环境内分泌干扰物暴露与儿童肥胖的研究进展
2016-03-23许韶君综述杨胜利审校
吴 浪,许韶君 综述 杨胜利 审校
环境内分泌干扰物暴露与儿童肥胖的研究进展
吴浪1,2,许韶君3综述杨胜利1审校
【摘要】环境内分泌干扰物(endocrine disruptor chemicals, EDCs)在环境中无处不在,通过污染的食物、水及暴露于EDCs的日常使用物品而被人体吸收,并可在人类的血液、尿液中检测到EDCs及其代谢产物。近年来,肥胖发生率逐年增加,已成为世界各国面临的一个严重公共卫生问题。大量研究表明,EDCs会干扰机体内脂肪代谢并可能引发肥胖。笔者对该因素与儿童肥胖之间关联的研究进展综述如下。
【关键词】内分泌干扰物;肥胖症;儿童
【中国图书分类号】R58;R17
作者单位: 1. 100039北京,武警总医院心内科;230032合肥,安徽医科大学:2.第二临床学院,3.公共卫生学院
儿童肥胖已成为一个倍受关注的全球性公共卫生问题。过去几十年里,无论是在发达国家还是发展中国家,儿童肥胖及其相关疾病的患病率均不断增高[1]。有研究表明,儿童超重和肥胖增加了成年期糖尿病、高血压、冠心病、脑卒中的发病率和过早死亡的风险[2]。
不良生活方式是肥胖的重要诱因,最近的研究表明EDCs可能是肥胖的另一个不容忽视的危险因素,并且研究已证实EDCs暴露对肥胖发生的影响,特别是在个体发育的关键时期。儿童处于人体的生长发育阶段,对EDCs暴露尤其敏感[3,4]。笔者将对EDCs暴露与儿童肥胖之间的关联进行综述。
1 EDCs暴露与儿童肥胖的关联
EDCs是指环境中存在的能够干扰生物体内源性激素的合成、释放、转运、结合或清除,从而影响机体的内环境稳定、生殖发育及行为的外源性物质。人类普遍暴露于EDCs中,双酚A、邻苯二甲酸酯(phthalates, PAEs)和持久性有机污染物(persistent organic pollutant, POPs)可以在美国各地几乎所有年龄组人群的脂肪、血液和尿液里被检测到[5]。然而,关于EDCs对人类健康影响的研究仍有限,无论是学术界还是管理部门对于绝大多数EDCs的危害及其作用机制等问题尚存在争议[6]。
1.1双酚A双酚A是世界上使用最广泛的工业化合物之一,作为生产聚碳酸酯塑料和环氧树脂的主要原料,用于生活用品如食品、饮料的包装等。Ryan等[7]研究发现,暴露于双酚A的儿童肥胖和代谢性疾病的发病风险增加。Trasande等[8]研究结果显示,尿中双酚A浓度与儿童青少年肥胖密切相关,尿中双酚A浓度≥1.5 ng/ml的儿童青少年肥胖风险比双酚A浓度<1.5 ng/ml的儿童青少年增大2.57倍。
1.2PAEsPAEs是塑料工业中使用最广泛、品种最多、产量最大的增塑剂,被普遍应用在日常及高分子塑胶产品生产中,如玩具、食品包装材料、乙烯地板和壁纸、润滑剂、洗涤剂、个人护理用品、医用血袋和胶管等。作为普遍存在的EDCs和新的致肥原(obesogens),PAEs是导致肥胖和代谢紊乱的重要危险因素之一[9]。PAEs主要通过呼吸道、消化道、皮肤等途径进入人体。美国国民健康和营养调查(the national health and nutrition examination survey, NHANES)数据表明,在调查儿童的血清中均可检测出PAEs及其代谢产物[10]。Teitelbaum等[11]在研究中发现,超重儿童尿液中PAEs的浓度与其体型呈正相关。
1.3POPsPOPs是指在环境中难降解、高脂溶性、可以在食物链中富集放大, 能够通过各种传输途径而进行全球迁移的一类半挥发性且毒性极大的污染物。尽管大多数POPs被禁止使用,但它们仍广泛应用于工农业生产过程,如农药、建材、纺织、化工、电子电器等,这意味着大多数人在日常生活中都会接触到这类有害物质。人体暴露于POPs中,无论其浓度高低均会导致代谢功能紊乱[12],尽管目前对儿童肥胖与POPs的关联研究有限,但仍有相关研究显示儿童早期接触POPs和肥胖有显著关系[13]。多氯联苯(polychlorinated biphenyl, PCB)是一种广泛应用于工业的POPs,有研究表明儿童的体重指数(body mass index, BMI) 与其血清PCB118浓度呈正相关,而且男孩的体重与其血清中六氯苯(hexachlorobezene, HCB)的浓度成正比[14]。
1.4重金属有机锡、三丁基锡(tributyhin, TBT)和三苯基锡(triphenltin, TPT)和砷是常见的具有内分泌干扰物特性的金属元素,另外,镉、镍、铅和汞也与肥胖相关。TBT作为杀菌剂、热稳定剂和化学催化剂被广泛用于农业和工业,具有促进脂肪生成和储存的作用[15]。动物研究结果表明,长期暴露于低剂量的TBT可以导致肥胖、肝脂肪变性并诱导胰岛素抵抗[16]。
2 EDCs促进肥胖的作用机制
越来越多的研究开始关注EDCs对肥胖的作用机制。现有证据表明EDCs可干扰人体的脂肪代谢和内分泌系统的正常功能,破坏控制体重的稳态机制[17]。EDCs可能通过改变体重和脂质平衡相关的细胞信号通路导致肥胖[18]。EDCs通过激活过氧化物酶体增殖物激活型受体(peroxisome proliferator activated receptor, PPAR),干扰雌激素的信号通路,影响脂肪因子的代谢,从而促进脂肪形成和脂质积累[19,20],见表1。
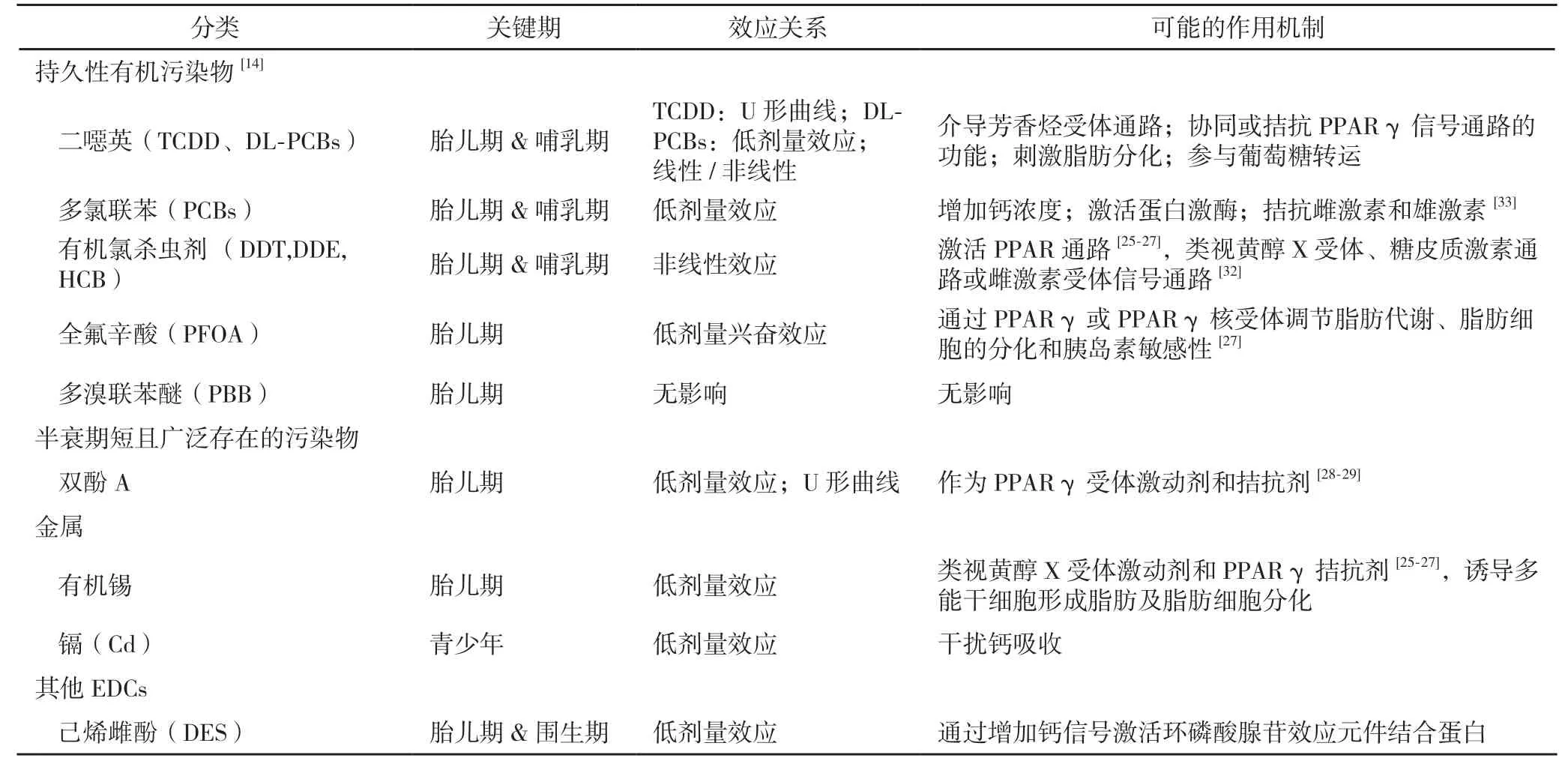
表1 EDCs致儿童肥胖可能的作用机制[13]
2.1激活PPAR核激素受体家族之一的PPAR是脂肪酸活化转录因子,具有调控脂肪代谢和糖代谢的作用。已知有三种亚型:PPAR-α,β/δ和γ,PPARα参与脂类分解,在脂肪酸氧化和脂蛋白代谢的过程中发挥重要作用,而PAEs和多氟烷基化合物可激活体内的PPARα[21,22]。PPARγ是成熟脂肪细胞分化和行使功能所需的核受体,在脂肪生成过程中经诱导表达[23],影响脂肪生成和胰岛素敏感性[24]。越来越多的证据显示EDCs如PAEs、TBT和三氟咪唑(triflumizole, TFZ)等均可激活PPARγ,诱导多能间质干细胞向脂肪细胞分化和增加体内脂肪量[25-27]。双酚A可以诱导激活PPARγ,使PPARγ相关的生理功能受损[28,29]。
2.2干扰雌激素的信号通路激素可以调节机体的生长发育和物质代谢。EDCs通过干扰人体正常激素的合成及代谢,影响内分泌激素的动态平衡[30]。大多数环境EDCs与类固醇激素受体具有较高的亲和力,其中包括雌激素受体(estrogen receptor, ER)、孕激素受体(progestrone receptor, PR)和雄激素受体(androgen receptor, AR)。目前已知的两种雌激素受体是ERα和ERβ。雌激素可激活ERα减少食物摄入和延缓体重增加[30];雌激素还可激活ERβ调节能量代谢过程,包括葡萄糖转运、糖酵解、三羧酸循环及脂肪酸β氧化和合成[31]。EDCs作为ER受体的激动剂或拮抗剂,可以协同或拮抗特定组织的雌激素反应,导致受体依赖的转录信号通路失调,从而扰乱人体的物质代谢平衡[32]。最近发现的雌激素相关受体(estrogen related receptor, ERR)家族的成员之一ERRγ,可以紧密结合双酚A,它们之间具有较高的亲和力,其亲和常数(affinity constant, KD)为5.5 nM[33]。PAE具有雌激素的特性及抗雄激素生物效应,可能会干扰脂肪和碳水化合物的代谢,增加机体肥胖的风险[34]。
2.3影响脂肪因子的代谢脂肪组织不仅被视为一个单纯的脂质贮藏室,现已经演变成一个内分泌器官,在能量平衡中发挥重要作用。脂肪组织可能是肥胖者体内最大的内分泌器官之一,可以分泌产生多种脂肪因子,包括瘦素、抵抗素、脂联素和内脂素等[35]。脂肪因子可导致胰岛素抵抗和中心性肥胖。大脑通过整合各种代谢信号控制能量和体重平衡。瘦素传达外周能量摄入和消耗的信号到大脑,产生抑制食欲和促进能量消耗的信号,以抑制体重增加[36]。脂联素——成熟脂肪细胞的标记物,调节胰岛素敏感性、葡萄糖稳态和脂质生成,对脂肪组织致胰岛素抵抗具有拮抗作用[37]。有研究显示,肥胖儿童脂联素的含量是降低的,尤其伴有代谢综合征的肥胖儿童,其体内的脂联素浓度较不伴有上述疾病的肥胖儿童低[38]。目前已确定一些EDCs影响上述脂肪因子代谢的机制。研究证明,BPA通过影响脂肪细胞分化、脂质积累、葡萄糖运输和脂联素分泌促进体重增加的发生发展[39]。在脂肪细胞的分化过程中,大多数EDCs能够影响瘦素和脂联素的释放,从而诱导脂质积累。
3 小 结
儿童期超重和肥胖对个体的身心健康有着不可忽视的影响,而大量数据表明EDCs可促进儿童肥胖的发生发展。EDCs在多种环境介质(空气、水、土壤和灰尘)中不断被检测到,可以认为EDCs广泛存在于环境中。机体可以通过污染的水和食物等途径吸收EDCs,故随着时间的推移,其对人类健康造成的潜在威胁将越来越显著。EDCs与肥胖及肥胖相关疾病的关联研究是一个令人兴奋的新领域,目前的数据表明其对儿童青少年的生长发育产生不利影响。但人们对EDCs引起肥胖具体作用机制的研究仍存在不足,需要进一步的探索研究。现有证据足以引起人们对EDCs的重视,并促使有关部门制定相关产业及环保方面法律法规的客观依据,并希望能尽快制定出适合我国国情的有效防治方案。
【参考文献】
[ 1 ]Dirinck E, Jorens P, Gaal LV. Obesity and obesity-related diseases: a consequence of our man-made chemical environment? [J]. Health, 2012, 4(12):1556-1561.
[ 2 ]Reilly J J, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review [J]. Int J Obes, 2011, 35(7): 891-898.
[ 3 ]Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity [J]. Birth Defects Res C Embryo Today, 2011, 93 (1): 34-50.
[ 4 ]Newbold R R. Impact of environmental endocrine disrupting chemicals on the development of obesity [J]. Hormones( Athens), 2010, 9(3): 206-217.
[ 5 ]Nadal A. Obesity: fat from plastics? Linking bisphenol A exposure and obesity [J]. Nat Rev Endocrinol, 2012, 9(1): 9-10.
[ 6 ]Sax L. Polyethylene terephthalate may yield endocrine disruptors [J]. Environ Health Perspect, 2010, 118(4): 445-448.
[ 7 ]Ryan K K, Haller A M, Sorrell J E, et al. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice [J]. Endocrinology, 2010, 151(6): 2603-2612.
[ 8 ]Trasande L, Attina T M, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents [J]. JAMA, 2012, 308(11): 1113-1121.
[ 9 ]Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption [J]. Annu Rev Physiol, 2010, 73(1): 135-162.
[10]Fourth national report on human exposure to environ-mental chemicals [EB/OL]. [2015-11-23]. http://www.cdc. gov/exposurereport/pdf/FourthReport.pdf
[11]Teitelbaum S L, Mervish N, Moshier E L, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children [J]. Environ Res, 2012, 112(112): 186-193.
[12]Ruzzin J. Public health concern behind the exposure to persistent organic pollutants and the risk of metabolic diseases [J]. BMC Public Health, 2012, 12(9): 1-8.
[13]Grant K L. Environmental contributions to obesity and type 2 diabetes [J]. J Environ Immunol Toxicol, 2013, 1(2): 89-91.
[14]Dhooge W, Den Hond E, KoppenG, et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships [J]. Environ Int, 2010, 36(4): 330-337.
[15]Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes [J]. J Steroid Biochem Mol Biol, 2011, 127(1-2): 9-15.
[16]Zuo Z, Chen S, Wu T, et al. Tributyltin causes obesity and hepatic steatosis in male mice [J]. Environ Toxicol, 2011, 26(1): 79-85.
[17]Thayer K A, Heindel J J, Bucher J R, et al. Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review [J]. Environ Health Perspect, 2012, 120(6): 779-789.
[18]Hatch E E, Nelson J W, Stahlhut R W, et al. Association of endocrine disruptors and obesity: perspectives from epidemiologic studies [J]. Int J Androl, 2010, 33(2): 324-332.
[19]Cock M D, Legler J, Bor M V D. Endocrine disrupting chemicals( EDCS) and childhood obesity: what do epidemiological studies tell us? [J]. Pediatric Research, 2011, 70(2): 368.
[20]Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity [J]. Int J Androl, 2012, 35(3): 437-448.
[21]Desvergne B, Feige J N, Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? [J]. Mol Cell Endocrinol, 2009, 304(1-2): 43-48.
[22]Abdelmegeed M A, Yoo S H, Henderson L E, et al. PPARalpha expression protects male mice from high fatinduced nonalcoholic fatty liver [J]. J Nutr, 2011, 141(4): 603-610.
[23]Eeckhoute J, Oger F, Staels B, et al. Coordinated regulation of PPARγ expression and activity through control of chromatin structure in adipogenesis and obesity [J]. PPAR Res, 2012, 2012(7): 1641-1640.
[24]Auwerx J. PPARgamma, the ultimate thrifty gene [J]. Diabetologia, 1999, 42(9): 1033-1049.
[25]le Maire A, Grimaldi M, Roecklin D, et al. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors [J]. EMBO Rep, 2009, 10(4): 367-373.
[26]Li X, Pham H T, Janesick A S, et al. Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma( PPAPγ) [J]. Environ Health Perspect, 2012, 120(12): 1720-1726 .
[27]Taxvig C, Dreisig K, Boberg J, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation [J]. Mol Cell Endocrinol, 2012, 361(1-2): 106-115.
[28]Riu A, le Maire A, Grimaldi M, et al. Characterization of novel ligands of ERα, Erβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites [J]. Toxicol Sci, 2011, 122(2): 372-382.
[29]Riu A, Grimaldi M, le Maire A, et al. Peroxisome proliferator-activated receptor γis a target for halogenated analogs of bisphenol A [J]. Environ Health Perspect, 2011, 119(9): 1227-1232.
[30]Brown L M, Gent L, Davis K, et al. Metabolic impact of sex hormones on obesity [J]. Brain Res, 2010, 1350(2): 77-85.
[31]Vom Saal F S, Nagel S C, Coe B L, et al. The estrogenic endocrine disrupting chemical bisphenol A(BPA) and obesity [J]. Mol Cell Endocrinol, 2012, 354(1-2): 74-84.
[32]Craig Z R, Wang W, Flaws J A. Endocrine-disrupting chemicals in ovarian function: effects on steroid genesis, metabolism and nuclear receptor signaling [J]. Reproduction, 2011, 142(5): 633-646.
[33]Babu S, Vellore N A, Kasibotla A V, et al. Molecular docking of bisphenol A and its nitrated and chlorinated metabolites onto human estrogen-related receptor-gamma [J]. Biochem Biophys Res Commun, 2012, 426(2): 215-220.
[34]Trasande L, Attina T M, Sathyanarayana S, et al. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample [J]. Environ Health Perspect, 2013,121(4): 501-506.
[35]Rho Y H, Solus J, Sokka T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis [J]. Arthritis Rheum, 2009, 60(7): 1906-1914.
[36]Morris D L, Rui L. Recent advances in understanding leptin signaling and leptin resistance [J]. Am J Physiol Endocrinol Metab, 2009, 297(6): e1247-e1259.
[37]Turer A T, Scherer P E. Adiponectin: mechanistic insights and clinical implications [J]. Diabetologia, 2012, 55(9): 2319-2326.
[38]Klünder-Klünder M, Flores-Huerta S, Garcia-Macedo R, et al. Adiponectin in eutrophic and obese children as a biomarker to predict metabolic syndrome and each of its components [J]. BMC Public Health, 2013, 13(2): 330-360.
[39]Rubin B S, Soto A M. Bisphenol A: perinatal exposure and body weight [J]. Mol Cell Endocrinol, 2009, 304(1-2):55-62.
(2016-01-05收稿2016-01-22修回)
(责任编辑宋宫儒)
Research progress of the effect of environmental endocrine disruptor chemicals on obesity in children
WU Lang1,2, XU Shaojun3, and YANG Shengli1. 1. Department of Cardiology, General Hospital of Chinese People’s Armed Police Forces, Beijing 100039, China;2. The Second Clinical College, 3. School of Public Health,Anhui Medical University, Hefei 230032, China
【Abstract】Environmental endocrine disruptor chemicals(EDCs) are ubiquitous in the environment. The absorption of EDCs into human body can be through EDC-exposed food, water and everyday items. The detection of EDCs and its metabolites in human blood and urine indicates that EDCs have a deleterious impact on human health. In recent years, the increasing incidence of obesity has become a serious public health problem in the world. A large number of studies have manifested that EDCs can disturb human natural lipometabolism and cause obesity. In this paper, the correlation between EDCs and obesity in children are reviewed.
【Keywords】endocrine disruptors; obesity; child
Corresponding author:YANG Shengli, E-mail: yangshengli@medmail.com.cn
通讯作者:杨胜利,E-mail:yangshengli@medmail.com.cn
作者简介:吴浪,硕士研究生在读,E-mail:wulang56@sina.com
DOI:10.13919/j.issn.2095-6274.2016.02.013
