白背飞虱细胞色素P450还原酶基因的分子特征与表达模式分析
2016-08-15余航刘苏朱晴子周文武梁庆梅史肖肖祝梓杰祝增荣
余航, 刘苏, 朱晴子, 周文武, 梁庆梅, 史肖肖, 祝梓杰, 祝增荣
(浙江大学昆虫科学研究所/水稻生物学国家重点实验室/农业部农业昆虫学重点实验室,杭州 310058)

白背飞虱细胞色素P450还原酶基因的分子特征与表达模式分析
余航, 刘苏, 朱晴子, 周文武, 梁庆梅, 史肖肖, 祝梓杰, 祝增荣*
(浙江大学昆虫科学研究所/水稻生物学国家重点实验室/农业部农业昆虫学重点实验室,杭州 310058)
从水稻重要害虫白背飞虱(Sogatellafurcifera)中克隆了一条编码细胞色素P450还原酶(cytochrome P450 reductase,CPR)的全长cDNA,命名为SfCPR,其开放阅读框全长为2 034 bp,编码一个含有677个氨基酸残基的蛋白质。SfCPR蛋白质的2级结构具有CPR的典型特征,例如N端跨膜区、黄素单核苷酸结合域、黄素腺嘌呤二核苷酸结合域和烟酰胺腺嘌呤二核苷酸磷酸结合域,以及保守的催化残基。系统进化分析结果显示,SfCPR与灰飞虱和褐飞虱的CPRs聚在同一分支。荧光定量聚合酶链式反应结果显示:SfCPR基因在白背飞虱各龄期均有表达,其中以成虫表达量最高;在白背飞虱成虫各组织中均能够检测到SfCPR基因的表达,其中,以腹部表达量最高。使用亚致死剂量的溴氰菊酯、吡虫啉和噻嗪酮处理白背飞虱3龄若虫后,试虫的SfCPR表达水平均显著上升。上述结果为进一步研究该基因的生理功能奠定了基础。
细胞色素P450还原酶; 白背飞虱; 杀虫剂; 表达模式
Summary Nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P450 reductase (CPR) is an electron supplier for various cytochrome P450 monooxygenases. Most P450-mediated catalytic reactions in insects require involvement of CPR, such as detoxification of insecticides and plant secondary metabolites. So far, CPR genes have been identified and characterized from many model and non-model insect species. Since insect P450 system is one of the most important metabolic adaptive traits involved in the degradation of xenobiotics and regulation of endogenous substrates. CPR, as the indispensable electron donor of P450 system, has attracted increasing attention as a potential candidate to develop novel chemical inhibitor to manage target insect pests. Rice planthoppers, such asNilaparvatalugens,LaodelphaxstriatellusandSogatellafurcifera, are considered to be the most serious pests of rice. Previously, some studies onL.striatellusandN.lugensfound that silencing the CPR gene by RNAi technology could increase their sensitivity to insecticides, but little was known aboutS.furcifera. This work firstly reports the identification of CPR gene inS.furciferaand up-regulation of the CPR transcription by chemical insecticides. The research will facilitate further study on the function of CPR inS.furcifera.
In this study, a full-length cDNA encoding CPR was cloned fromS.furcifera. The phylogenetic relationships ofSfCPRwith other insect CPRs were estimated by neighbor-joining method, and its distribution in various tissues and different developmental stages were analyzed by real-time quantitative polymerase chain reaction (qPCR). Finally, after exposure of deltamethrin, buprofezin and imidacloprid at sublethal concentrations for 6, 12 and 24 h, the relative expression levels ofSfCPRin the third-instar nymphs were investigated.
By searching the transcriptome data sets ofS.furcifera, a cDNA fragment encoding putative CPR (namedSfCPR) was identified. This cDNA fragment was then amplified by PCR and sequenced in order to confirm that the sequence was not chimeric. TheSfCPRcDNA contained a complete open reading frame (ORF) of 2 034 bp nucleotides, encoding a protein of 677 amino acid residues. The theoretical isoelectric point (pI) and calculated molecular mass of SfCPR protein are 5.48 and 76.762 ku, respectively. The secondary structure of SfCPR protein showed the marked features of typical CPRs, such as N-terminal transmembrane region, FMN-, FAD- and NADPH-binding domains and conserved catalytic residues. In addition, a transmembrane anchor was predicted in the N-terminus of the protein, indicating that SfCPR is an endoplasmic reticulum-located protein. Phylogenic analysis was used to gain insight into the phylogenetic relationships among CPR proteins from diverse insect species. We found that SfCPR and CPRs from other two planthoppers were clustered together. Real-time quantitative PCR showed that the expression ofSfCPRwas detectable in all developmental stages and the level in adults was the highest. TheSfCPRtranscripts were also expressed in all the tested tissues of the adults, and most were strongly expressed in the abdomen. The exposure at sublethal concentrations of deltamethrin, buprofezin and imidacloprid led to significantly elevated expression ofSfCPR. The expression ofSfCPRwas activated soon (6 h) after treatment with buprofezin and imidacloprid, while the response ofSfCPRexpression to deltamethrin was relatively slow (12 h).
In conclusion, this work is the first report about the cDNA sequence information, secondary structure and transcription profiles of CPR gene in theS.furcifera. These findings provide foundation for further research on the physiological function ofSfCPR.
烟酰胺腺嘌呤二核苷酸磷酸-细胞色素P450还原酶(cytochrome P450 reductase,CPR)是细胞色素P450单加氧酶(cytochrome P450 monooxygenases,CYP)的电子供体,在P450介导的诸多催化反应中起着非常重要的作用[1]。对于昆虫而言,大多数P450介导的对杀虫剂和植物次生代谢物的解毒过程中都需要有CPR的参与[2-3]。CPR也会参与昆虫的其他生理途径,例如蜕皮激素和表皮碳氢化合物的合成与代谢等[4-5]。
尽管昆虫CPR的晶体结构目前尚无报道,但是对大鼠和酵母CPR的3维结构研究表明,该酶由3个功能结构域组成:黄素单核苷酸(flavin mononucleotide,FMN)结合域、黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)结合域和烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate,NADPH)结合域,它们均参与电子穿梭过程[6-7]。此外,CPR在N端有一较短的疏水性跨膜区,该区域有助于CPR定位于细胞内质网上[2,6]。结构生物学研究表明,CPR在电子传递中自身结构会发生变化,以促进与NADPH的结合和电子转移[8-9]。
目前,多种模式和非模式昆虫的CPR基因已被鉴定,如黑腹果蝇(Drosophilamelanogaster)[5,10]、冈比亚按蚊(Anophelesgambiae)[11]、床虱(Cimexlectularius)[12]、二化螟(Chilosuppressalis)[13]、棉铃虫(Helicoverpaarmigera)[14]和小菜蛾(Plutellaxylostella)[15]等,这对深入研究CPR的生理功能和利用CPR来开发新型的害虫无公害管理技术均具有重要意义。由于CPR是P450s的电子供体,所以目前利用RNA干扰技术(RNA interference,RNAi)沉默昆虫的CPR基因进而抑制P450s的活性也受到越来越多的关注。例如:当注射双链RNA(double-stranded RNA,dsRNA)沉默冈比亚按蚊和床虱的CPR基因后,试虫对拟除虫菊酯类杀虫剂的敏感性均显著上升[11-12];当黑腹果蝇的CPR基因被沉默后,试虫表皮碳氢化合物合成代谢受阻,试虫的存活率和羽化率均显著下降[10]。
稻飞虱是水稻的一类重大害虫。近10年来,褐飞虱(Nilaparvatalugens)、灰飞虱(Laodelphaxstriatellus)和白背飞虱(Sogatellafurcifera)及其传播的病毒病相继大规模爆发[16-18]。目前,防治这类害虫仍然依赖大量使用化学杀虫剂;而杀虫剂的无节制使用,不仅造成环境污染,而且还诱使飞虱产生抗药性[19]。此前,针对褐飞虱和灰飞虱CPR基因的研究发现,通过RNAi技术沉默该基因后,试虫对杀虫剂敏感性显著上升,因此,可以利用该基因开发新型的无公害防治技术[20-21]。但白背飞虱的CPR基因至今尚无报道。
本研究首先克隆了重要水稻害虫白背飞虱的CPR基因(SfCPR)全长cDNA序列,并对SfCPR基因的分子特征进行了分析;其次,使用实时荧光定量聚合酶链式反应(real-time quantitative polymerase chain reaction,qPCR)检测了该基因在白背飞虱不同组织和不同发育阶段的表达模式;最后,检测了SfCPR基因在受到3种杀虫剂(溴氰菊酯、吡虫啉和噻嗪酮)胁迫后表达水平的变化。本研究结果可为进一步揭示SfCPR的生理功能奠定基础。
1 材料与方法
1.1供试虫源
本试验所用白背飞虱种群采集自浙江大学华家池校区(杭州),用感虫水稻品种TN1(Taichung Native 1)在人工气候室内饲养繁殖35代以上。饲养条件:温度(25±1) °C,相对湿度80%,光周期16 h光照/8 h黑夜。
1.2RNA提取和第1链cDNA合成
取白背飞虱3龄若虫100头,用Trizol试剂(美国Invitrogen公司)提取总RNA。提取的RNA样本用无RNA酶的DNA酶(大连宝生物工程有限公司)处理,以消除可能的基因组DNA污染。RNA的浓度和质量用Nanodrop 2000微量分光光度计(美国Thermo Scientific公司)测定。取1 μg总RNA,使用PrimeScript试剂盒(大连宝生物工程有限公司)合成第1链cDNA。
1.3PCR扩增
通过搜索已公开的白背飞虱转录组数据库[22],得到一条编码CPR的cDNA序列,命名为SfCPR。根据SfCPR的序列信息设计1对基因特异性引物(表1),扩增其开放阅读框(open reading frame,ORF)区域。使用KOD FX DNA聚合酶(日本东洋纺公司)进行PCR扩增。PCR产物经琼脂糖凝胶电泳鉴定,用凝胶回收试剂盒(美国Axygen公司)回收纯化,克隆入pMD18-T载体(大连宝生物工程有限公司),转化大肠杆菌DH5α菌株,并测序验证。
1.4序列分析
使用ExPASy服务器(http://www.expasy.org/tools/protparam.html)计算蛋白质理论分子质量和等电点。使用NCBI的BLAST服务器(http://blast.ncbi.nlm.nih.gov/Blast.cgi)搜索序列一致性及同源基因。使用TMHMM程序(http://www.cbs.dtu.dk/services/TMHMM)预测跨膜区。通过搜索NCBI的保守结构域数据库(http://www.ncbi.nlm.nih.gov/structure/cdd/cdd.shtml)确定保守结构域及关键催化残基。使用ClustalW 2.0程序(http://www.ebi.ac.uk/tools/msa/clustalw2/)连配SfCPR与其他昆虫CPR的氨基酸序列,连配结果导入MEGA 5.05软件[23],以邻接法构建系统发育树,各分支置信度通过自举法进行1 000次重复检验获取。
1.5实时荧光定量聚合酶链式反应
使用实时荧光定量聚合酶链式反应(qPCR)检测SfCPR基因在白背飞虱不同发育阶段(1~5龄若虫和成虫)以及短翅型成虫不同组织(头、胸、腹、足)中的相对表达水平。试验设3个生物学重复,每个重复含试虫50头。提取总RNA的方法同1.2节,使用Revertra Ace qPCR 反转录试剂盒(日本东洋纺公司)合成第1链cDNA,并用超纯水稀释至10 ng/μL。qPCR扩增体系为20 μL,包含1 μL (10 ng) cDNA模板,10 μL SYBR Green Realtime PCR Master Mix试剂(日本东洋纺公司),上下游引物各0.2 μL (0.2 μmol/L),超纯水8.6 μL。选择白背飞虱的18S rRNA作为内参基因。qPCR所用引物见表1。qPCR所用仪器为Bio-Rad CFX96型实时定量系统(美国Bio-Rad Laboratories公司)。PCR反应采用2步法,热循环参数:95 ℃,2 min,1个循环;95 ℃,10 s,60 ℃,20 s,40个循环。基因的相对表达水平使用2-ΔΔCT方法[24]计算。
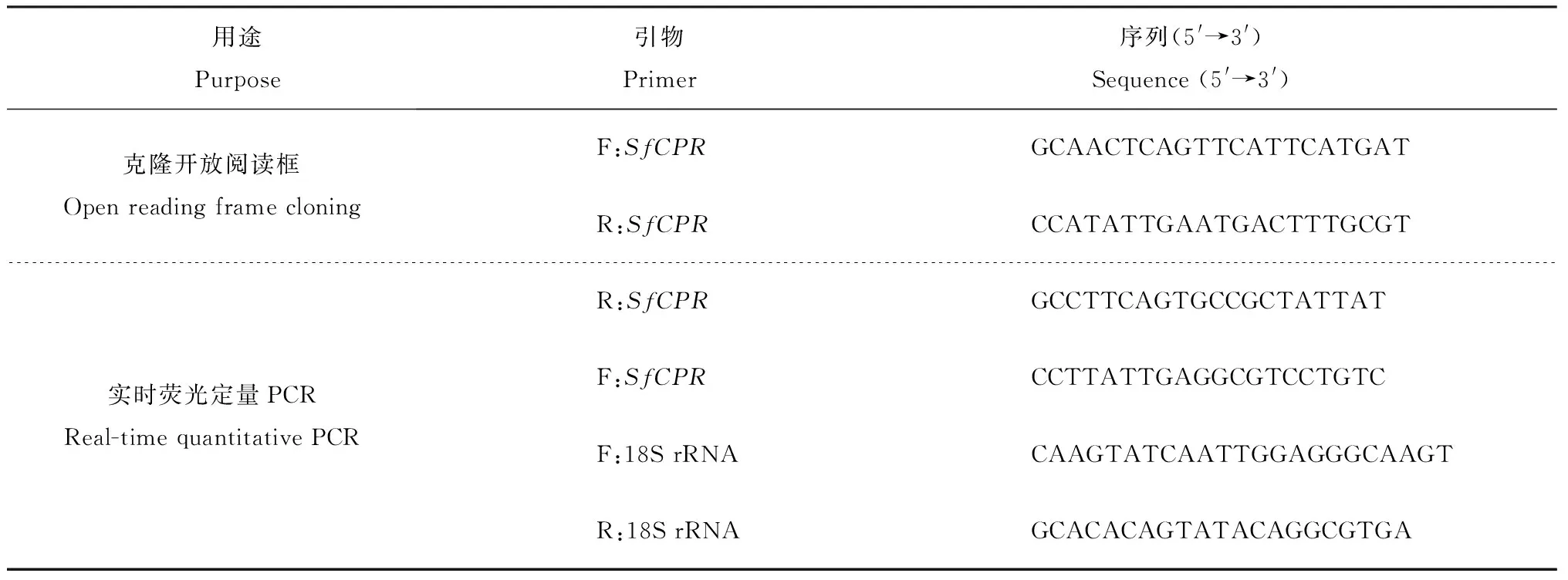
表1 研究所用引物
1.6杀虫剂处理
溴氰菊酯、吡虫啉和噻嗪酮购自浙江省台州市新农公司,纯度≥95%。使用时先用丙酮配制成储存液,再逐级稀释至亚致死浓度(溴氰菊酯、吡虫啉和噻嗪酮均为0.5 ng/μL)。使用手动微量点滴仪(美国Hamilton公司)对白背飞虱3龄若虫进行杀虫剂处理,每试虫滴定0.1 μL。处理6、12和24 h后,随机选择30头试虫提取RNA,检测SfCPR基因表达水平的变化。以丙酮处理的试虫作为对照。试验设3个生物学重复。qPCR步骤同1.5节。
1.7数据统计
使用DPS V9.50软件[25]对试验数据进行统计分析。原始数据经反正弦转换,用单因素方差分析进行处理,并用最小显著差别法比较多个样本之间是否存在显著差异(显著性水平设为P<0.05)。
2 结果
2.1序列分析
通过搜索白背飞虱转录组数据库,获得了1条编码SfCPR的cDNA序列。该序列经PCR扩增和测序予以验证。SfCPR的ORF区域全长为2 034 bp,编码一个由677个氨基酸残基组成的蛋白质。SfCPR的理论分子质量为76.762 ku,理论等电点为5.48。该蛋白质与半翅目昆虫CPR的氨基酸序列具有非常高的一致性(68%~97%),与非半翅目昆虫CPR的一致性也较高(65%~72%)(表2)。SfCPR序列已登录至GenBank数据库,登录号为KJ017970。
为了确定白背飞虱是否还存在其他种类的CPR旁系同源基因,以模式昆虫的CPR氨基酸序列为模板,使用本地BLAST程序在白背飞虱转录组数据库中搜索,结果没有发现新的与CPR同源的蛋白质序列。这说明在白背飞虱体内,在转录水平上只有1种CPR表达形式。为分析SfCPR蛋白质的2级结构特征,将SfCPR与其他几种昆虫的CPR进行连配。结果(图1)表明,SfCPR具有CPR的典型特征,例如N端有一个由23个氨基酸残基组成的跨膜区,保守的FMN、FAD和NADPH结合区域,以及关键催化残基。
2.2系统进化分析
为了探知昆虫CPRs之间的进化关系,从GenBank下载半翅目(Hemiptera)、膜翅目(Hymenoptera)、鞘翅目(Coleoptera)、鳞翅目(Lepidoptera)和双翅目(Diptera)昆虫已被注释的CPR氨基酸序列,连同SfCPR一起构建系统发育树。结果(图2)显示,虽然这些蛋白质的同源性很高,但不同物种的CPR仍被分在不同的进化支上。其中,白背飞虱(S.furcifera)、灰飞虱(L.striatellus)和褐飞虱(N.lugens)的CPRs聚在同一分支上:表明它们之间的亲缘关系最接近。

表2 白背飞虱和其他昆虫CPRs氨基酸序列的一致性百分数

Sfur:白背飞虱;Lstr:灰飞虱;Nlug:褐飞虱;Agam:冈比亚按蚊;Bmor:家蚕;Clec:床虱;Mdom:家蝇。实线方框内为预测的跨膜区;灰色、黄色和紫色分别表示构成FMN、FAD和NADPH结合域的关键氨基酸残基;虚线框内为FAD结合域的精氨酸、酪氨酸和丝氨酸;黑色箭头处为丝氨酸、半胱氨酸、天冬氨酸和色氨酸4个催化残基。Sfur: Sogatella furcifera; Lstr: Laodelphax striatellus; Nlug: Nilaparvata lugens; Agam: Anopheles gambiae; Bmor: Bombyx mori; Clec: Cimex lectularius; Mdom: Musca domestica. The putative transmembrane region is boxed, and the amino acid residues constituting the binding site in the FMN-, FAD- and NADPH-binding domains are highlighted in gray, yellow and purple, respectively. FAD-binding motifs (Arg-X-Tyr-Ser) are indicated by dotted box, and four catalytic residues (Ser, Cys, Asp and Trp) are marked by black arrows. 图1 白背飞虱和其他昆虫的CPRs氨基酸多序列比对Fig.1 Multiple alignment of amino-acid sequences of CPRs from S. furcifera and other insect species

下划线处为白背飞虱的CPR;各序列的GenBank登录号见表2。标尺表示在不同昆虫的CPR蛋白质中5%的氨基酸残基有差异。 CPRs from S. furcifera are underlined. GenBank accession numbers of the sequences used are listed in Table 2. Scale represents 5% of the amino acid residues changed in the CPR proteins from different insect species.图2 基于邻接法的昆虫CPRs系统进化分析Fig.2 Phylogenic analysis of insect CPRs based on neighbor-joining method
2.3组织和发育阶段SfCPR表达模式分布
通过qPCR分析发现:SfCPR基因在白背飞虱短翅型成虫的各组织(头、胸、腹、足)中均有表达,其中以腹部的表达量最高,显著高于头部和足部(P<0.05),但与胸部表达水平间的差异无统计学意义(P>0.05);SfCPR基因在足部的表达水平最低(图3A)。同样,在白背飞虱的各个发育期均能检测到SfCPR基因的表达,其中成虫期的SfCPR表达量最高,2龄若虫次之,4龄若虫表达量最低,且4龄若虫SfCPR的表达水平与2龄若虫和成虫之间差异有统计学意义(P<0.05)(图3B)。
2.4杀虫剂处理对SfCPR表达的影响
使用亚致死浓度的溴氰菊酯、吡虫啉和噻嗪酮处理白背飞虱若虫后,SfCPR基因的表达水平均有不同程度的上调(图4):SfCPR对吡虫啉和噻嗪酮的响应较迅速,使用这2种杀虫剂处理6 h后,SfCPR的表达水平即显著上升,较之丙酮处理组分别提高了16.04倍和5.72倍;但SfCPR对溴氰菊酯的响应较慢,处理12 h后,该基因的表达水平才显著升高;用3种杀虫剂处理24 h后,sfCPR基因的表达水平仍然显著高于丙酮对照组。

短栅上的不同小写字母表示在P<0.05水平差异有统计学意义。Different lowercase letters above bars indicate statistically significant differences at the 0.05 probability level. 图3 SfCPR在白背飞虱不同组织(A)和不同发育阶段(B)的相对表达水平Fig.3 Tissue-specific (A) and developmental stage-dependent (B) expression of S. furcifera CPR gene SfCPR
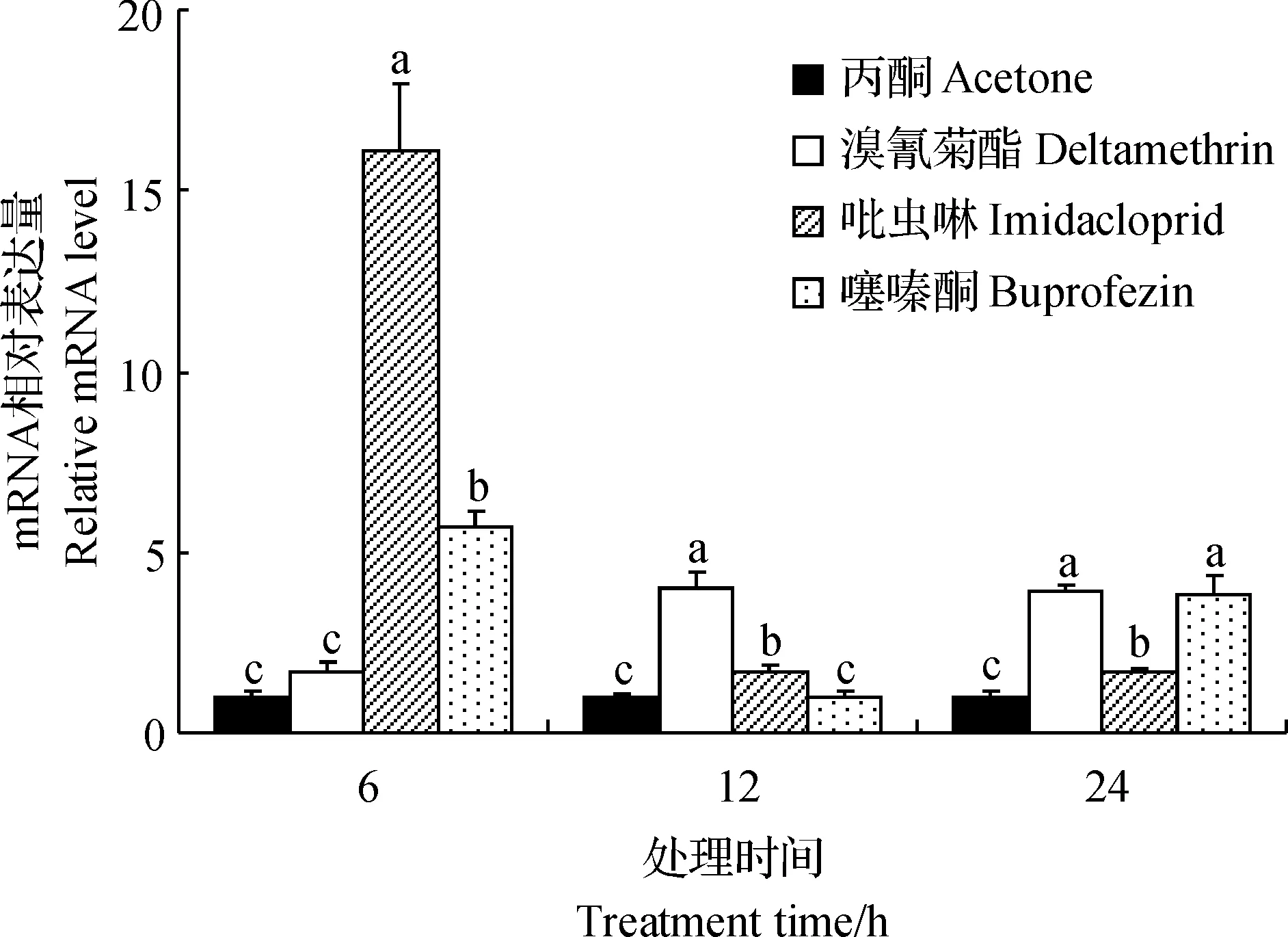
短栅上的不同小写字母表示在P<0.05水平差异有统计学意义。Different lowercase letters above bars indicate statistically significant differences at the 0.05 probability level. 图4 3种杀虫剂滴定后SfCPR表达水平变化Fig.4 Relative mRNA levels of SfCPR after exposure of three insecticides
3 讨论
本研究克隆了白背飞虱SfCPR的cDNA片段,分析了SfCPR的分子特征,使用qPCR检测了SfCPR在白背飞虱不同组织、不同发育阶段以及在受到不同杀虫剂胁迫后的表达模式。CPR为细胞色素P450单加氧酶(CYPs)提供电子,在各种内源性和外源性化合物代谢过程中起着重要作用。本试验结果为后续SfCPR功能研究以及利用该基因开发白背飞虱的新型防治技术奠定了基础。
大多数昆虫、脊椎动物和真菌基因组中只表达1个CPR基因。而在一些维管植物如水稻(Oryzasativa)和棉花(Gossypiumhirsutum)中表达2个以上的CPR基因[26-27]。本研究通过BLAST搜寻转录组数据,发现在白背飞虱转录组中只有1个CPR基因的转录本。这与在其他昆虫上的研究结果[11-12]一致。
SfCPR蛋白质的2级结构具有CPR的典型特征,例如:保守的FMN、FAD和NADPH结合区域,这些区域在电子传递过程中有重要作用;N端的跨膜区,这一区域可将CPR蛋白锚定在细胞内质网上[6]。有研究表明,虽然N端跨膜区与CPR的活性无关,但这一区域若缺失,CPR就不能传递电子给CYP,催化反应也不能进行[2,28]。
多序列比对结果表明,SfCPR与其他昆虫CPR的氨基酸序列一致性均较高,尤其是3种飞虱的CPR之间一致性超过95%。但同时也发现,在3种飞虱CPR的氨基酸序列中,FAD和NADPH结合区域之间的氨基酸残基差异较大。哺乳动物CPR的晶体结构显示,FMN和FAD结构域之间的连接区域与辅酶的定向有关,并且有助于稳定CPR的3维结构[9]。但FAD和NADPH结构域之间的连接区域是否具有同样的功能,还有待进一步研究。
SfCPR基因在白背飞虱成虫的不同组织中均能检测到表达,其中以腹部表达量最高,足部最低。这与褐飞虱和灰飞虱中相应CPR基因的表达模式相似[20-21]。同样,SfCPR在白背飞虱不同发育期也均有表达,其中以成虫表达量最高,4龄若虫最低。这一模式与褐飞虱和灰飞虱略有不同,可能是由于物种之间的差异所致[20-21]。此前针对棉铃虫和小菜蛾CPR的研究发现,该基因在杀虫剂抗性种群中的表达水平显著高于敏感种群,而且当试虫受到杀虫剂胁迫后,CPR基因的表达量均显著上升。本研究使用3种不同作用类型的杀虫剂处理白背飞虱若虫后,SfCPR基因的表达水平同样有不同程度的上调。其中,SfCPR对吡虫啉和噻嗪酮的响应较快,处理后6 h表达水平即显著上升;相较而言,SfCPR对溴氰菊酯的响应较慢,处理后12 h表达水平才显著上调。这是首次发现飞虱科昆虫的CPR基因对杀虫剂胁迫产生响应,也暗示了SfCPR基因很可能参与了P450介导的对杀虫剂的解毒过程。此前的报道显示,注射dsRNA沉默褐飞虱和灰飞虱的CPR基因后,试虫对杀虫剂的敏感性显著增加[20-21]。因此,本研究结果也为利用SfCPR开发针对白背飞虱的新型防治技术奠定了前期基础。
[1]FEYEREISEN R. Insect P450 enzymes.AnnualReviewofEntomology, 1999,44:507-533.
[2]ANDERSEN J F, UTERMOHLEN J G, FEYEREISEN R. Expression of housefly CYP6A1 and NADPH-cytochrome P450 reductase inEscherichiacoliand reconstitution of an insecticide-metabolizing P450 system.Biochemistry, 1994,33:2171-2177.
[3]WEN Z, PAN L, BERENBAUM M R,etal. Metabolism of linear and angular furanocoumarins byPapiliopolyxenesCYP6B1 co-expressed with NADPH cytochrome P450 reductase.InsectBiochemistryandMolecularBiology, 2003,33:937-947.
[4]HORIKE N, TAKEMORI H, NONAKA Y,etal. Molecular cloning of NADPH-cytochrome P450 oxidoreductase from silkworm eggs.EuropeanJournalofBiochemistry, 2000,267:6914-6920.
[5]QIU Y, TITTIGER C, WICKER-THOMAS C,etal. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis.ProceedingsoftheNationalAcademyofSciencesoftheUSA, 2012,109:14858-14863.
[6]WANG M, ROBERTS D L, PASCHKE R,etal. Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes.ProceedingsoftheNationalAcademyofSciencesoftheUSA, 1997,94:8411-8416.
[7]LAMB D C, KIM Y, YERMALITSKAYA L V,etal. A second FMN binding site in yeast NADPH-cytochrome P450 reductase suggests a mechanism of electron transfer by diflavin reductases.Structure, 2006,14:51-61.
[8]ELLIS J, GUTIERREZ A, BARSUKOV I L,etal. Domain motion in cytochrome P450 reductase: Conformational equilibria revealed by NMR and small-angle X-ray scattering.JournalofBiologicalChemistry, 2009,284:36628-36637.
[9]XIA C, HAMDANE D, SHEN A L,etal. Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding.JournalofBiologicalChemistry, 2011,286:16246-16260.
[10]HOVEMANN B T, SEHLMEYER F, MALZ J.DrosophilamelanogasterNADPH-cytochrome P450 oxidoreductase: Pronounced expression in antennae may be related to odorant clearance.Gene, 1997,189:213-219.
[11]LYCETT G J, MCLAUGHLIN L A, RANSON H,etal.AnophelesgambiaeP450 reductase is highly expressed in oenocytes andinvivoknockdown increases permethrin susceptibility.InsectMolecularBiology, 2006,15:321-327.
[12]ZHU F, SAMS S, MOURAL T,etal. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug,Cimexlectularius.PLoSONE, 2012,7:e31037.
[13]LIU S, LIANG Q M, HUANG Y J,etal. Cloning, functional characterization, and expression profiles of NADPH-cytochrome P450 reductase gene from the Asiatic rice striped stem borer,Chilosuppressalis(Lepidoptera: Pyralidae).ComparativeBiochemistryandPhysiologyPartB:Biochemistry&MolecularBiology, 2013,166:225-231.
[14]ZHAO C, TANG T, FENG X,etal. Cloning and characterisation of NADPH-dependent cytochrome P450 reductase gene in the cotton bollworm,Helicoverpaarmigera.PestManagementScience, 2014,70:130-139.
[15]CHEN X E, ZHANG Y L. Identification and characterization of NADPH-dependent cytochrome P450 reductase gene and cytochrome b5gene fromPlutellaxylostella: Possible involvement in resistance to beta-cypermethrin.Gene, 2015,558(2):208-214.
[16]程家安,朱金良,祝增荣,等.稻田飞虱灾变与环境调控.环境昆虫学报,2008,30(2):176-182.
CHENG J A, ZHU J L, ZHU Z R,etal. Rice planthopper outbreak and environment regulation.JournalofEnvironmentalEntomology, 2008,30(2):176-182. (in Chinese with English abstract)
[18]祝增荣,程家安.中国水稻害虫治理对策的演变及其展望.植物保护,2013,39(5):25-32.
ZHU Z R, CHENG J A. The evolution and perspective of rice insect pest management strategy in China.PlantProtection, 2013,39(5):25-32. (in Chinese with English abstract)
[19]娄永根,程家安.稻飞虱灾变机理及可持续治理的基础研究.应用昆虫学报,2011,48(2):231-238.LOU Y G, CHENG J A. Basic research on the outbreak mechanism and sustainable management of rice planthoppers.ChineseJournalofAppliedEntomology, 2011,48(2):231-238. (in Chinese with English abstract)
[20]LIU S, LIANG Q M, ZHOU W W,etal. RNA interference of NADPH-cytochrome P450 reductase of the rice brown planthopper,Nilaparvatalugens, increases susceptibility to insecticides.PestManagementScience, 2015,71(1):32-39.
[21]ZHANG Y L, WANG Y M, WANG L H,etal. Knockdown of NADPH-cytochrome P450 reductase results in reduced resistance to buprofezin in the small brown planthopper,Laodelphaxstriatellus(Fallén).PesticideBiochemistryandPhysiology, 2015,127:21-27.
[22]XU Y, ZHOU W W, ZHOU Y J,etal. Transcriptome and comparative gene expression analysis ofSogatellafurcifera(Horváth) in response to southern rice black-streaked dwarf virus.PLoSONE, 2012,7:e36238.
[23]TAMURA K, PETERSON D, PETERSON N,etal. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.MolecularBiologyandEvolution, 2011,28:2731-2739.
[24]LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod.Methods, 2001,25:402-408.
[25]TANG Q Y, ZHANG C X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research.InsectScience, 2013,20:254-260.
[26]YANG C Q, LU S, MAO Y B,etal. Characterization of two NADPH: Cytochrome P450 reductases from cotton (Gossypiumhirsutum).Phytochemistry, 2010,71:27-35.
[27]PARK S, KIM Y S, RUPASINGHE S G,etal. Rice P450 reductases differentially affect P450-mediated metabolism in bacterial expression systems.BioprocessandBiosystemsEngineering, 2013,36:325-331.
[28]LAMB D C, WARRILOW A G S, VENKATESWARLU K,etal. Activities and kinetic mechanisms of native and soluble NADPH-cytochrome P450 reductase.BiochemicalandBiophysicalResearchCommunications, 2001,286:48-54.
Molecular characterization and expression profiles of cytochrome P450 reductase gene in Sogatella furcifera (Hemiptera: Delphacidae).JournalofZhejiangUniversity(Agric. &LifeSci.), 2016,42(4):391-400
YU Hang, LIU Su, ZHU Qingzi, ZHOU Wenwu, LIANG Qingmei, SHI Xiaoxiao, ZHU Zijie, ZHU Zengrong*
(StateKeyLaboratoryofRiceBiology/KeyLaboratoryofAgriculturalEntomology,MinistryofAgriculture/InstituteofInsectSciences,ZhejiangUniversity,Hangzhou310058,China)
cytochrome P450 reductase;Sogatellafurcifera; insecticide; expression profile
农业部公益性行业(农业)科研专项(201403030);国家自然科学基金(31371935);国家重点基础研究发展计划(973计划)(2010CB126200);亚洲发展银行-国际水稻研究所(ADB-IRRI)稻飞虱项目(RETA13,RETA14,RETA15)。
Corresponding author):祝增荣(http://orcid.org/0000-0002-3247-1486),Tel:+86-571-88982355,E-mail:zrzhu@zju.edu.cn
联系方式:余航(http://orcid.org/0000-0002-6651-0797),E-mail:hangyubig@qq.com
2015-12-17;接受日期(Accepted):2016-03-02;网络出版日期(Published online):2016-07-18
S 435.112.3; Q 963
A
URL:http://www.cnki.net/kcms/detail/33.1247.S.20160718.2038.016.html
