7种槭树释放挥发性有机化合物组分分析
2016-06-30刘华红张汝民浙江农林大学亚热带森林培育国家重点实验室培育基地浙江临安311300
王 琦,刘华红,王 彬,张汝民,高 岩(浙江农林大学 亚热带森林培育国家重点实验室培育基地,浙江 临安311300)
7种槭树释放挥发性有机化合物组分分析
王琦,刘华红,王彬,张汝民,高岩
(浙江农林大学 亚热带森林培育国家重点实验室培育基地,浙江 临安311300)
摘要:为探讨槭树Acer spp.释放挥发性有机化合物(VOCs)的组分,采用动态顶空气体循环法对苦茶槭A. ginnala,鸡爪槭A. palmatum,三角槭A. buergerianum,樟叶槭A. cinnamomifolium,羊角槭A. yangJuechi,毛脉槭A. pubinerve和青榨槭A. davidii等7种植物释放VOCs进行收集,利用热脱附/气相色谱/质谱(TDS-GC-MS)联用技术对其组分进行分析。结果表明:不同树种释放VOCs种类与相对含量差异明显。苦茶槭和青榨槭分别释放17种和20种成分,以酯类、醛类和醇类物质为主,相对含量较多的有乙酸叶醇酯、癸醛、(Z)-3-己烯-1-醇和壬醛;鸡爪槭、三角槭和毛脉槭分别释放15种、19种和23种成分,以萜类、酯类和醛类物质为主,相对含量较多的为罗勒烯、乙酸叶醇酯、癸醛、长叶烯和壬醛;樟叶槭释放24种成分,以萜类化合物为主,相对含量较多的有罗勒烯、α-蒎烯、3-蒈烯、β-蒎烯和松油烯;羊角槭释放25种成分,以萜类、醛类和醇类物质为主,相对含量较多的有癸醛、长叶烯、2-乙基-1-己醇、石竹烯和壬醛。以上7种槭树均可作为保健型园林植物材料。图3表1参29
关键词:植物学;槭树;挥发性有机化合物;热脱附/气相色谱/质谱联用技术
浙江农林大学学报,2016,33(3):524-530
Journal of ZheJiang A&F University
植物通过次生代谢释放的挥发性有机化合物(volatile organic compounds,VOCs)主要包括萜烯类、苯丙酸类/苯环型和脂肪酸衍生物[1-2]。这些VOCs是植物生长[3]、发育[4]和繁衍[5]以及抵抗不利条件[6-8]的重要手段,在人居环境中影响空气质量[9]和人体健康[10-12]。随着核磁共振和色谱等分析技术的发展,对园林树木释放VOCs的研究逐渐增多。目前,国内研究集中在油松Pinus tabuliformis,侧柏Platycladus orientalis等针叶树上[11, 13],而对阔叶树较缺乏系统研究。槭树Acer spp.隶属槭树科Aceraceae槭树属Acer阔叶乔木或灌木,主产于北温带地区,是温带落叶阔叶林、针阔混交林以及亚热带山地森林的建群种和重要组成,也是针叶林的伴生种,中国槭树种类世界最多,许多槭树为优良荒山绿化和园林造景树种[14]。糖槭A. saccharum,五角枫A. mono,元宝枫A. truncatum,复叶槭A. negundo和挪威槭A. platanoides等释放的VOCs具有信号传导[15]、抑制昆虫[16-17]和真菌[18]的作用,关于其他槭树释放VOCs尚未见报道。因此,本研究以槭树为试验材料,采用活体植株动态顶空气体循环采集法与热脱附/气相色谱/质谱(TDS-GC-MS)联用技术测定不同槭树释放VOCs,旨在探索槭树释放VOCs组分与规律,为进一步研究植物VOCs对环境质量的影响以及植物配置提供依据。
1 材料与方法
1.1材料
以浙江农林大学东湖校区7种不同槭树苦茶槭Acer ginnala,鸡爪槭A. palmatum,三角槭A. buergerianum,樟叶槭A. cinnamomifolium,羊角槭A. yangJuechi,毛脉槭A. pubinerve和青榨槭A. davidii为材料。采集健康无损伤,树龄15 a左右植株枝叶释放VOCs。
1.2 VOCs采集
于2013年7月10-20日上午10:00-11:00,采用动态顶空气体循环法[11]采集7种槭树枝叶释放VOCs。选择生长一致的叶片,采集叶片40片·次-1,3次重复。采气袋容积为0.1 m3,采气时间30 min,气体流量0.1 m3·min-1。
1.3VOCs分析
VOCs分析采用TDS-GC-MS联用技术,仪器及参数设置条件参考文献[11]。TDS(德国GERSTEL公司TD3型)工作条件:系统载气压力20 kPa,进样口温度250℃,脱附温度250℃,10 min,冷阱温度-100℃,保持3 min,冷阱进样时温度骤然升至260℃。GC(7890A,Agilent安捷伦科技有限公司)工作条件:色谱柱为30.00 m×250.00 μm×0.25 μm的HP-5 MS柱;程序升温;初始温度40℃,4 min后以6℃·min-1的速率升至250℃,保持3 min后以10℃·min-1的速率升至270℃,保持5 min。MS (5975C,Agilent安捷伦科技有限公司)工作条件:电离方式为EI,电子能量为70 eV,质量范围为4.67× 10-27~75.02×10-27,接口温度280℃,离子源温度230℃,四级杆温度150℃。
1.4数据处理
采用NIST 2008谱图库兼顾色谱保留时间,同时结合手工检索确定VOCs成分,利用峰面积归一化法测定各组分的百分含量,数据处理采用Origin 8软件。
2 结果与分析
2.1槭树科7种植物释放VOCs成分分析
槭树科7种植物释放的VOCs通过TDS-GC-MS分析(图1),扣除本底空气中的杂质后,共鉴定出48种化合物(表1)。其中苦茶槭鉴定出17种化合物,主要是酯类、醛类和醇类,包括乙酸叶醇酯(63.0%),癸醛(6.5%)和2-乙基-1-己醇(5.6%)等10种化合物,占VOCs总量的89.7%;鸡爪槭检测出15种化合物,主要是酯类、萜类和醇类,包括乙酸叶醇酯(49.6%),长叶烯(9.7%),2-乙基-1-己醇(11.7%)等11种化合物,占VOCs总量的85.5%;三角槭检测出19种化合物,主要是萜类、醛类和酯类,包括罗勒烯(20.3%),长叶烯(10.6%),乙酸叶醇酯(13.0%),癸醛(11.3%)和壬醛(9.2%)等14种化合物,占VOCs总量的84.9%;樟叶槭检测出24种化合物,主要为罗勒烯(24.4%),α-蒎烯(15.6%)和3-蒈烯(11.9%)等18种萜类化合物,占VOCs总量的96.6%;羊角槭检测出25种化合物,主要是萜类、醛类和醇类,包括长叶烯(12.0%),石竹烯(10.1%),癸醛(14.9%),壬醛(8.6%)和2-乙基-1-己醇(11.8%)等17种化合物,占VOCs总量的81.1%;毛脉槭检测出23种化合物,主要为萜类和酯类,包括罗勒烯(11.4%),长叶烯(8.9%)和乙酸叶醇酯(18.3%)等18种化合物,占VOCs总量的79.0%;青榨槭检测出20种化合物,主要是醇类、酯类和醛类,包括乙酸叶醇酯(23.7%),癸醛(15.0%),壬醛(10.1%),(Z)-3-己烯-1-醇(11.1%)和2-乙基-1-己醇(7.7%)等11种化合物,占VOCs总量的80.9%。
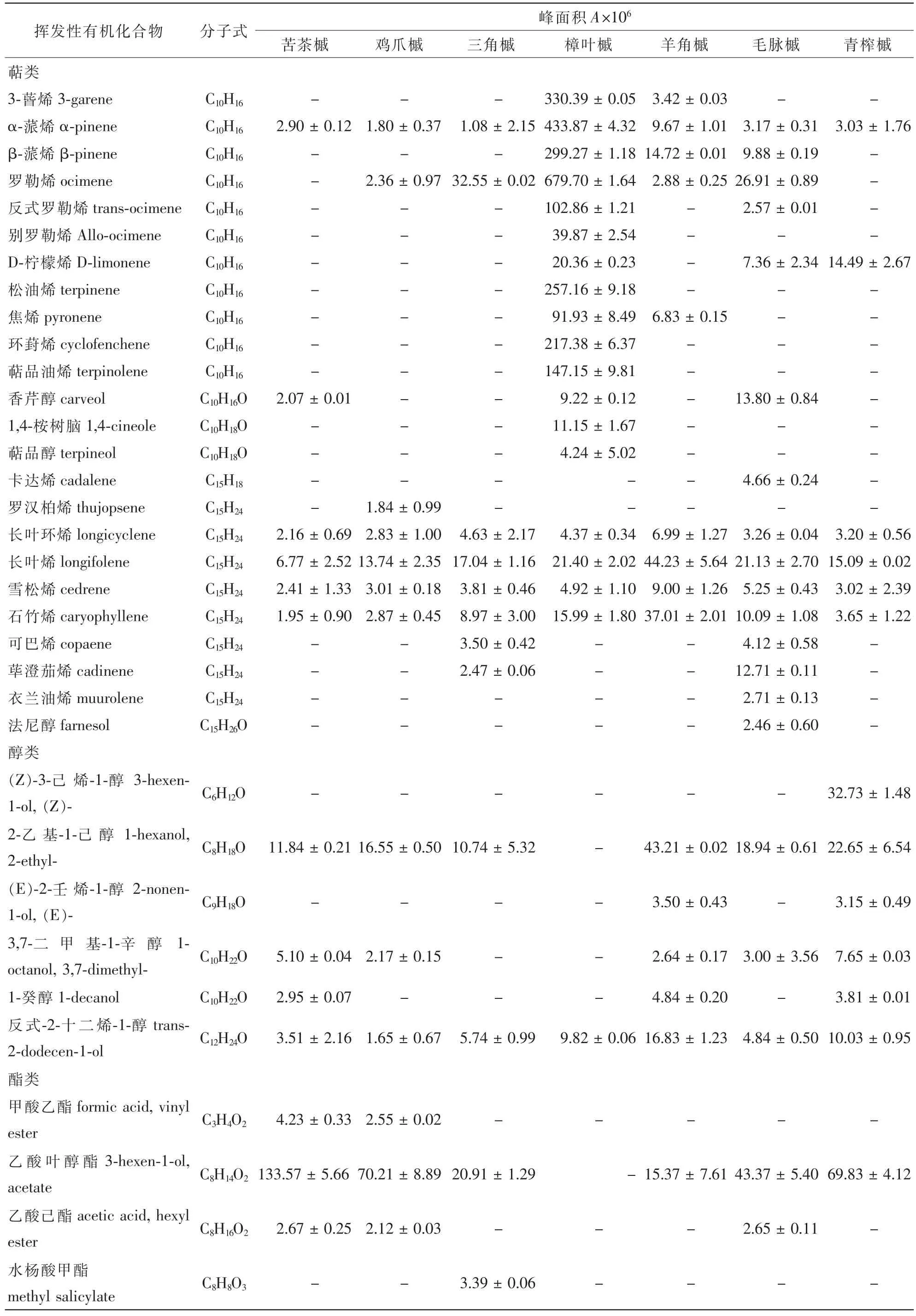
表1 7种槭树释放挥发性有机化合物(VOCs)主要组分(平均值±标准偏差)Table 1 Main components of the volatile organic compounds released from branches and leaves in 7 Acer species(mean±SD)
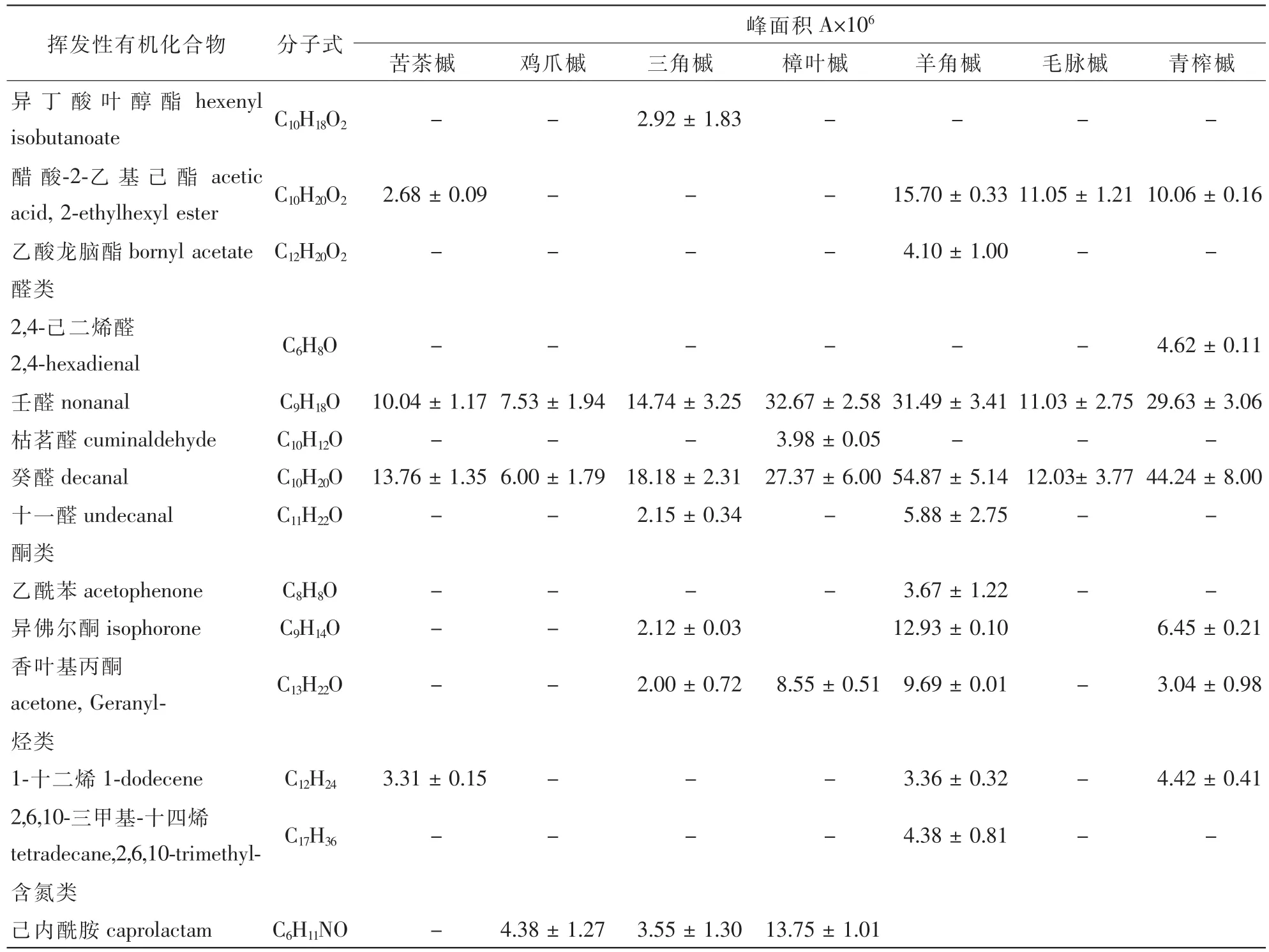
表1 (续)Table 1 (Continued)
槭树科7种植物释放VOCs的共有成分是α-蒎烯、长叶烯、长叶环烯、雪松烯、石竹烯、反式-2-十二烯-1-醇、壬醛和癸醛等8种化合物,分别占苦茶槭、鸡爪槭、三角槭、樟叶槭、羊角槭、毛脉槭和青榨槭各总量的20.5%,26.7%,46.2%,19.8%,57.2%,29.9%和38.0%。常绿树樟叶槭与落叶树苦茶槭、鸡爪槭、三角槭、羊角槭、毛脉槭和青榨槭共有成分分别为21.5%,32.6%,70.0%,67.4%,55.4%和43.9%。特有成分最多的是樟叶槭(24.4%),其次是青榨槭(12.7%)、毛脉槭(4.2%)、三角槭(3.9%)、羊角槭(3.3%)和鸡爪槭(1.3%)。
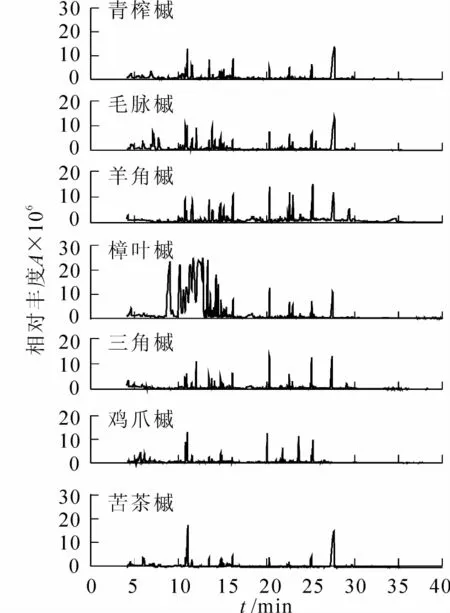
图1 7种槭树释放VOCs的总离子流图Figure 1 Total ion current of volatile organic compounds released from branches and leaves in 7 Acer species
2.2槭树科7种植物释放VOCs种类及差异性比较
7种槭树科植物释放VOCs种类和相对含量存在显著差异(图2)。苦茶槭共有5类化合物,萜类6种(8.6%),醇类4种(11.0%),酯类4种(67.6%),醛类2种(11.2),烃类1种(1.6%);鸡爪槭含有萜类、烃类、醛类等5类化合物,萜类7种(20.1%),醇类1种(11.9%),酯类3种(52.9%),醛类2种(9.6%),含氮化合物1种(3.1%);三角槭包括萜类、酮类、醛类等6类化合物:萜类8种(46.1%),醇类2种(10.3%),酯类3种(17.0%),醛类3种(21.9%),酮类2种(2.6%),含氮化合物1种(2.2%);樟叶槭含有萜类、醇类、醛类等5类化合物:萜类18种(96.6%),醇类1种(0.4%),醛类3种(2.3%),酮类1种(0.3%),含氮化合物1种(0.5%);羊角槭含有萜类、醇类、酯类等6类化合物,萜类9种(36.7%),醇类5种(19.3%),酯类3种(9.6%),醛类3种(25.1%),酮类3种(7.2%),烃类2种(2.1%);毛脉槭含有萜类、醇类、酯类等4类化合物,萜类15种(54.9%),醇类3种(11.3%),酯类3种(24.1%),醛类2种(9.7%);青榨槭含有萜类、醇类、脂类等6类化合物,萜类6种(14.4%),醇类6种(27.2%),酯类2种(27.1%),醛类3种(26.6%),酮类2种(3.2%),烃类1种(1.5%)。萜类化合物含量最高的是樟叶槭,其相对含量分别是苦茶槭、鸡爪槭、三角槭、羊角槭、毛脉槭和青榨槭的14.6倍、4.8倍、2.1倍、2.6倍、2.8倍和6.7倍。在苦茶槭VOCs中脂类化合物相对含量最高,其相对含量是鸡爪槭、三角槭、羊角槭、毛脉槭和青榨槭的1.3倍、4.0倍、7.1倍、2.8倍和2.5倍,在樟叶槭中未检测到。

图2 7种槭树释放VOCs的相对含量Figure 2 Relative contents of VOCs from branches and leaves in 7 Acer species

图3 7种槭树释放VOCs的种类Figure 3 Constituents of VOCs from branches and leaves in 7 Acer species
3 结论与讨论
本研究对华东地区生长的7种槭树释放VOCs研究表明:同属不同种间植物释放VOCs种类和相对含量差异明显。常绿树樟叶槭与落叶类释放VOCs差异较大,说明槭树中常绿类与落叶类释放VOCs差异可能不完全反应组系差异。落叶类槭树间释放VOCs差异较小,共有成分较高(占63.0%~96.0%),其中鸡爪槭在落叶类中共有成分最高(占91.0%~94.0%),可能为所测落叶类槭树释放VOCs的核心类型。本研究中苦茶槭和鸡爪槭主要成分是酯类物质(50.0%以上),与张风娟等[16]测定华北地区生长的4种落叶类槭树释放成分一致;羊角槭释放的α-蒎烯、β-蒎烯、乙酸叶醇酯、长叶烯、长叶环烯和石竹烯等物质,在宋秀华等[19]测试的元宝枫7月释放VOCs中也检测到。这可能与采集方法、发育节律[19]、外界条件[20]、生长地域及亲缘关系等因素有关,槭树释放VOCs调控规律还需深入研究。
萜类化合物在药剂预防和治疗心血管疾病、癌症以及抗菌、抗炎、抗病毒、抗氧化剂、抗高血糖等生物活性方面扮演着一定角色[21]。石竹烯具有镇静、抗焦虑、抗抑郁[22],抗炎[22]和抗肿瘤活性[23];α-蒎烯[24]、3-蒈稀[25]、β-蒎烯[26]能抗炎镇痛;罗勒烯是重要信号分子,抗菌杀虫[27],抗白血病肿瘤细胞增殖[28];萜品油烯能有效抑制低密度脂蛋白氧化[29]。槭树均释放α-蒎烯、石竹烯等萜类物质,樟叶槭富含罗勒烯、α-蒎烯、3-蒈烯、β-蒎烯和萜品油烯,三角槭和毛脉槭主要释放罗勒烯,羊角槭主要释放石竹烯,推测所测槭树有不同程度的保健功能,可作为保健型园林植物材料。苦茶槭和鸡爪槭富含的乙酸叶醇酯(63.1%,49.6%)是一种具有香蕉气味的高级香料,推测其还可种植提取香精。萜类及C6~C10醇醛类物质对细菌、真菌和放线菌有抑制作用[11,18,21],说明槭树具有良好杀菌价值。建议在公园或小区的林荫步道、锻炼区、保健区等活动场所适量配置槭树,以抑制微生物、改善空气质量、预防疾病,发挥槭树资源优势,构建优美人居环境。
4 参考文献
[1]DUDAREVA N, PICHERSKY E. Biochemical and molecular genetic aspects of floral scents[J]. Plant Physiol, 2000, 122(3):627 - 634.
[2]DIXON R A. Natural products and plant disease resistance[J]. Nature, 2001, 411(6839):843 - 847.
[3]左照江,张汝民,王勇,等.冷蒿挥发性有机化合物主要成分分析及其地上部分结构研究[J].植物生态学报,2010,34(4):462 - 468. ZUO Zhaojiang, ZHANG Rumin, WANG Yong, et al. Analysis of main volatile organic compounds and study of aboveground structures in Artemisia frigid[J]. Chin J Plant Ecol, 2010, 34(4):462 - 468.
[4]PICHERSKY E, GERSHENZON J. The formation and function of plant volatiles:perfumes for pollinator attraction and defense[J]. Curr 0pin Plant Biol, 2002, 5(3):237 - 243.
[5]BALDWIN I T, HALITSCHKE R, PASCHOLD A, et al. Volatile signaling in plant-plant interactions:“talking trees”in the genomics era[J]. Science, 2006, 311(5762):812 - 815.
[6]SINGSAAS E L, LERDAU M, WINTER, K., et al. Isoprene increases thermotolerance of isoprene-emitting species [J]. Plant Physiol, 1997, 115(4):1413 - 1420.
[7]LORETO F, VELIKOVA V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes[J]. Plant Physiol, 2001, 127(4):1781 - 1787.
[8]LORETO F, PINELLI P, MANES F, et al. Impact of ozone on monoterpene emissions and evidence for an isoprenelike antioxidant action of monoterpenes emitted by Quercus ilex leaves[J]. Tree Physiol, 2004, 24(4):361 - 367.
[9]CALFAPIETRA C, FARES S, MANES F, et al. Role of biogenic volatile organic compounds(BVOC)emitted by urban trees on ozone concentration in cities:a review[J]. Environ Pollut, 2013, 183:71 - 80.
[10]郑华,金幼菊,周金星,等.活体珍珠梅挥发物释放的季节性及其对人体脑波影响的初探[J].林业科学研究,2003,16(3):328 - 334. ZHENG Hua, JIN Youju, ZHOU Jinxing, et al. A preliminary study on human brain waves influenced by volatiles released from living Sorbaria kirilowii(Regel)Maxim. in different seasons[J]. For Res, 2003, 16(3):328 - 334.
[11]GAO Yan, JIN Youju, LI Haidong, et al. Volatile organic compounds and their roles in bacteriostasis in five conifer species[J]. J Integr Plant Biol, 2005, 47(4):499 - 507.
[12]LEE J, PARK B J, TSUNTESUGU Y, et al. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects[J]. Public Health, 2011, 125(2):93 - 100.
[13]李娟,王成,彭镇华,等.侧柏春季挥发物浓度日变化规律及其影响因子研究[J].林业科学研究,2011,24 (1):82 - 90. LI Juan, WANG Cheng, PENG Zhenhua, et al. The diuranal variation and influence factors of VOC of Platycladus orientalis in spring[J]. For Res, 2011, 24(1):82 - 90.
[14]徐廷志.槭树科的地理分布[J].云南植物研究,1996,18(1):43 - 50. XU Tingzhi. Phytogeography of the family Aceraceae[J]. Acta Bot Yunnan, 1996, 18(1):43 - 50.
[15]BALDWIN I T, SCHULTZ J C. Rapid changes in tree leaf chemistry induced by damage:evidence for communication between plants[J]. Science, 1983, 221(4607):277 - 279.
[16]张风娟,金幼菊,陈华君,等.光肩星天牛对4种不同槭树科寄主植物的选择机制[J].生态学报,2006,26 (3):870 - 877. ZHANG Fengjuan, JIN Youju, CHEN Huajun, et al. The selectivity mechanism of Anoplophora glabripennison four different species of maples[J]. Acta Ecol Sin, 2006, 26(3):870 - 877.
[17]张风娟,金幼菊.茉莉酸甲酯喷施和光肩星天牛Anoplophora glabripennis(Motschulsky)咬食后五角枫释放的挥发物[J].生态学报,2007,27(7):2990 - 2996. ZHANG Fengjuan, JIN Youju, Comparison of volatiles from Anoplophora glabripennis(Motsch.)and methyl jas-monate(MeJA)-applied Acer mono Maxim to identify wound signal transduction pathways[J]. Acta Ecol Sin, 2007, 27(7):2990 - 2996.
[18]张风娟,李继泉,徐兴友,等.皂荚和五角枫挥发性物质组成及其对空气微生物的抑制作用[J].园艺学报,2007,34(4):973-978. ZHANG Fengjuan, LI Jiquan, XU Xingyou, et al. The volatiles of two greening tree species and the antimicrobial activity[J]. Acta Hortic Sin, 2007, 34(4):973-978.
[19]宋秀华,李传荣,许景伟,等.元宝枫叶片挥发物成分及其季节差异[J].园艺学报,2014,41(5):915 - 924. SONG Xiuhua, LI Chuanrong, XU Jingwei, et al. The analysis of volatile organic compounds and seasonal differences emitted from leaves of Acer truncatum[J]. Acta Hortic Sin, 2014, 41(5):915 - 924.
[20]LI Jianguang, JIN Youju, LUO Youqing, et al. Leaf volatiles from host tree Acer negundo:Diurnal rhythm and behavior responses of Anoplophora glabripennis to volatiles in field[J]. Acta Bot Sin, 2003, 45(2):177 - 182.
[21]BAKKALI F, AVERBECK S, AVERBECK D, et al. Biological effects of essential oils-a review[J]. Food Chem Toxicol, 2008, 46(2):446 - 475.
[22]GHELARDINI C, GALEOTTI N, MANNELLI L D C, et al. Local anaesthetic activity of β-caryophyllene[J]. Il Farmaco, 2001, 56(5):387 - 389.
[23]da SILVA S L, FIGUEIREDO P, YANO T. Chemotherapeutic potential of the volatile oils from Zanthoxylum rhoifolium Lam leaves[J]. Eur J Pharmacol, 2007, 576(1):180 - 188.
[24]ORHAN I, KÜPELI E, ASLAN M, et al. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L.[J]. J Ethnopharmacol, 2006, 105(1):235 - 240.
[25]OCETE M A, RISCO S, ZARZUELO A, et al. Pharmacological activity of the essential oil of Bupleurum gibraltaricum:anti-inflammatory activity and effects on isolated rat uteri[J]. J Ethnopharmacol, 1989, 25(3):305 - 313.
[26]LIAPI C, ANIFANDIS G, ANIFANTIS G, et al. Antinociceptive properties of 1, 8-Cineole and beta-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents[J]. Planta Med, 2007, 73(12):1247 - 1254.
[27]SINGH G, SINGH O P, de LAMPASONA M P, et al. Studies on essential oils. Part 35:chemical and biocidal investigations on Tagetes erecta leaf volatile oil[J]. Flavour Frag J, 2003, 18(1):62 - 65.
[28]SAAB A M, TUNDIS R, LOIZZO M R, et al. Antioxidant and antiproliferative activity of Laurus nobilis L.(Lauraceae)leaves and seeds essential oils against K562 human chronic myelogenous leukaemia cells[J]. Nat Prod Res, 2012, 26(18):1741 - 1745.
[29]GRASSMANN J, HIPPELI S, SPITZENBERGER R, et al. The monoterpene terpinolene from the oil of Pinus mugo L. in concert with α-tocopherol and β-carotene effectively prevents oxidation of LDL[J]. Phytomedicine, 2005, 12(6):416 - 423.
Component analysis of volatile organic compounds from branches and leaves in seven Acer species
WANG Qi, LIU Huahong, WANG Bin, ZHANG Rumin, GAO Yan
(The Nurturing Station for the State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Lin’an 311300, Zhejiang, China)
Abstract:To analyze the volatile organic compounds(VOCs)released in Acer spp., VOCs from the branches and leaves of Acer ginnala, Acer palmatum, Acer buergerianum, Acer cinnamomifolium, Acer yangJuechi, Acer pubinerve, and Acer davidii were collected and analyzed by the dynamic headspace air-circulation method and thermal desorption system/gas chromatograhpy/mass spectrum(TDS-GC-MS). Results showed that the species of VOCs and their relative proportions varied significantly with species of Acer spp., A. ginnala and A. davidii released 17 and 20 kinds of VOCs, respectively, most of which were esters, aldehydes, and alcohols, such as 3-hexen-1-ol acetate, decanal,(Z)-3-hexen-1-ol, and nonanal. A. palmatum, A. buergerianum, and A. pubinerve released 15, 19, and 23 kinds, respectively, most of which were terpenes, esters, and aldehydes, such as ocimene, 3-hexen-1-ol acetate,(Z)-decanal, longifolene, and nonanal. A. cinnamomifolium released 24 kinds of VOCs, most of which were terpenes, such as ocimene,α-pinene, 3-carene,β-pinene, and terpinene. A. yangJuechi released 25 kinds, most of which were terpenes, aldehydes, and alcohols, such as decanal, longifolene, 2-ethyl-1-hexanol, caryophyllene, and nonanal. Thus, the health function of VOCs from these Acer species could be utilized in healthcare gardens.[Ch, 3 fig. 1 tab. 29 ref.]
Key Words:botany;Acer;volatile organic compounds;TDS-GC-MS
中图分类号:S718.3;S685.99
文献标志码:A
文章编号:2095-0756(2016)03-0524-07
doi:10.11833/j.issn.2095-0756.2016.03.022
收稿日期:2015-01-24;修回日期:2015-12-10
基金项目:国家自然科学基金资助项目(31270756,31470704)
作者简介:王琦,从事园林植物研究。E-mail:hankywang@hotmail.com。通信作者:王彬,实验师,从事植物生理生态研究。E-mail:wangbin@zafu.edu.cn
