)0,(b)0.5,(c)1,(d)2 equimolar CFCOOH33Theworking and counterelectrodeswere all Pt slide,the reference electrodew as SCE;scan ratewas100mV∙s-1.
Because the electronic communication in dimer 1∙1 occurs through the quadruple hydrogen-bondde interface,itwasexpected that the electronic communication can also be controlled by addition of CF3COOH/Et3N,considering that thequadruplehydrogen bondsof homodimer 1∙1 can begradually dissociated/associated upon titrationw ith CF3COOH/Et3N in CHCl3asevidenced by1H NMR spectra.From the cyclic voltammetry shown in Fig.7,upon addition of 0.5equimolarof CF3COOH,theΔE representing the magnitude of electronic communication wasdecreased from 260mV(Fig.7a)to 150mV(Fig.7b),and was further reduced to 100mV when themolar ratio of CF3COOH to compound 1 was increased to 1:1,because of the partial dissociation of the quadruple hydrogen-bonded bridge by the successive addition of CF3COOH.When themolar ratio of CF3COOH to compound 1 w as increased to 2:1,the tw o separated oxidation wavesw ere integrated into one new wave at 0.41 V with theΔE of 0mV(Fig.7d).This isbecause the electronic communication between the ferrocenes units was effectively inhibited due to the completely destroying of quadruple hydrogen-bonded bridge and dissociation of homodimer1∙1.In addition,above phenomenon is highly reversible,the initial voltammogram can be stepw ise recovered by successive addition of Et3N(Supporting Information).This shows that the stepw ise association-dissociation of homodimer 1∙1 by reversible protonation/deprotonation process can be used to tune the level of electronic communication effectively.
4Conclusions
In summary,in this paper an ureidopyrim idinonemodified ferrocene derivative that self-assembled into stable hydrogenbonded ferrocene dimer in polar solvent CHCl3and CH2Cl2was prepared.The assembled ferrocene dimer shows controllable behavior of the electronic communication by protonation of the pyrimidinone unit.The CV data of thehydrogen-bonded dimer in CHCl3showed splitting of the oxidativewavesof the ferrocene centers,revealing the existence of electronic communication across hydrogen-bonded bridge.Upon protonation/deprotonation, the electronic communication of the tw o ferrocene units w as gradually sw itched off/on due to the controllable dissociation/ association of the hydrogen-bonded dimer.Thehigh reversibility and greatbinding strength of theureidopyrimidinonehydrogenbonded unit play the important role in the formation of a controllable conversion system of redox-protonation coupled reaction. Further utilization of the ureidopyrimidinone hydrogen-bonded unitasbridgemay comprise anew chemistry ofmixed-valence system.
Supporting In formation:available free of charge via the internetathttp://www.whxb.pku.edu.cn.
Refe ren ces
(1)Venkatasubbaiah,K.;Zakharov,L.N.;Kassel,W.S.; Rheingold,A.L.;Jäkle,F.Angew.Chem.2005,117(34),5564. doi:10.1002/ange.200502148
(2)Nishihara,H.Bull.Chem.Soc.Jpn.2001,74(1),19.doi: 10.1246/bcsj.74.19
(3)Ceccon,A.;Santi,S.;Orian,L.;Bisello,A.Coordin.Chem. Rev.2004,248,683.doi:10.1016/j.ccr.2004.02.007
(4)Muraoka,H.;Watanabe,Y.;Takahashi,A.;Kamoto,H.; Ogawa,S.Heteroatom Chem.2014,25,473.doi:10.1002/ hc.21156
(5)Xu,G.L.;Crutchley,R.J.;DeRosa,M.C.;Pan,Q.J.;Zhang, H.X.;Wang,X.;Ren,T.J.Am.Chem.Soc.2005,127(38), 13354.doi:10.1021/ja0534452
(6)Xu,G.L.;Xi,B.;Updegraff,J.B.;Protasiew icz,J.D.;Ren,T. Organometallics 2006,25(22),5213.doi:10.1021/om0607550
(7)Iyoda,M.;Kondo,T.;Okabe,T.;Matsuyama,H.;Sasaki,S.; Kuwatani,Y.Chem Lett.1997,26(1),35.doi:10.1246/ cl.1997.35
(8)Li,Y.;Josow icz,M.;Tolbert,L.M.J.Am.Chem.Soc.2010, 132(30),10374.doi:10.102/ja101585z
(9)Hu,Y.Q.;Zhu,N.;Han,L.M.Acta Phys.-Chim.Sin.2015,31 (2),227.[胡宇强,竺宁,韩利民.物理化学学报,2015,31 (2),227.]doi:10.3866/PKU.WHXB201411061
(10)Mahmoud,K.;Long,Y.T.;Schatte,G.;Kraatz,H.B. J.Organomet.Chem.2004,689,2250.doi:10.1016/j. jorganchem.2004.04.016
(11)Yoshida,J.;Kuwahara,K.;Yuge,H.J.Organomet.Chem. 2014,756,19.doi:10.1016/j.jorganchem.2014.01.018
(12)Tanaka,Y.;Koike,T.;Akita,M.Eur.J.Inorg.Chem.2010, 3571.doi:10.1002/ejic.201000661
(13)Moriuchi,T.;Hirao,T.Tetrahedron Lett.2007,48(29),5099. doi:10.1016/j.tetlet.2007.05.095
(14)DiPietro,C.D.;Serroni,S.;Campagna,S.;Gandolfi,M.T.; Ballardini,R.;Fanni,S.;Browne,W.R.;Vos,J.G.Inorg. Chem.2002,41(11),2871.doi:10.1021/ic0112894
(15)Alvarez,J.;Kaifer,A.E.Organometallics1999,18(26),5733. doi:10.1021/om990678r
(16)Tannai,H.;Tsuge,K.;Sasaki,Y.Inorg.Chem.2005,44(15), 5206.doi:10.1021/ic050672w
(17)deRege,P.J.;Williams,S.A.;Therien,M.J.Science1995, 269,1409.doi:10.1126/science.7660123
(18)Sánchez,L.;Sierra,M.;Martín,N.;Myles,A.J.;Dale,T.J.; Rebek,J.;Seitz,W.;Guldi,D.M.Angew.Chem.Int.Edit. 2006,45(28),4637.doi:10.1002/anie.200601264
(19)Wilkinson,L.A.;McNeill,L.;Meijer,A.J.H.M.;Patmore,N. J.J.Am.Chem.Soc.2013,135(5),1723.doi:10.1021/ ja312176x
(20)Goeltz,J.C.;Kubiak,C.P.J.Am.Chem.Soc.2010,132(49), 17390.doi:10.1021/ja108841k
(21)Sun,H.;Steeb,J.;Kaifer,A.E.J.Am.Chem.Soc.2006,128 (9),2820.doi:10.1021/ja060386z
(22)Beijer,F.H.;Sijbesma,R.P.;Kooijman,H.;Spek,A.L.; Meijer,E.W.J.Am.Chem.Soc.1998,120(27),6761.doi: 10.1021/ja974112a
(23)Sijbesma,R.P.;Meijer,E.W.Chem.Commun.2003,5.doi: 10.1039/B205873C
(24)Alexander,A.M.;Bria,M.;Brunklaus,G.;Caldwell,S.; Cooke,G.;Garety,J.F.;Hewage,S.G.;Hocquel,Y.; McDonald,N.;Rabani,G.;Rosair,G.;Smith,B.O.;Spiess, H.W.;Rotello V.M.;Woisel,P.Chem.Commun.2007,2246. doi:10.1039/B 703070C
(25)Zhao,Y.P.;Zhao,C.C.;Wu,L.Z.;Zhang,L.P.;Tung,C.H.; Pan,Y.J.J.Org.Chem.2006,71(5),2143.doi:10.1021/ jo051932u
(26)Demadis,K.D.;Hartshorn,C.M.;Meyer,T.J.Chem.Rev. 2001,101(9),2655.doi:10.1021/cr990413m
Ureidopyrimidinone Quadruple Hydrogen-Bonded Ferrocene Dimer:Control of Electronic Communication
WANG Su-Min ZHAORong-Na WANGQi-Guan*GUO Hao LIJin-Hua ZHANGWen-Hui
(ShaanxiKey Laboratory ofPhotoelectric FunctionalMaterialsand Devices,SchoolofMaterialsand Chemical Engineering, Xi′an TechnologicalUniversity,Xi′an 710032,P.R.China)
A ferrocene homodimerwas assemb led via the ureidopyrim idinone quadrup le hydrogen-bonded module in this paper.Remarkab le e lectronic communication was found between the two ferrocene centers ac ross the u reidopyrim id inone bridge in chlo rofo rm.The separa tion between the tw o redox poten tials(ΔE)of the ferrocenylmoietieswas 260mV.Upon protonation of the hydrogen-bonded bridge by successive addition o f 0.5,1,and 2 equivalents of trifluoroacetic acid,the extent of the electronic communication between the subunits gradua lly lowered,withΔE decreasing to 150,100,and 0mV,respectively,because of the stepw ise dissociation of the pyrim idinone hydrogen-bonded bridge.This phenomenon is reversib le,and the initial vo ltamm og ram can be recovered stepw ise by successive add ition of triethylam ine,dem onstra ting effective controlo f the e lectronic communication between two ferrocene centers.
Ureidopyrim idinone;Quad rup le hydrogen bonding;Electronic communication;Contro l; Ferrocene
1 Introduction
Ferrocene dimers and oligomers have attracted enormous interestsin thepastyearsdue to theirpotentialuseasgood candidate formolecular w ire1,2.In ferrocene dimers,electronic communi-cation between Fe(II)and Fe(III)sites in themixed valence could form the basis for well-defined molecular devices of energy conversion,sensing,ormolecular electronics,thus promoting continued research in thisdirection3-5.A variety ofπandσbonds have been generally chosen asbridgesof redox centers to support theelectronic communication process.It is suggested thatelectronic communication was strongly affected by geometry,electronic structure,and length of the linkersbetween redox centers6-9, and extensive electron delocalization is the critical factor for efficientelectronic communication over large distance.
November9,2015;Revised:December24,2015;Published onWeb:January 4,2016.
O648
10.3866/PKU.WHXB201601044
*Corresponding author.Email:qiguanwang@163.com;Tel:+86-29-86173324.
The projectwas supported by theNationalNatural Science Foundation of China(21103133),Scientific Research Foundation for theReturned
Overseas Chinese Scholars,Ministry of Education,Natural Science Foundation of ShaanxiProvince,China(2015JM5224),ShaanxiProvincial
Education Department Program,China(2013JK 0678),and National Training Programs of Innovation and Entrepreneurship for Undergraduates,
China(201510702030).
国家自然科学基金(21103133),教育部留学归国科研启动基金,陕西省自然科学基金(2015JM5224),陕西省教育厅科研计划项目(2013JK0678)和大学生创新创业训练计划项目(201510702030)资助©Editorialoffice of Acta Physico-Chim ica Sinica
