1-丁腈-3-甲基咪唑双三氟甲基磺酸亚胺的性质
2016-09-13刘青山刘惠牟林
刘青山 刘惠 牟林
(1沈阳农业大学理学院,沈阳110866;2上海环境卫生工程设计院有限公司,上海200232;3上海污染场地修复工程技术研究中心,上海200232)
1-丁腈-3-甲基咪唑双三氟甲基磺酸亚胺的性质
刘青山1,*刘惠2,3牟林1,*
(1沈阳农业大学理学院,沈阳110866;2上海环境卫生工程设计院有限公司,上海200232;3上海污染场地修复工程技术研究中心,上海200232)
制备了功能化离子液体1-丁腈-3-甲基咪唑双三氟甲基磺酸亚胺。在T为283.15-353.15K温度范围内,测定了该功能化离子液体的密度、动力粘度、电导率及折光率。讨论了亚甲基的增减对该类功能化离子液体的密度、动力粘度、电导率及折光率等性质的影响,并与传统咪唑类、吡啶类离子液体物理化学性质的变化趋势进行了对比。通过经验方程计算了该功能化离子液体的热膨胀系数、分子体积、标准摩尔熵及晶格能等热力学性质参数。讨论了Vogel-Fulcher-Tamm an(VFT)方程和Arrhenius方程的适用性,得出VFT方程适用于该功能化离子液体,而Arrhenius方程并不适用。有关研究对新型离子液体的合成及其工业化的应用具有十分重要的意义。
功能化离子液体;密度;动力粘度;电导率;折光率
Egashira etal.6-8have introduced the cyano group on the imidazolium FILs and quaternary ammonium FILs,respectively.The FILs have been applied in the lithium batteries as electrolyte components.The FILshave showed an improved cycle behavior compared w ith the electrolyte based on a tetraalkylammonium ionic liquidw ithouta cyano group.The quaternary ammoniumbased FILs containing a cyano group showed the better stability of the cathodic than the im idazolium-based FILs.Hardacre etal.9,10have synthesized two seriesof pyridinium-based FILs in order to compare the effectsof differentelectron-withdraw ing functional groups on theirability to form ionic liquids.The presenceof the electron-withdrawing functionalgroups nitrile or trifluoromethyl substituent on the pyridinium ring leads to salts w ith higher melting temperature than traditional ILs.On thebasis,theauthors have observed the liquid charge-transfer complex form on contacting electron-rich aromaticsw ith electron withdrawing group appended 1-alkyl-4-cyanopyridinium ionic liquids.Zhang etal.11have studied the solubilitiesof C2H4and CO2in the cyano type im idazolium FILs through the gas chromatography.Compared w ith the 1,3-dialkylimidazolium type ILs,the cyano type FILs result in a remarkable decrease of the interactions of hydrocarbons.The cyano type ILs have exhibited the advantageous properties.As the solvent,it can be applied as suitable reaction mediaand ligands in catalytic reaction,aselectrolyte in lithium battery,as solvent for extraction of metals and dissolution of cellulose.
Although the cyano type FILshavebeen applied in somearea, the physico-chem ical propertiesare notenough for the application. Recently,the 1-(cyanomethyl)-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]im ide[MCNMIM][NTf2]has been synthesized in our group12.The density,dynam ic viscosity,electrical conductivitywere determined by the traditionalmethods.In order to study theeffectof themethyleneon the properties for the series ILs,the 1-(cyanopropyl)-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide[PCNMIM][NTf2]was synthesized and the propertieswere determ ined.The com parison of the propertiewas also discussed with the non-functional traditional ILs.The traditional ILsare1-ethyl-3-methylimidazolium bis[(trifluoromethyl) sulfonyl]imide([EMIM][NTf2]),1-butyl-3-methylimidazolium bis [(trifluoromethyl)sulfonyl]im ide([BMIM][NTf2]),1-ethyl-2,3-methylim idazolium bis[(trifluoromethyl)sulfony l]im ide([EMMIM][NTf2]),1-butyl-2,3-methylimidazolium bis[(trifluoromethyl)sulfonyl]im ide([BMMIM][NTf2]).Someof the thermodynam ic propertieswere estimated from themeasurementdata by theempiricalequations.The studiesof the above FIL properties are important for thenovel IL synthesisand industrialapplication.
2 Experimental
2.1Materia ls
The putiries,moleculer weight(MW),and company name (Source)of the 1-(cyanomethyl)-3-methylim idazolium cloride ([PCNMIM][Cl])and lithium bis[(trifluoromethyl)sulfonyl]imide ([Li][NTf2])are listed in Table S1 of the Supporting Information.
The hydrophobic FIL[PCNMIM][NTf2]was obtained in the distilled water by the traditional ion exchange reaction according to the literature12.The[PCNMIM][Cl]was dissolved by distilled w ater in flask,the equivalent amount of LiNTf2saltwas added into the flask under stirring.The bottom colorless phase was washed several times with distilled water until no Cl-(itwas determined by AgNO3/HNO3solution,the content of Cl-was estimated to lower than 0.005%(inmass fraction).The products w ere finally dried in high vacuum for 48 h at 353 K.The FIL [PCNMIM][NTf2]was characterized by1H NMR and13C NMR spectra(See the Figs.S1 and S2 of the Supporting Information). The finalmass fraction and molecular weight of the FIL[PCNMIM][NTf2]were also listed in Table S1 of the Supporting Information.
In order to compare the differences of the structure,the [PCNMIM][NTf2],[MCNMIM][NTf2],([EMIM][NTf2]),and ([BMIM][NTf2])are presented in Fig.1.
2.2Measu rem en ts o f the dens ity,e lec trical conduc tivity,dynam ic viscosity,and refrac tive index
Theweightcontentof thewaterwas determined by a Cou-Lo Aquamax Karl Fischermoisturemeter v.10.06(Mettler Toledo, Switzerland),and was found to be<0.02%(inmass fraction). Recently,Jacquem in etal.16have studied the effectof thewater contenton the hydrophobic IL[EMIM][NTf2]properties(density and dynamic viscosity)from 0.005%to 1.98%(mass fraction).So, thewater content can be ignored in thisw ork.
The density,electrical conductivity,dynam ic viscosity,and refractive index were determ ined by a previousmethod at atmosphere12.The uncertaintiesof theexperimentalvaluesare also estimated according to literature17-20.
Thedensity and dynam ic viscosity of the FIL[PCNMIM][NTf2] was determined by a automated SVM3000Anton Paar rotational Stabinger viscometer-densimeter(Anton Paar GmbH,Austria) w ith cylinder geometry at((288.15-338.15)±0.01)K12.The principlewasbased on amodified Couetteaccording to a rapidly rotating outer tubeand a relative slow ly rotating innermeasuring bob.The valueswere recorded every 5K after attaining thermal equilibrium 30m in.The electrical conductivity wascarried outon a MP522 conductivity instrument(Shanghai San-Xin Instrumentation,P.R.China)w ith the cellconstantsof1 cm-1(the cellw as calibratedw ith theaqueous KClsolution)at((288.15-338.15)± 0.05)K12.Themeasurement frequency and voltageof theelectrical conductivity were 50Hz and 9 V,respectively.The refractive indexwas carried outaccording to the literature21.The datawere carried on an Abbe refractometer(ShanghaiShen Guang InstrumentCo.,P.R.China)and the frequencywas50Hz.The degassed w ater refractive index was determ ined by the instrument at(298.15±0.05)K and the valuewas in good agreementw ith the literature22.Each valuewas the averageof the three timesand the standard uncertaintiesw ere±0.0002 g∙cm-3for density at 0.65confidence level,±1%for dynamic viscosity at0.95confidence level,and±1%for dynamic viscosity at0.95confidence level.The uncertainty valueswere estimated from theeffectof themachine, room temperatureand pressure,waterand halogen contentetc.
The above experimental valuesare listed in Table 1.
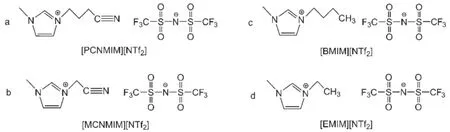
Fig.1 Structuresof[PCNMIM][NTf2],[BMIM][NTf2],[MCNMIM][NT f2],and[EMIM][NTf2]
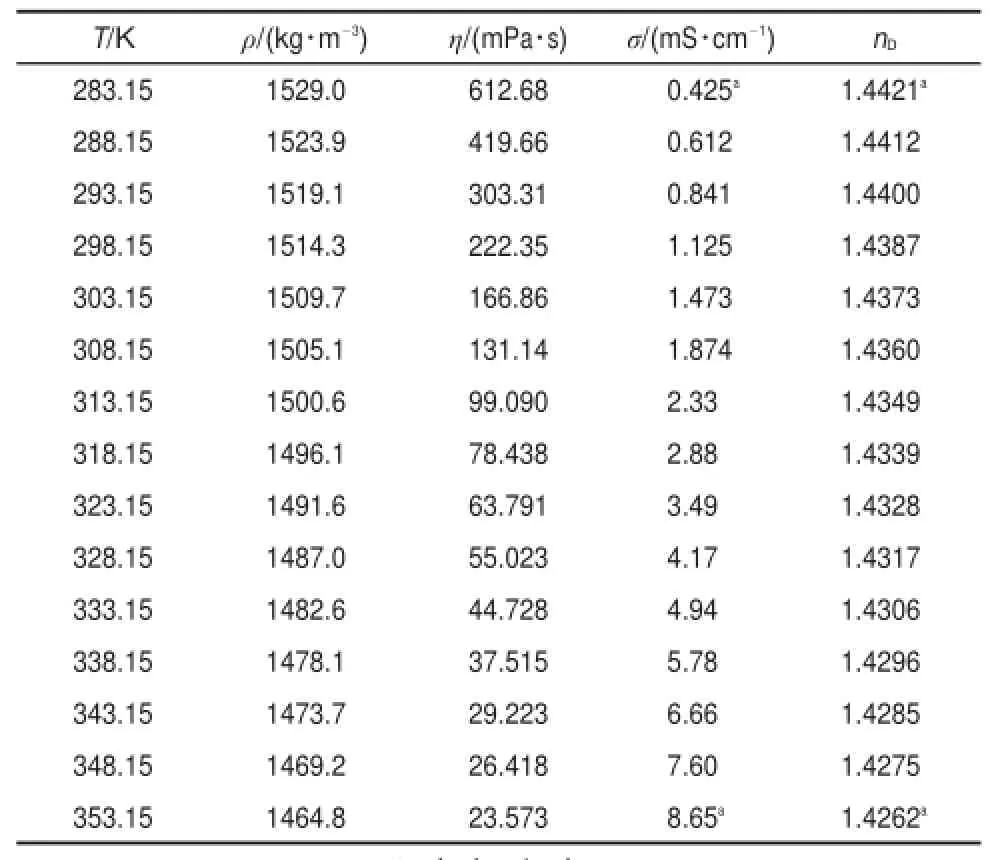
Table1 Experimentalvaluesofdensity,ρ,dynam ic viscosity,η, electricalconductivity,σ,and refractive index,nD,of[PCNMIM] [NTf2]from 283.15to353.15K underatmospherepressure
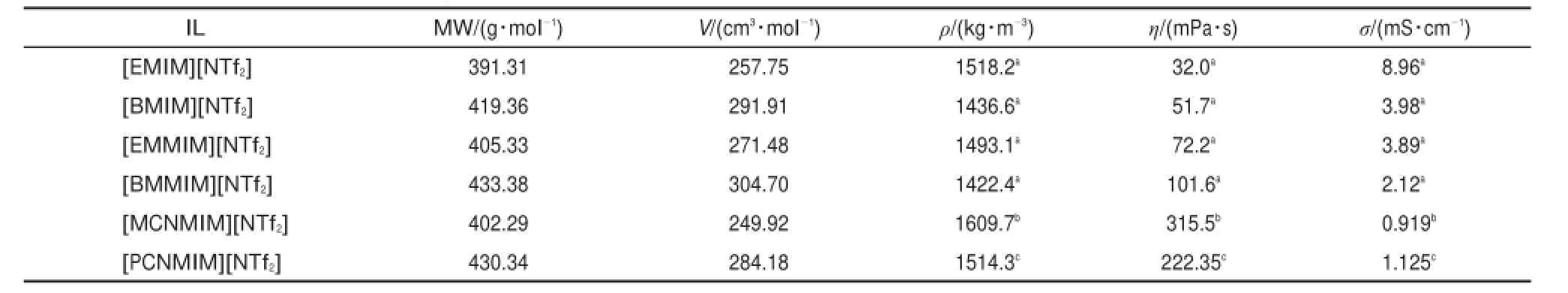
Tab le 2 Com parison of density,dynam ic viscosity,and electricalconductivity of[EMIM][NTf2],[BMIM][NTf2],[EMMIM][NTf2], [BMMIM][NTf2],[MCNMIM][NTf2],and[PCNMIM][NTf2]at298.15K under atm osphere pressure
3 Results and discussion
From the Table 1,the valueof electrical conductivity is1.125mS∙cm-1for FIL[PCNMIM][NTf2]and the relative deviation is 6.25%with the literature valueof 1.2mS∙cm-1at298.15K7.
In order to compare the influence ofmethylene on IL properties, the values of density,dynamic viscosity,and electrical conductivity for three series ILsare listed in Table2 at298.15K.The ILs are[EMIM][NTf2],[BMIM][NTf2],[EMMIM][NTf2],[BMMIM] [NTf2],[MCNMIM][NTf2],and[PCNMIM][NTf2],respectively. From Table 2,the three series ILs have exhibited the same tendency for density after the introduction of themethylene on alky l side chain.Usually,for dynam ic viscosity and electrical conductivity,the introduction of themethylene leads to the increase of dynamic viscosity and decrease of electrical conductivity.However,for the tw o FILs,the valueshave exhibited the contrary tendency w ith the traditional ILs.Compared w ith the literature12,the dynamic viscosity valuesof FIL[PCNMIM][NTf2] are lower than FIL[MCNMIM][NTf2]and the electrical conductivity values of FIL[PCNMIM][NTf2]are higher than FIL [MCNMIM][NTf2]in the temperature range.Theabnormal results have been also discoveried for traditional pyridinium type ILs from our group23-27.For the pyridinium type ILs,the electrical conductivity values increasewhen themethylgroup is introduced on position 4.The dynam ic viscosity values decreasewhen the methyl group is introduced on position 4.We believe that the groupsof the electron w ithdraw ing and electron donating play the important role to theeffectof the properties.―CN is theelectron withdrawing group,―CH2―and―CH3are theelectron donating groups.For the two series ILs,the introduction of the―CH2―and―CH3leads to the cation having the relatively symmetry structure.Then,the cation and anion are relatively far away and the force of them becomesweak.So,the ILs exhibit the high fluidity after the introduction of―CH2―and―CH3.
Based on the sameanion,at298.15K,the density follows the order of ILs[C44mpy][NTf2]<[BMMIM][NTf2]<[BMIM][NTf2]<[C4py][NTf2]<[PCNMIM][NTf2],whereas theexperimentaldensity valuesare1.5143 g∙cm-3for[PCNMIM][NTf2], 1.4366g∙cm-3for[BMIM][NTf2]28,1.4224g∙cm-3for[BMMIM] [NTf2]28,1.4547g∙cm-3for1-ethylpyridiniumbis[(trifluoromethyl) sulfonyl]imide[C4py][NTf2]25,and1.4187g∙cm-3for4-methyl-1-butylpyridinium bis[(trifluoromethyl)sulfonyl]im ide[C44mpy] [NTf2]26,The dynam ic viscosity follows theorderof ILs[BMIM] [NTf2]<[C44mpy][NTf2]<[C4py][NTf2]<[BMMIM][NTf2]< [PCNMIM][NTf2],whereas the values are 222.35mPa∙s for [PCNMIM][NTf2],51.7mPa∙s for[BMIM][NTf2]28,101.6mPa∙s for[BMMIM][NTf2]28,58.3 m Pa∙s for[C4py][NTf2]27,and 55.14mPa∙s for[C44mpy][NTf2]26.The electrical conductivity follows the order of ILs[PCNMIM][NTf2]<[C4py][NTf2]< [BMMIM][NTf2]<[C44mpy][NTf2]<[BMIM][NTf2],whereas the valuesare1.125mS∙cm-1for[PCNMIM][NTf2],2.12mS∙cm-1for[BMMIM][NTf2]28,3.98m S∙cm-1for[BMIM][NTf2]28, 1.67mS∙cm-1for[C4py][NTf2]27,and 3.41mS∙cm-1for[C44mpy] [NTf2]26.
From the above result,the introduction of the―CN functional group on imidazolium ring leads to the density and dynam ic viscosity increasing,electrical conductivity decreasing.The reason of the above resultmay be the increasing of the van derWaals force between the functional group w ith cation or anion and insufficient side-chainmobility.Theeffectorder of the interaction force is―CN>―CH3.
The temperatures dependences on density,electrical conductivity,dynam ic viscosity,and refractive index of the FIL[PCNMIM][NTf2]are plotted in Figs.2 and 3.From the Fig.2,it can be seen that the linesof density and refractiveare linearonewith the increasing of the temperature.From the Fig.3,the electrical conductivity and dynam ic viscosity exhibite non-linear onew ith temperature increasing.
3.1Density
From Fig.2,the lnρvs T can be fitted according to the follow ing straight line.The thermal expansion coefficient,α,can be obtained.

herein,ρis the density values,b isan empirical constant,αis the thermalexpansion coefficient,T is the experimeantal temperature. The thermal expansion coefficient is6.09×10-4K-1.The value is in the range of 5×10-4and 7×10-4K-1w hich obtained by Jacquemin etal.16.
According to Glasser29,the empirical equations are used for estimation of themolecular volume,Vm,standardmolarentropy, S0,and latticeenergy,UPOTfrom density values.

herein,Mismolarmass,N isAvogadro′s constant,The calculated values of the above are 0.4721 nm3formolecular volume,617.9 J∙K-1∙mol-1for standardmolarentropy,and 405kJ∙mol-1for latticeenergy,respectively.Accoring to the literature12,the value of themolecular volume is0.4151 nm3.The contribution of the methylene to themolecular volume is0.0285nm3for the cyano type FILs.Thevalue isin good agreementwith the reported values of 0.0277 nm3for[Cn3mpy][NTf2](n=3,4,6)23,0.0289 nm3for [Cn4mpy][NTf2](n=2,4,6)23,0.0280nm3for ILs[Cnpy] [NTf2]24,30,0.0282 nm3for[Cnmim][NTf2]29,and 0.0279 nm3for Aminoacid at298.15K31-34.The latticeenergy ismuch lower than traditional salts,like UPOT,CsI=613 kJ∙mol-122.Usually,the ILs exhibit the low lattice energy23-34.And it is the reason that the ILs have the relatively low melting temperature,can existas liquid stateat room temperature.
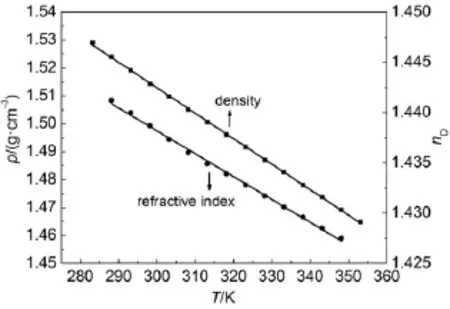
Fig.2 Density and refractive index vs tem perature plots for FIL[PCNMIM][NTf2]
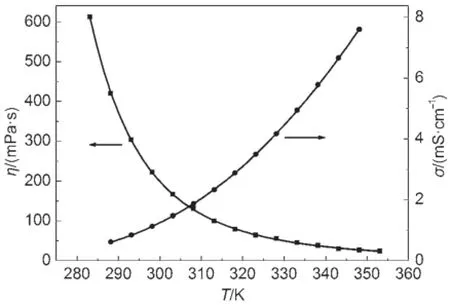
Fig.3 Dynam ic viscosity and electrical conductivity vs tem perature p lots for[PCNMIM][NTf2]
3.2Mo lar electrica l conductivity
Themolar electrical conductivities can be calculated from density and electrical conductivity by the following equation:

where Λ is themolar electrical conductivity,σis the electrical conductivity,Mis themolarmass,andρis the density.Themolar electricalconductivity valuesare listed in Table3.
3.3Electrical conduc tivity
Recently,Vila etal.35havebuilt the relationaship of theVogel-Fulcher-Tamman(VFT)and Arrheniusequations as themodified VFT equation.Theelectrical conductivity activation energies for the four FILscan be calculated by themodified VFT equation.The VFTequation,Arrheniusequation,andmodified VFTequation are below:
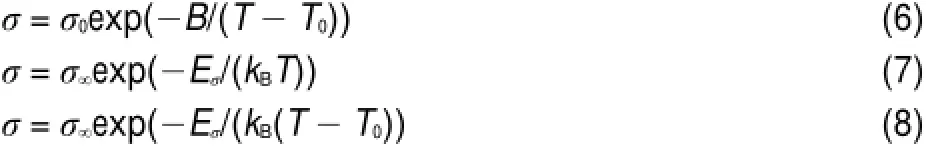
hereσis theelectrical conductivity;σ0,B are fitting parameters;is theactivation energy,which indicates theenergy needed for an ion to hop to a free hole;is themaximum electrical conductivity,kBis the Boltzmann constant,
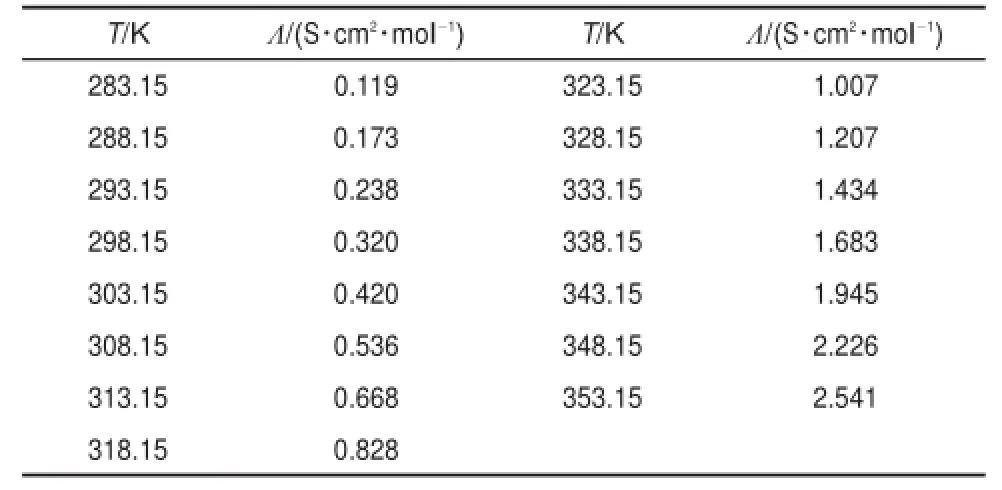
Table 3 Calcu lated valuesofmolar electrical conductivity [PCNMIM][NTf2]at288.15-338.15K under atmospheric pressure
The fitted equation isσ=491.6exp(661.8/(T+189.3))by the equation(6).And the correlation coefficient,R,is0.99997,which indicates that theVFT equation can bewellused for theelectrical conductivity fitting.The calculated valueof electrical conductivity activation energy is 57.1 kJ∙mol-1for FIL[PCNMIM][NTf2] according to equation(8).According to literature12,the electrical conductivity activation energies are 53.6kJ∙mol-1for FIL [MCNMIM][NTf2]and 47.6kJ∙mol-1for FIL[EOHMIM][NTf2], respectively.The FIL[PCNMIM][NTf2]exhibits the highest energy than other two FILs[MCNMIM][NTf2]and[EOHMIM] [NTf2].
By theArrheniusequation,the lnσvs1000/T wasplotted for the FIL in Fig.4.
3.4Dynam ic viscosity
Like the electrical conductivity,the VFT equation,Arrhenius equation,andmodified VFT equation are below:

whereηis the dynamic viscosity;η0,B are fitting parameters;Eηis the activation energy for dynam ic viscosity;s themaximum dynamic viscosity,kBis the Boltzmann constant,
The best fitted equation isη=0.16exp(882.1/(T+176.2))by the equation(9).And the correlation coefficient,R,is 0.99989, w hich indicates that the VFT equation can also be used for the dynamic viscosity fitting.The dynamic viscosity activation energy can also be calculated and the value is 76.1 kJ∙mol-1for FIL [PCNMIM][NTf2]according to equation(11).According to literature12,the dynam ic viscosity activation energies are 62.9 kJ∙mol-1for FIL[MCNMIM][NTf2]and 59.7 kJ∙mol-1for FIL [EOHMIM][NTf2],respectively.Like theelectrical conductivity activation energies,the FIL[PCNMIM][NTf2]have also exhibited the highestenergy than other two FILs[MCNMIM][NTf2]and [EOHMIM][NTf2].
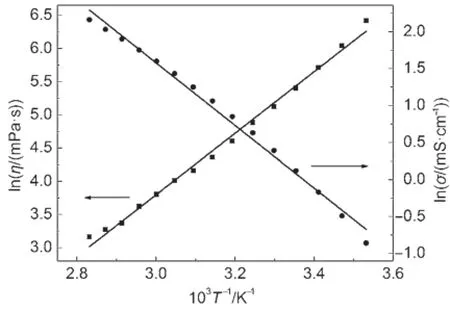
Fig.4Plots of lnηand lnσvs1000/T for FIL[PCNMIM][NTf2]
According to equation(10),the lnηvs 1000T-1w as also described in Fig.4likeelectrical conductivity for the FIL[PCNMIM] [NTf2].
In Fig.4,the black lines are straight lines for fitting of lnσvs 1000T-1and lnηvs 1000T-1.From the Fig.4,the experimental points are distributed on both sidesof the black straight lines.It indicated that theArrhenius equation isnotwell fit for the fitting of the electrical conductivity and dynamic viscosity on temperature.
3.5Walden p roduct
The relationship ofmolar conductivity and dynam ic viscosity can bebuiltby the followingWalden equation:

whereαis the fitting parameter,k is a temperature dependent constant.The description of lgΛvs lgη-1is shown in Fig.5for the FIL[PCNMIM][NTf2]from 283.15to 353.15K.The slope,α,can be obtained and the value is 0.927 for FIL[PCNMIM][NTf2].In order to compare the relationship,the fiveothers imidazolium type ILs12,23arealso ploted at298.15K in Fig.5.The solid straight line is the ideal line for aqueousKClsolutions36.
From Fig.5,like theothers five ILs,theWalden pointof the FIL [PCNMIM][NTf2]isbelow and close to the ideal line,so,the the FIL[PCNMIM][NTf2]isalso called“subionic”37.Itmeans that the FIL[PCNMIM][NTf2]did not yield the expected conductivity from the high fluiditiesbecause on average the proton transfer is incomplete37.From the Fig.5,theWalden pointshaveexhibited the differentchange tendency for the three series ILsat298.15K.For the traditional ILs,the Walden points of[BMIM][NTf2]and [BMMIM][NTf2]are lower than[EMIM][NTf2]and[EMMIM] [NTf2]follow ing the ideal line,respectively.However,the FIL is reversible,theWalden pointof[MCNMIM][NTf2]is lower than [PCNMIM][NTf2].We think that the FIL[PCNMIM][NTf2]has a better fluidity than[MCNMIM][NTf2].The result can also be explained according to the dynam ic viscosity and electrical conductivity.Comparedwith the literature12,the dynamic viscosity valuesof FIL[PCNMIM][NTf2]are lower than FIL[MCNMIM] [NTf2]and theelectrical conductivity valuesof FIL[PCNMIM][NTf2]arehigher than FIL[MCNMIM][NTf2]in the temperature range.
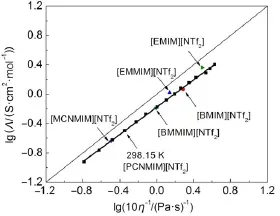
Fig.5Plotof lgΛvs lgη-1for the FIL[PCNMIM][NTf2]in the wholem easu rem en t tem peraturew ith other imidazolium type ILsat298.15KThesolid straightline is the ideal line for aqueousKClsolutions.
3.6Re fractive index
The relationship of refractive index and temperature can also be described for the FIL[PCNMIM][NTf2]by a linear equation:

where nDis the refractive index;A,B are fitting parameters;T isexperimentaltemperature.The fitted linearequation is nD=1.5064-2.27×104T.The correlation coefficient is 0.997 from the experimental values.It indicates that the linear equation is fit for the fitting of the refractive index on temperature.
4Conclusions
The density,electrical conductivity,dynam ic viscosity,and refractive index of the FIL[PCNMIM][NTf2]were determ ined at atmospheric pressureat283.15-353.15K.The FIL[PCNMIM] [NTf2]exhibited the lower lattice energies than the traditional melt salts,it is the reason that the ionic liquid is liquid at room temperature.The dynamic viscosity of[PCNMIM][NTf2]is lower than[MCNMIM][NTf2]and the electrical conductivity of [PCNMIM][NTf2]is higher than[MCNMIM][NTf2].The FIL [PCNMIM][NTf2]exhibits a better fluidity than[MCNMIM] [NTf2],it is reversiblewith the traditional imidazolium type ionic liquids.The VFT equation can be well used for fitting of the electrical conductivity and dynam ic viscosity on temperature, however,the Arrhenius equation not fit.The temperature dependence on refractive index can be well fitted by the linear equation.The property study of the FIL[PCNMIM][NTf2]willbe significant for industrialand engineering applications.
Supporting In form ation:The1HNMRand13CNMRspectra havebeen included.Thisinformation isavailable freeofcharge via theinternetathttp://www.whxb.pku.edu.cn.
Refe ren ces
(1)Welton,T.Chem.Rev.1999,99,2071.doi:10.1021/cr980032t
(2)Rantw ijk,F.V.;Sheldon,R.A.Chem.Rev.2007,107,2757. doi:10.1021/cr050946x
(3)Greaves,T.L.;Drummond,C.J.Chem.Rev.2008,108,206. doi:10.1021/cr068040u
(4)Hapiot,P.;Lagrost,C.Chem.Rev.2008,108,2238.doi: 10.1021/cr0680686
(5)Jessop,P.G.;Subramaniam,B.Chem.Rev.2007,107,2666. doi:10.1021/cr040199o
(6)Egashira,M.;Okada,S;Yamaki,S.J.I.;Dri,D.A.;Bonadies, F.;Scrodati,B.J.Power Sources 2004,138,240.doi:10.1016/ j.jpowsour.2004.06.022
(7)Egashira,M.;Todo,H.;Yoshimoto,N;Morita,N.M.;Yamaki, J.I.J.Power Sources 2007,174,560.doi:10.1016/j. jpow sour.2007.06.123
(8)Egashira,M.;Nakagawa,M.;Watanabe,I.;Okada,S.;Yamaki, J.I.J.Power Sources 2005,146,685.doi:10.1016/j. jpow sour.2005.03.069
(9)Hardacre,C.;Holbrey,J.D.;Mullan,C.L.;Nieuwenhuyzen, M.;Reichert,W.M.;Seddon,K.R.;Teat,S.J.New J.Chem. 2008,32,1953.doi:10.1039/b805063e
(10)Hardacre,C.;Holbrey,J.D.;Mullan,C.L.;Nieuwenhuyzen, M.;Youngs,T.G.A.;Bow ron,D.T.;Teat,S.J.Phys.Chem. Chem.Phys.2010,12,1842.doi:10.1039/b921160h
(11)Zhang,J.;Zhang,Q.;Qiao,B.;Deng,Y.J.Chem.Eng.Data 2007,52,2277.doi:10.1021/je700297c
(12)Liu,Q.S.;Liu,J.;Liu,X.X.;Zhang,S.T.J.Chem. Thermodyn.2015,90,39.doi:10.1016/j.jct.2015.06.010
(13)Bates,E.D.;Mayton,R.D.;Ntai,I.;Davis,J.H.,Jr.J.Am. Chem.Soc.2002,124,926.doi:10.1021/ja017593d
(14)Cai,Y.;Peng,Y.;Song,G.Catal.Lett.2006,109,61.doi: 10.1007/s10562-006-0057-3
(15)Hu,S.;Jiang,T.;Zhang,Z.;Zhu,A.;Han,B.;Song,J.;Xie,Y.; Li,W.Tetrahedron Lett.2007,48,5613.doi:10.1016/j. tetlet.2007.06.051
(16)Jacquemin,J.;Husson,P.;Padua,A.A.H.;Majer,V.Green Chem.2006,8,172.doi:10.1039/B513231B
(17)Chen,Y.;Zhuo,K.;Chen,J.;Bai,G.J.Chem.Thermodyn. 2015,86,13.doi:10.1016/j.jct.2015.02.017
(18)Chen,Y.;Zhang,H.;Li,A.;Zhuo,K.Fluid Phase Equilibr. 2015,388,78.doi:10.1016/j.fluid.2014.12.038
(19)Wei,J.;Chang,C.;Zhang,Y.Y.;Hou,S.Y.;Fang,D.W.; Guan,W.J.Chem.Thermodyn.2015,90,310.doi:10.1016/j. jct.2015.04.029
(20)Ma,X.X.;Wei,J.;Guan,W.;Pan,Y.;Zheng,L.;Wu,Y.;Yang, J.Z.J.Chem.Thermodyn.2015,89,51.doi:10.1016/j. jct.2015.02.025
(21)Tong,J.;Hong,M.;Chen,Y.;Wang,H.;Guan,W.;Yang,J.Z. J.Chem.Thermodyn.2012,54,352.doi:10.1016/j. jct.2012.05.012
(22)Lide,D.R.Handbook ofChemistryand Physics,82nd ed.; CRCPress:BocaRaton,FL,2001-2002.
(23)Liu,Q.S.;Li,P.P.;Welz-Biermann,U.;Chen,J.;Liu,X.X.J.Chem.Thermodyn.2013,66,88.doi:10.1016/j. jct.2013.06.008
(24)Liu,Q.S.;Yang,M.;Yan,P.F.;Liu,X.M.;Tan,Z.C.;Welz-Biermann,U.J.Chem.Eng.Data 2010,55,4928.doi: 10.1021/je100507n
(25)Liu,Q.S.;Li,P.P.;Welz-Biermann,U.;Liu,X.X.;Chen,J.J. Chem.Eng.Data 2012,57,2999.doi:10.1021/je3004645
(26)Liu,Q.S.;Yan,P.F.;Yang,M.;Tan,Z.C.;Li,C.P.;Welz-Biermann,U.Acta Phys.-Chim.Sin.2011,27,2762.[刘青山,颜佩芳,杨淼,谭志诚,李长平,Welz-Biermann U.物理化学学报,2011,27,2762.]doi:10.3866/PKU.WHXB20112762
(27)Zhang,Q.G.;Wei,Y.;Sun,S.S.;Wang,C.;Yang,M.;Liu,Q. S.;Gao,Y.A.J.Chem.Eng.Data 2012,57,2185.doi: 10.1021/je300153f
(28)Cheng,Z.;Lee,J.M.J.Phys.Chem.B 2014,118,2712.doi: 10.1021/jp411904w
(29)Glasser,L.Thermochim.Acta 2004,421,87.doi:10.1016/j. tca.2004.03.015
(30)Liu,Q.S.;Yang,M.;Li,P.P.;Sun,S.S.;Welz-Biermann,U.; Tan,Z.C.;Zhang,Q.G.J.Chem.Eng.Data 2011,56,4094. doi:10.1021/je200534b
(31)Fang,D.W.;Tong,J.;Guan,W.;Wang,H.;Yang,J.Z.J.Phys. Chem.B 2010,114,13808.doi:10.1021/jp107452q
(32)Fang,D.W.;Guan,W.;Tong,J.;Wang,Z.W.;Yang,J.Z.J. Phys.Chem.B 2008,112 7499.doi:10.1021/jp801269u
(33)Tong,J.;Song,B.;Wang,C.X.;Li,L.;Guan,W.;Fang,D.W.; Yang,J.Z.Ind.Eng.Chem.Res.2011,50,2418.doi:10.1021/ ie101903t
(34)Xu,W.G.;Ma,X.X.;Li,L.;Tong,J.;Guan,W.Ind.Eng. Chem.Res.2012,51,4105.doi:10.1021/ie201530b
(35)Vila,J.;Ginés,P.;Pico,J.M.;Franjo,C.;Jiménez,E.;Varela, L.M.;Cabeza,O.Fluid Phase Equilibr.2006,242,141.doi: 10.1016/j.fluid.2006.01.022
(36)Schreiner,C.;Zugmann,S.;Hartl,R.;Gores,H.J.J.Chem. Eng.Data 2010,55,1784.doi:10.1021/je900878j
(37)Belieres,J.P.;Angell,C.A.J.Phys.Chem.B 2007,111,4926. doi:10.1021/jp067589u
Properties of 1-(Cyanopropyl)-3-methylimidazolium Bis[(trifluoromethy l)sulfonyl]imide
LIUQing-Shan1,*LIU Hui2,3MOU Lin1,*
(1School ofScience,Shenyang Agricultural University,Shenyang 110866,P.R.China;2ShanghaiEnvironmental Sanitation Engineering Design Institute Co.,Ltd.,Shanghai200232,P.R.China;3ShanghaiEngineering Research CenterofContaminated SitesRemediation,Shanghai200232,P.R.China)
The functionalionic liquid(FIL)1-(cyanopropyl)-3-methylim idazolium bis[(trifluoromethyl)sulfonyl] im ide[PCNMIM][NTf2]was synthesized using an ion-exchange me thod.Density,dynam ic viscosity,electrical conductivity,and refractive indexwere determ ined in the temperature range 283.15-353.15K.The e ffectof methylene group introduction is discussed for the FILs and im idazolium ionic liquids(ILs).The thermalexpansion coefficient,mo lecular volume,standard molarentropy,and lattice energy were determ ined by the empirical equations from themeasurementvalues.The temperature dependence on electrical conductivity and dynam ic viscosity of the FILs were fitted by the Vogel-Fulcher-Tammann(VFT)equation.The adaptability of the Arrhenius equation was also discussed for the electrica l conductivity and dynam ic viscosity.The study of the thermodynam ic properties of the FIL is important for synthesis ofnew ILs and theirapplication.
Functionalionic liquid;Density;Dynam ic viscosity;Electricalconductivity;Refractive index
1 Introduction
Ionic liquids(ILs)have exhibited outstanding physico-chem ical properties1-5,likegood solvation,negligible vapor pressure,good thermalstability,and designability etc.ILs havebeen applied forindustrial and scientific areaas thegreen solvents.The functional ionic liquids(FILs)havebeen paidmuchmoreattention because of the designability6-15.The physical chem ical properties can be designed according to the introduction of the functionalgroups, like―CN,―OH,―CH2―O―CH3,etc.
August3,2015;Revised:December17,2015;Published onWeb:December17,2015.
O642
10.3866/PKU.WHXB201512171
*Corresponding authors.LIUQing-Shan,Email:liuqingshan@dicp.ac.cn;Tel:+86-13478787524.MOU Lin,Email:myname-mulin@tom.com.
The projectwas supported by theNationalNatural Science Foundation of China(21203193)and Program for Liaoning Excellent Talents in
University,China(LJQ2015099).
国家自然科学基金(21203193)和辽宁省高等学校优秀人才支持计划(LJQ2015099)资助©Editorialoffice of Acta Physico-Chim ica Sinica
