分离培养适用于染色体分选的新生小鼠皮肤成纤维细胞
2016-08-06晁天柱徐福意周宇荀肖君华
晁天柱, 徐福意, 徐 伟, 李 凯, 周宇荀, 肖君华
(东华大学 生物科学与技术研究所,上海 201620)
分离培养适用于染色体分选的新生小鼠皮肤成纤维细胞
晁天柱, 徐福意, 徐伟, 李凯, 周宇荀, 肖君华
(东华大学 生物科学与技术研究所,上海 201620)
摘要:采用酶消化法分离培养新生小鼠皮肤成纤维细胞(mouse skin fibroblasts, MSFs).通过噻唑蓝(MTT)比色法和流式细胞术(flow cytometry, FCM),深入探讨不同培养基和血清浓度对MSFs增殖率和同步化效率的影响.结果表明:酶消化法能快速获取大量健康、高活力的MSFs;在杜氏培养液(DMEM)传代培养条件下,P2代MSFs具有高增殖率和有丝分裂指数;地美可辛工作浓度为0.05 μg/mL时,阻断同步化获取M期MSFs的效率最佳.研究结果为流式细胞术分选染色体提供实验材料和研究基础.
关键词:成纤维细胞; 噻唑蓝(MTT); 细胞同步化; 流式细胞术
纯化的染色体可应用于物理作图[1-2]、荧光原位杂交(fluorescence in situ hybridization, FISH)[3-4]、构建DNA文库[5-6]、有丝分裂相关蛋白质[7-8]的研究和二代测序.染色体纯化和测序技术相结合能快速、高效地获取目标染色体的DNA序列信息[9],并广泛应用于动植物个体,如小麦[10]、山羊草[11]、人[12]、小鼠[13]和仓鼠[14]等.在染色体纯化方法中,显微切割技术虽能获得高纯度染色体,但耗时、耗力,得率低[15];磁珠分选法的纯度低,且染色体不完整[16];而流式细胞术(FCM)是染色体纯化效率最高的方法[17].
虽然各种细胞类型都能用于流式细胞术分选染色体[18],但细胞的质量、稳定性和有丝分裂指数,严重影响单染色体悬液的质量和流式分辨率[19].成纤维细胞在适当体外培养条件下可迅速增长繁殖,而具有较高有丝分裂指数的胎鼠成纤维细胞(mouse embryonic fibroblasts, MEFs)是最佳选择[20].但原代培养MEFs需处死母鼠和子鼠,因此针对珍贵的数量性状基因座(quantitative trait locus, QTL)定位新策略“野生小家鼠来源一号染色体替换系”群体(population chromosome substitution strains, PCSSs)[21],选取快捷、方便的新生小鼠皮肤是更合适的选择.
通过优化细胞培养条件提高细胞有丝分裂指数,结合秋水仙酰胺[20]或地美可辛[22]提高有丝分裂中期(mitosis metaphase, M-phase)细胞的比例,能有效降低影响流式分辨率的细胞碎片数量[18,23].本文以PCSSs群体新生小鼠皮肤为样本,利用酶消化法分离培养新生小鼠皮肤成纤维细胞(mouse skin fibroblasts, MSFs),并进一步通过噻唑蓝(MTT)比色法和流式细胞周期检测技术获得MSFs最佳培养条件.
1材料和方法
1.1实验动物
遵守1988年《实验动物管理条例》,在东华大学生物科学与技术研究所屏障动物实验室设施进行实验,获取清洁级PCSSs群体新生小鼠,并在冰冷的碘酊和75%酒精中,分别处理2~3min,备用.
1.2新生小鼠皮肤成纤维细胞培养
取新生小鼠皮肤,磷酸盐缓冲液(PBS,Hyclone)漂洗后用手术刀将其切成尽量小块,胶原酶(1mg/mL, Sigma)37℃消化处理30min;1 500 r/min离心5min,弃上清;PBS重悬清洗,胰酶(0.5mg/mL,含乙二胺四乙酸(EDTA),上海吉泰生物科技有限公司)37℃消化处理20min;离心弃上清;加入含特级胎牛血清(FBS,体积分数为15%,Gibico)和双抗(250 μg /mL青霉素和400 μg/mL链霉素,南京凯基生物科技发展有限公司)的杜氏培养液和F12培养液混合培养基(DMEM/F12,体积比为1∶1,Gibco)培养基,重悬吹散沉淀并接种于T75培养瓶(Corning)中,置入37℃,5% CO2培养箱.用胰酶(2.5 mg/mL)消化传代融合在85%的MSFs,传代培养条件分别为含双抗(50 μg /mL青霉素和80 μg/mL链霉素)的DMEM-15%FBS,DMEM-10%FBS,DMEM/F12-15%FBS和DMEM/F12-10%FBS.取P1~P5代MSFs开展实验.
1.3MTT法评估细胞增殖状况
MSFs以1×104细胞/孔于96孔培养板上接种细胞,培养48h后,加入质量浓度为0.5mg/mL的MTT溶液,37℃,4h后弃上清并加入100μL二甲基亚砜(DMSO,Sigma),15min后采用Multiskan MK3(Thermo)型酶标仪测定490nm处的吸光度值.
1.4MSFs阻断同步化
取P2代MSFs以1×105细胞/孔于6孔培养板接种细胞,在细胞密度约为70%时,分别加入质量浓度为0.20,0.10,0.05 μg/mL的地美可辛.放入培养箱,分别在0,12,14,16,18,20,22 h,用胰酶消化法收集细胞,PBS清洗2次,缓慢加入1mL体积分数为75%的乙醇重悬细胞,-20℃保存备用.
1.5细胞周期分析
取酒精固定MSFs,PBS清洗2次,加入500μL 含核糖核酸酶(100 μg/mL,Sigma)、乙二胺四乙酸(0.003 7 mg/mL,Sigma)和碘化丙啶(50 μg/mL,上海生工生物工程技术有限公司)的混合溶液,避光4℃染色1h.通过BD Accuri C6检测MSFs细胞周期,用BD Csampler software软件分析MSFs细胞周期各时期细胞的百分比.
2结果与分析
2.1新生小鼠成纤维细胞分离培养
利用酶消化法获取大量、高活力新生小鼠成纤维细胞如图1所示.由图1(a)可知,初始培养12h后原代培养的MSFs散布贴壁,细胞呈纺锤形或不规则三角形,有少量上皮细胞,细胞核清晰,无集落.培养2d,细胞生长成单层细胞,呈典型梭形,旋涡状排列或纵横交错(图1(b)).3d左右单层细胞铺满瓶底,可消化传代进行传代培养(图1(c)).传代培养48h细胞可铺满皿底(图1(d)).
2.2MSFs增殖率检测
采用MTT比色法检测各世代MSFs增殖率,测试结果如图2所示.由图2可知,在DMEM培养条件下,P1~P2代MSFs的吸光度最高(吸光度与细胞数量成正比),说明此时MSFs增殖率最高,P3代开始快速下降;DMEM/F12培养条件下,MSFs增殖率逐代下降,且在P3~P4代开始凋亡.P1~P2代MSFs的增殖率,在DMEM和DMEM/F12两种培养基之间具有极显著差异(P<0.000 1),而10%FBS和15% FBS对MSF增殖率无显著影响(P>0.1),说明影响P1~P2代MSF增殖率的主要因素为培养基.

(a) MSFs初始培养12 h(×50)
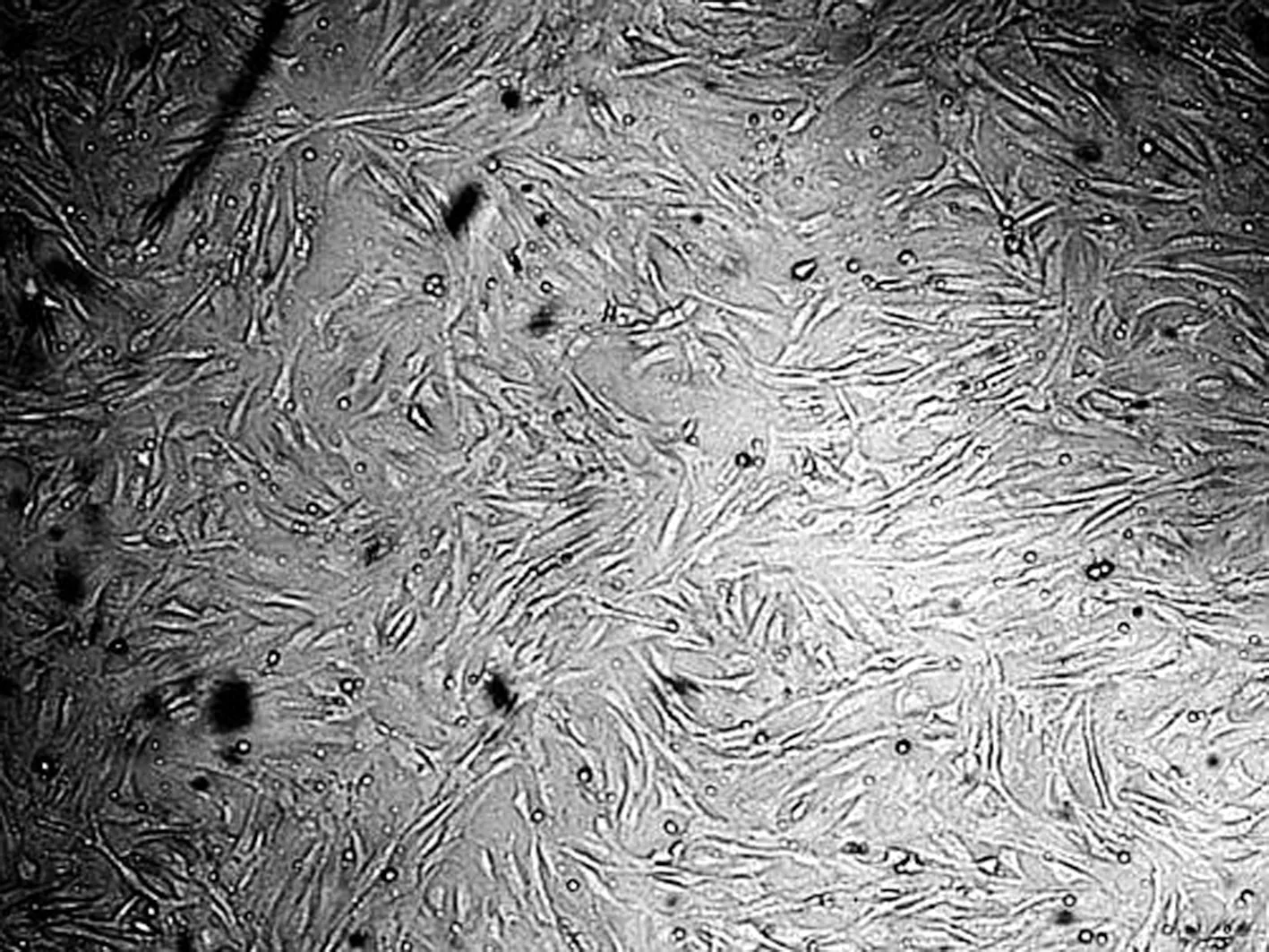
(b) MSFs初始培养2 d(×100)
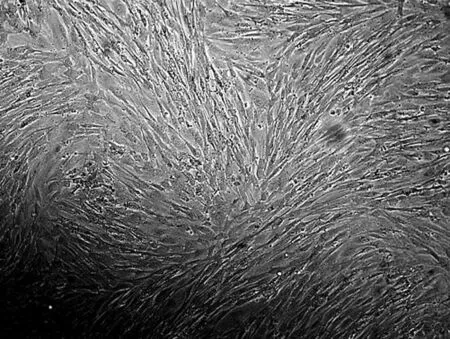
(d) MSFs传代培养48 h(×100)
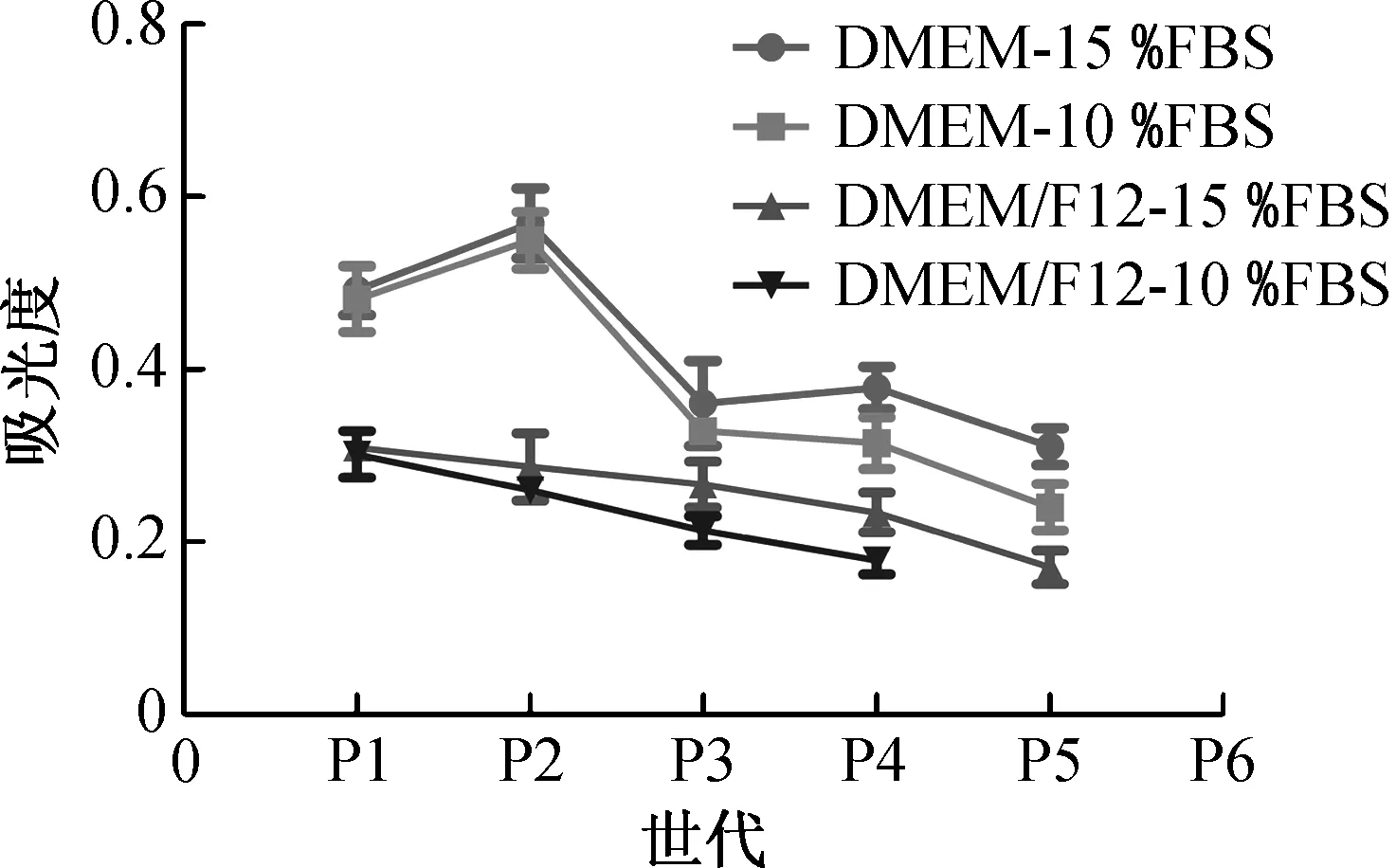
图2 MSFs的增殖率Fig.2 The proliferation rate of MSFs
高浓度双抗既抑制细菌滋生也抑制细胞增殖,MSFs初始培养的双抗浓度高于传代培养,因此P1→P2代MSFs的增殖率逐渐上升.MSFs对氧化压力敏感,在20% O2的培养条件下易发生氧化损伤和传代危机[24-25],氧化压力是导致P3代MSFs活力急速下降的可能原因.虽然DMEM/F12能促进MSFs贴壁和增殖,但会降低MSFs传代次数,是P1~P2代MSFs低增殖率和有丝分裂指数以及P3代MSFs开始凋亡的原因.
2.3流式细胞术检测MSFs细胞周期
采用流式细胞术检测细胞周期,根据单细胞中DNA含量的差异将细胞周期分为 G0/G1期(2倍体)、S期(2~4倍体)和G2/M期(4倍体).表1列举了4种培养条件下,地美可辛(0.05, 0.10,0.20 μg/mL)阻断同步化MSFs停滞在M期,导致G2/M期4倍体MSFs的百分数变化情况.由表1可知,0.05 μg/mL地美可辛阻断同步化MSFs的效率最佳:在DMEM-15%FBS培养条件下,阻断同步化MSFs 18h,G2/M期细胞百分数达到最大(56.4%±0.19%);DMEM-10%FBS为18h(58.6%±0.53%);DMEM/F12-15%FBS为16h(57.4%±0.52%);DMEM/F12-10%FBS为22 h(58.5%±0.69%),都高于0.10和0.20μg/mL地美可辛阻断同步化的效率,且具有显著差异(P<0.05).在DMEM传代培养条件下,指数生长期MSFs(对照组)的有丝分裂指数更高,即G2/M期细胞比例高于DMEM/F12传代培养的MSFs且存在极显著差异(P<0.000 1),表明DMEM更适于MSFs传代培养.
随着地美可辛阻断同步化时间的延长,凋亡MSFs的比例逐渐增加(如表2所示),G2/M期细胞的百分数达到最大值(阻断同步化16~18h)后逐渐下降,说明阻断同步化16h后,M期细胞凋亡率高于其增加率.地美可辛阻断同步化超过16h诱发M期细胞凋亡,生成降低流式分辨率的亚细胞结构[19],因此地美可辛同步化MSFs的时间应小于16h.
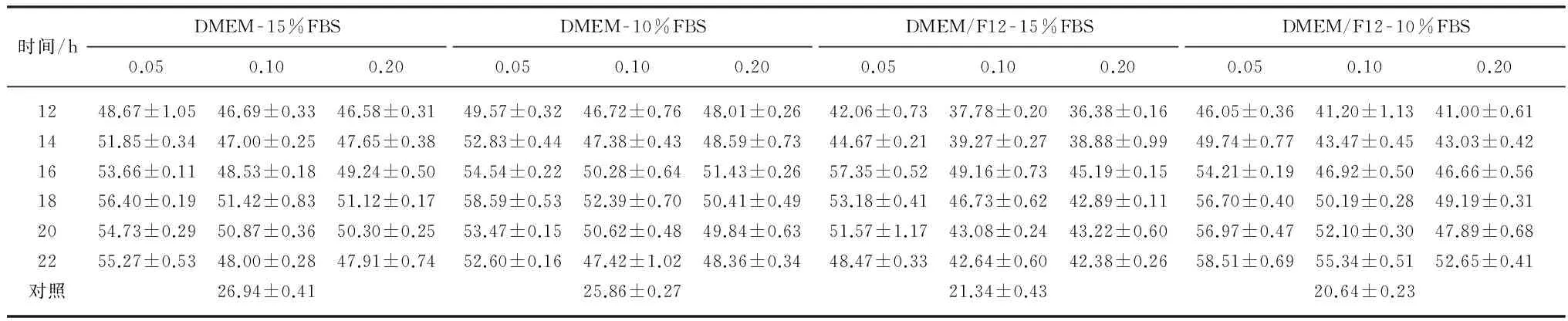
表1 G2/M期MSFs百分数(平均值±标准差)
注:对照样本和地美可辛处理样本之间G2/M期细胞百分数存在极显著差异(P<0.001).
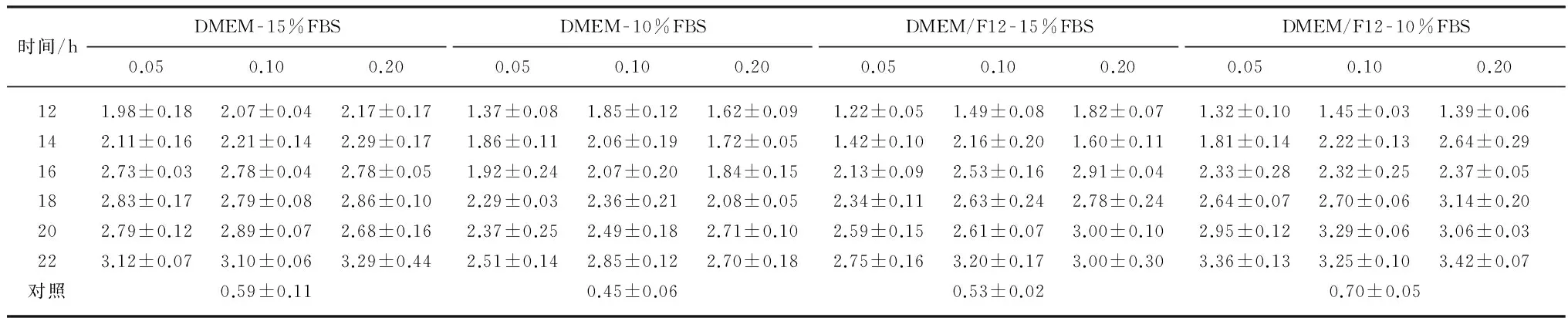
表2 凋亡MSFs百分数(平均值±标准差)
注:4种培养条件下,地美可辛(0.05 ,0.10,0.20 μg/mL)阻断同步化MSFs,凋亡MSFs的百分数.对照样本和地美可辛处理样本之间凋亡细胞百分比数存在极显著差异(P<0.001).
3结语
本文通过酶消化法能快速获得大量具有高增殖率的新生小鼠皮肤成纤维细胞;相比DMEM/F12,在DMEM传代培养条件下MSFs具有更高的增殖率和有丝分裂指数;FCM分析MSFs在不同地美可辛浓度下G2/M期百分数,发现0.05 μg/mL地美可辛阻断同步化MSFs 的效率最高.根据地美可辛处理条件下MSFs凋亡和G2/M百分数的变化规律,发现地美可辛阻断同步化时间应小于16h.
参考文献
[1] NEELY R K, DEEN J, HOFKENS J. Optical mapping of DNA: Single-molecule-based methods for mapping genomes[J]. Biopolymers, 2011, 95(5):298-311.
[2] YONG N D, DEBELLE F, OLDROYD G E, et al. The medicago genome provides insight into the evolution of rhizobial symbiose [J]. Nature, 2011,480:520-524.
[3] HAN Y H, ZHANG Z H, HUANG S W, et al. An integrated molecular cytogenetic map of cucumis sativus L chromosome 2 [J]. BMC Genet, 2011, 12(1):18-24.
[4] SZINAY D, BAI Y, VISSER R, et al.FISH applications for genomics and plant breeding strategies in tomato and other solanaceous crops [J]. Cytogenet Genome Res, 2010, 129(1/2/3):199-210.
[5] KORSTANJE R, GILLISSEN G F, DEN BIEMAN M G, et al.Mapping of rabbit chromosome 1 markers generated from a microsatellite enriched chromosomespecific library [J]. Anim Genet, 2001, 32(5):308-312.
[6] SAFAR J, BARTO S J, JANDA J, et al. Dissecting large and complex genomes: Flow sorting and BAC cloning of individual chromosomes from bread wheat [J]. Plant J, 2004, 39(6):960-968.
[7] FUKUI K. Structural analyses of chromosomes and their constituent proteins [J]. Cytogenet Genome Res, 2009, 124(3/4):215-227.
[8] BIRCHLER J A, GAO Z, HAN F P. A tale of two centromeresdiversity of structure but conservation of function in plants and animals [J]. Funct Integr Genom, 2009, 9(1):7-13.
[9] DOLEŽEL J, VRNA J, SAFJ, et al. Chromosomes in the flow to simplify genome analysis [J]. Funct Integr Genomics, 2012, 12(3): 397-416.
[10] CHOULE F, ALBERTI A, FEUILLET C, et al. Structural and functional partitioning of bread wheat chromosome 3B [J]. Science, 2014, 345: 1249721.
[11] AKPINA R BA, LUCAS S J, BUDAK H, et al. Sequencing chromosome 5D of aegilops tauschii and comparison with its allopolyploid descendant bread wheat (Triticum aestivum) [J]. Plant Biotechnology Journal, 2015,13(6):740-752.
[12] YANG H, CHEN X, WONG W H. Completely phased genome sequencing through chromosome sorting [J]. Proc Natl Acad Sci USA, 2011, 108(1): 12-17.
[13] SUDBERY I, STALKER J, ADAMS D J, et al. Deep shortread sequencing of chromosome 17 from the mouse strains A/J and CAST/Ei identifies significant geimline varitaion and candidate genes that regulate liver triglyceride levels [J]. Genome Biol, 2009, 10(10): R112.1-R112.19.
[14] KURIKI H, SONATA S, MURATA K. Flow karyotype analysis and sorting of theChinese hamster chromosome: Comparing the effects of the isolation buffer [J]. J Clin Lab Anal, 1993, 7(2): 119-122.
[15] DI BUCCHIANICO S, POMA A M, GIARDI M F,et al. Atomic force microscope nanolithography on chromosomes to generate single-cell genetic probes [J]. J Nanobiotechnology, 2011, 9: 27-33.
[16] VITHARANA S N, WILSON G S. Fractionation of chromosome 15 with an affinity-based approach using magnetic beads [J]. Genomics, 2006, 87(1): 158-164.
[17] BARTHOLDI M F, PARSON J D, ALBRIGHT K A, et al.System for flow sorting chromosomes on the basis of pulse shape [J]. Cytometry, 1990, 11(1): 165-172.
[18] TRASK B. Chromosome isolation procedures [M]//GRAY J. Flow Cytogenetics. New York: Academic Press, 1989: 43-60.
[19] METEZEAU P, SCHMITZ A, FRELAT G. Analysis and sorting of chromosomes by flow cytometry: New trends [J]. Bio Cell, 1993, 78(1/2): 31-39.
[20] NG B L, CARTER N P. Factors affecting flow karyotype resolution [J]. Cytometry A, 2006, 69(9): 1028-1036.
[21] XIAO J H, LIANG Y M, LI K, et al. A novel strategy for genetic dissection of complex traits: The population of specific chromosome substitution strains from laboratory and wild mice [J]. Mamm Genome, 2010, 21(7/8): 370-376.
[22] LI Z, CHEN X, SUN X, et al. Nuclear transfer of Mphase ferret fibroblasts synchronized with the microtubule inhibitor demecoline [J]. J Exp Zool A Comp Exp Bio, 2005, 303(12):1126-1134.
[23] LOZES C. Cell culture for chromosome isolation [M]//GRAY J. Flow Cytogenetics. New York: Academic Press, 1989: 35-42.
[24] SELUANOV A, HINE C, GORBNOVA V, et al. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan [J]. Aging Cell, 2008, 7(6): 813-823.
[25] PARRINELLO S, SAMPER E, CAMPISI J, et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts [J]. Nat Cell Bio, 2003, 5(8): 741-747.
文章编号:1671-0444(2016)03-0390-05
收稿日期:2015-06-08
基金项目:国家自然科学基金面上资助项目(31371257);上海市科委关键资助项目(12140900404)
作者简介:晁天柱(1982—),男,山东枣庄人,博士研究生,研究方向为医学分子遗传学.E-mail:chaotianzhu@126.com 肖君华(联系人),男,教授,E-mail:xiaojunhua@dhu.edu.cn
中图分类号:Q 952
文献标志码:A
Isolation and Culturing of Fibroblasts from Newborn Mouse Skin for Chromosome Sorting
CHAOTian-zhu,XUFu-yi,XUWei,LIKai,ZHOUYu-xun,XIAOJun-hua
(Institute of Biological Sciences and Biotechnology,Donghua University, Shanghai 201620, China)
Abstract:The newborn mouse skin fibroblasts (MSFs) were isolated from the skin of newborn mouse using collagenase and trypsin digestion method. And the proliferation and synchronization rate of MSFs were measured by methylthiazolyl-tetrazolium (MTT ) assay and flow cytometry (FCM ) under different blood serum concentration and medium, respectively. Results show that the healthy and highly active MSFs were isolated using enzyme digestion method. In different blood serum concentration and medium, MSFs have the optimum synchronization rate with 0.05 μg/mL demecocline, and highest proliferation rate and mitoticindex under the culture of Dulbecco’s modified eagle medium (DMEM) in passage two. And the research result provides the experimental materials and research foundations for the flow chromosome sorting.
Key words:fibroblast; methylthiazolyl-tetrazolium (MTT ); cell synchronization; flow cytometry
