Reversal effects of desipramine on resistance of U251/TR cells to temozolomide
2016-02-16MAJianYANGYanruLIUJingjingLIFangfangCHENMeihuaWANGHaoWANGLeiSUNLiliWANGFengzeWANGDecaiHantingZHANG
MA Jian*,YANG Yan-ru*,LIU Jing-jing,LI Fang-fang,CHEN Mei-hua,WANG Hao, WANG Lei,SUN Li-li,WANG Feng-ze,WANG De-cai,Han-ting ZHANG,3
(1.Institute of Pharmacology,2.School of Life Science,Taishan Medical College,Taian 271016,China;3.Departments of Behavioral Medicine&Psychiatry and Physiology&Pharmacology,West Virginia University College of Medicine,Morgantown,WV 26506,USA)
·ORIGINAL ARTICLES·
Reversal effects of desipramine on resistance of U251/TR cells to temozolomide
MA Jian1*,YANG Yan-ru1*,LIU Jing-jing1,LI Fang-fang1,CHEN Mei-hua1,WANG Hao1, WANG Lei1,SUN Li-li1,WANG Feng-ze2,WANG De-cai1,Han-ting ZHANG1,3
(1.Institute of Pharmacology,2.School of Life Science,Taishan Medical College,Taian 271016,China;3.Departments of Behavioral Medicine&Psychiatry and Physiology&Pharmacology,West Virginia University College of Medicine,Morgantown,WV 26506,USA)
OBJECTIVETo examine the reversal effect of desipramine(DMI)on resistance to temozolomide(TMZ)in U251/TR cells and explore its mechanism.METHODSU251/TR cells were exposed to DMI(20-80 μmol·L-1)or TMZ(0.5-10 mmol·L-1)for 24 h,cell viability was determined by cell counting kit-8 assay with IC50calculated.The cytotoxicity of U251/TR cells treated with TMZ(1 or 2 mmol·L-1)in combination with DMI(20,30 or 40 μmol·L-1)for 24 h was detected using CCK-8 assay.Synergism between DMI and TMZ was analyzed by the JIN Zheng-jun method.Apoptosis of U251/TR cells induced by TMZ 1 mmol·L-1,DMI 30 μmol·L-1,or their combination was examined by Hoechst33258 stains and caspase 3 activity was detected by luminescence analysis.Expression of C/EBP homologous protein (CHOP)was measured using quantitative real-time PCR and Western blotting.The survival rate of U251/TR cells treated with TMZ 1 mmol·L-1and/or DMI 30 μmol·L-1was also assessed after silencing CHOP expression by small interference RNA(siRNA).RESULTSDMI or TMZ alone inhibited the growth of U251/TR cells significantly in a concentration-dependent manner(r2=0.983,0.982,P<0.05), with the IC50(33.6±0.5)μmol·L-1and(2.5±0.6)mmol·L-1,respectively.The cell viability inhibitory rate of U251/TR cells by TMZ(1 or 2 mmol·L-1)combined with DMI(20,30,or 40 μmol·L-1)was greater than that by TMZ or DMI alone(P<0.05).The JIN Zheng-jun analysis revealed that combination of DMI and TMZ produced synergistic cytotoxicity(Q>1.15),ie,compared with TMZ alone,TMZ(1 mmol·L-1)com⁃bined with DMI(30 μmol·L-1)produced significant nuclear fragmentation and condensation(P<0.05). In addition,DMI and TMZ in combination activated caspase 3 activity in U251/TR cells(P<0.05).Knock⁃down of CHOP by specific siRNA attenuated the synergistic effect of DMI in the presence of TMZ,the survival rate of the combined drug group raised from 51.8%to 62.2%(P<0.05).CONCLUSIONThe results suggest that DMI reverse resistance of U251/TR cells to TMZ through activation of the CHOP-depend⁃ently apoptosis pathway.
glioma;desipramine;temozolomide;drug resistance;C/EBP homologous protein
Glioma is the most common and primary malignant brain tumor[1].However,glioma is difficult to be resected completely in surgery because of the special location.As a result,the current standard therapy for glioma is surgical resection followed by radiotherapy plus concomitant chemotherapy. Temozolomide (TMZ)is the mosteffective chemotherapeutic agent for treatment of glioma[2]. However,the efficacy of glioma treatment is severely reduced by the resistance to TMZ.It is of importance to develop therapeutic approaches to overcome this issue.A large number of preclinical and clinical studies have been carried out attempting to find drugs that can enhance the efficacy of TMZ[3-5].One example is the combined use of antidepressants[6-7]. Clinical studies have demonstrated that depressive disorders are common comorbidity in patients with advanced cancer,leading to the use of antide⁃pressants as conventional adjuvant agents in treatment of cancer comorbid with depression[8-9]. The direct biological effects of antidepressant drugs on tumor cells have attracted much attention in recent years.Desipramine(DMI),a classic antidepressant of TCAs,improves the mood of patients with depressive disorders.It also exerts various off-target effects such as analgesic and antitumor actions[10-11].DMI has been reported to inhibitcancer cellproliferation and induce apoptosis[12-13].Our previous study has also demonstrated that DMI induces apoptosis of glioma C6 cells[14].In the present study,we investigated the ability of DMI to reverse drug resistance to TMZ in the U251/TR glioma cellline and explored the related mechanisms.
1 MATERIALS AND METHODS
1.1 Drugs
DMI and TMZ were purchased from Sigma-Aldrich(St.Louis,MO,USA).The antibody against CHOP was purchased from Cell Signaling Technology(Danvers,MA,USA),whilethe antibody against β-actin and horseradish peroxidaseconjugated anti-mouse or anti-rabbit IgG were from Santa Cruz Biotechnology(Santa Cruz,CA, USA).
1.2 Cell culture
U251 cells were purchased from the Chinese Academy of Sciences Cell Bank(Shanghai,China). The U251/TR cell line was induced by the stepwise revulsion with TMZ.U251/TR cells were cultured in Dulbecco′s modified Eagle′s medium(DMEM)( (Invitrogen,Carlsbad,CA),supplemented with 10% fetal bovine serum (Invitrogen,Carlsbad, CA)and 1%penicillin/streptomycin(Invitrogen, Carlsbad,CA),and maintained in a humidified in⁃cubator with 5%CO2at 37℃.
1.3 CCK-8 assay for detection of cell viability
The viability of cells was analyzed by cell counting kit-8(CCK-8)kit(Dojindo Laboratories,Ku⁃mamoto,Japan).U251/TR cells were seeded on a 96-well plate at a density of 5000 cells/well and were cultured overnight.Various concentrations of DMI(20,30,40,50,60,70,or 80 μmol·L-1),TMZ (0.5,0.7,1,3,5,7,or 10 mmol·L-1),or the com⁃bination of TMZ(1 or 2 mmol·L-1)and DMI(20, 30 or 40 μmol·L-1)were then added to the wells and cultured for 24 h.Cell viability was assessed following the procedures described in the manu⁃facturer′s instructions.
1.4 Hoechst33258 staining for observation of cell apoptosis
U251/TR cells(1×105per well)were seeded on sterile cover glasses placed in the six-well plates and cultured overnight.The cells were then exposed to DMI 30 μmol·L-1+TMZ 1 mmol·L-1for 24 h,after which cells were fixed and washed twice with phosphate-buffer saline(PBS)and stained with Hoechst 33258 staining for 1 h(Bey⁃otime,Jiangsu,China).Stained nuclei were ob⁃served under a confocal microscope(Leica,Hei⁃delberg,Germany).
1.5Luminescence analysisof caspase 3 activity
U251/TR cells(3000 per well)were seeded on a 96-well microplate and incubated with DMI 30 μmol·L-1+TMZ 1 mmol·L-1for 24 h,followed by mixing with 100 μL of Caspase-Glo®3/7 Reagent(Promega,Madison,USA)at room tem⁃perature for 1 h.The luminescence intensities were analyzed with Multimode Reader (Tecan, Männedorf,Switzerland);enzyme activity was ex⁃pressed as a relative luminescence unit.
1.6 Quantitative real-time(RT-PCR)for deter⁃mining CHOP expression
U251/TR cells(5×105per well)were cultured on a 6-well plate and exposed to DMI(30 μmol·L-1) and TMZ(1 mmol·L-1)for 24 h.Total RNA extraction was isolated from cells by using RNAprep Pure Cell/Bacteria Kit(Tiangen,Beijing,China). The first strand cDNA synthesis was carried out using Quantscript RT cDNA Kit(Tiangen,Beijing, China).The RT mixture was mixed with Real Master Mix(SYBR green)agent(Tiangen,Beijing, China)and gene-specific primers in a final volume of 20 μL.Primers used for human CHOP were 5′-GAAACGGAAACAGAGTGGTCATTCCCC-3′and 5′-GTGGGATTGAGGGTCACATCATTGGCA-3′; for human β-actin were 5′-AGACGCAGGATG⁃GCATGGG-3′and 5′-GAGACCTTCAACACCCCAGCC-3′.The quantitative real-time RT-PCR was determined in ABI 7500 fast Real Time PCR (Life Technologies,CA,USA)using the following amplification conditions:hold for 2 min at 95℃, followed by 40 cycles of 95℃ for 30 s,60℃ for 20 s and 68℃ for 60 s.Data are expressed as the fold differences in target gene expression compared with both the endogenous control gene expression and the calibrator,using the 2-△△Ctmethod.
1.7 Western blotting analysis for detection of CHOP expression
U251/TR cells(5×105per well)were cultured on a 6-well plate overnight.Cells were incubated with DMI(30 μmol·L-1)and TMZ(1 mmol·L-1)for 24 h,followed by washing with ice-cold 1×PBS for twice and lysed cells in cold RIPA buffer. Identical amounts of cell lysates were separated by 10%or 12%SDS-PAGE,and proteins were electro-transferred onto PVDF membranes(Millipore, Bedford,MA,USA).Membraneswerethen incubated in blocking buffer(5%nonfat milk in TBS containing 0.1%Tween 20)at room temper⁃ature for 2 h.After washing in TBST buffer〔10 mmol·L-1Tris-HCl(pH 8.3),0.05%Tween-20 (Sigma Ultra,St.Louis,MO,USA)〕,membranes were incubated with primary antibodies against CHOP(1∶1000)and β-actin(1∶1000)as the control at 4℃ overnight before incubating with HRP-conjugated secondary antibodies(1∶5000, Zhongshan,Beijing,China)at room temperature for 2 h.The signal was visualized by chemilumi⁃nescence detection kit(Pierce Chemical,Rock⁃ford,IL,USA).
1.8 Small interference RNA(siRNA)experiments
The CHOP-specific siRNAs-(5′-GCUAGCUGAAGAGAAUGAATT-3′and 5′-UUCAUUCU⁃CUUCAGCUAGCTT-3′),scrambled siRNA(5′-UUCUCCGAACGUGUCACGUTT-3′and 5′-AC⁃GUGACACGUUCGGAGAATT-3′and negative control)were designed and synthesized by GenePharma(GenePharma,Shanghai,China). For transfection,cells were plated in a 12-well plate or a 96-well plate overnight before trans⁃fecting with siRNAs using Lipofectamine 2000 (Invitrogen Life Technologies,Carlsbad,CA) after 60%confluence was achieved.The knock⁃down effect was measured by quantitative realtime RT-PCR.Cells were then incubated with DMI 30 μmol·L-1+TMZ 1 mmol·L-1for 24 h,and then washed with medium before CCK-8 assays.
1.9 Statistical analysis
Data are presented asx±sand were analyzed by analysis ofvariances(ANOVA) followed by Dunnett′sttest,whereP<0.05 indicated a significantdifference.Synergistic analysis is presented with Q value,which was calculated by the JIN Zheng-jun method[15].Q>1.15 represents synergism,Q=0.85-1.15 addition, and Q<0.85 antagonism.
2 RESULTS
2.1 Effects of DMI and/or TMZ on U251/TR cell viability
As shown in Fig.1,DMI(20-80 μmol·L-1)or TMZ(0.5-10 mmol·L-1)treatment alone for 24 h reduced cellviability,as demonstrated by concentration-dependent inhibition of cell proliferation(Fig.1A,B);the IC50was(33.6±0.5)μmol·L-1for DMI and(2.5±0.6)mmol·L-1for TMZ.To examine the potential synergistic cytotoxicity,U251/TR cells were treated with lower concentrations of DMI (20,30 and 40 μmol·L-1)and TMZ(1 and 2 mmol·L-1)for 24 h,either alone or in combination. The combination of DMI and TMZ at certain concentrations produced remarkable potentiation in decreasing viability of U251/TR cells(P<0.05) (Fig.1C).Synergistic analysis is presented with Q values(Tab.1).It was observed that combination of DMI and TMZ produced a significant synergistic effect with the Q value>1.15.

Fig.1 Effect of desipramine(DMI)and temozolomide (TMZ)on U251/TR cell viability.U251/TR cells were exposed to DMI,TMZ,or their combination for 24 h.x±s,n=3.**P<0.01,compared with corresponding normal control group;#P<0.05,compared with corresponding TMZ alone group;△P<0.05,compared with corresponding DMI alone group.

Tab.1 Q values of combined administration of DMI and TMZ
2.2 Effects of DMI and/or TMZ on U251/TR apoptosis
Fig.2 showed that DMI(30 μmol·L-1)or TMZ (1 mmol·L-1)treatment alone for 24 h produced remarkable morphological changes in U251/TR cells.The nuclear size of U251/TR cells was decreased and the Hoechst fluorescence intensity in nuclei was increased,indicating the typical phenotype of apoptosis.In combination treatment, the morphological changes in nuclei of U251/TR cells were even more pronounced.

Fig.2 Effect of TMZ and DMI alone or in combination on U251/TR cell morphological changes by laser scanning confocal microscope. U251/TR cells were exposed to TMZ(1 mmol·L-1),DMI(30 μmol·L-1),or both for 24 h.
2.3 Effects of DMI and/or TMZ on caspase 3 activity of U251/TR cells
As shown in Fig.3,after treatment with DMI(30 μmol·L-1)or TMZ(1 mmol·L-1)alone, caspase 3 activity in U251/TR cells was signifi⁃cantly increased.This effect was further potentiated by combination of both treatments(P<0.05).
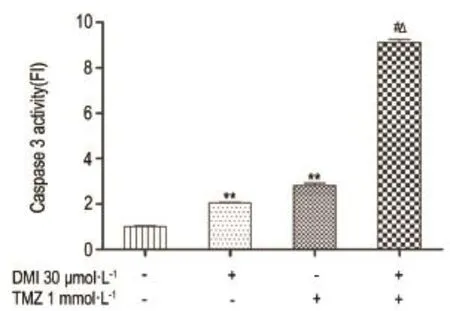
Fig.3 Effect of TMZ and DMI alone or in combination on caspase 3 activity of U251/TR cell.See Fig.2 for the treatment.x±s,n=3.**P<0.01,compared with normal control group;#P<0.05,compared with DMI alone group;△P<0.05,compared with TMZ alone group.
2.4Effects of DMIand/or TMZ on CHOP mRNA and protein expression in U251/TR cells
Treatment with DMI(30 μmol·L-1)or TMZ (1 mmol·L-1)alone robustly upregulated CHOP mRNAs in U251/TR cells(Fig.4);protein expression of CHOP was concomitantly increased following DMI or TMZ treatment(Fig.5).The effects of TMZ on CHOP mRNA or protein levels were markedly potentiated in the presence of DMI(P<0.05).
2.5 Effects of DMI and/or TMZ on CHOP-specific siRNA transfected U251/TR cell viability

Fig.4 Effect of TMZ and DMI alone or in combination for 24 h on CHOP mRNA expression in U251/TR cells detected by quantitative real-time RT-PCR.x±s,n=3.*P<0.05,compared with DMI alone;#P<0.05,compared with TMZ alone.

Fig.5 Effect of TMZ and DMI alone or in combination for 24 h on CHOP protein expression in U251/TR cells by Western blotting.B was the semi-quantitative result of A.x±s,n=3.*P<0.05,compared with normal control group;#P<0.05,compared with DMI alone group;△P<0.05,compared with TMZ alone group.
To further determine whether CHOP was involved in the synergistic effect of the combination treatment,CHOP expression was silenced by CHOP-specific siRNAs.The mRNA level of CHOP in U251/TR cells was significantly reduced to 19.2%by transfection of CHOP specific siRNAs. The survival rate of the combined drug group raised from 51.8%to 62.2%(P<0.05)after CHOP knockdown by CHOP-specific siRNA(Fig.6). These results suggest thatDMI increase sensitivity of glioma cells to TMZ by activating CHOP.

Fig.6 Effect of TMZ or DMI alone or in combination on survival of U251/TR cells after CHOP knockdown by CHOP-specific siRNAs(si-CHOP).U251/TR cells were firstly transfected with CHOP-specific siRNAs or scrambled siRNAs (si-SCR)for 24 h,respectively.Cells were then exposed to DMI and/or TMZ for another 24 h,after which the survival rate of U251/TR cells was detected by CCK-8 assays.x±s,n=3.**P< 0.01,compared with corresponding si-SCR group.
3 DISCUSSION
In the present study,DMI synergistically enhanced TMZ-induced inhibition of proliferation. The synergistic effect appears to be involved by apoptosis,which may be triggered by the combined treatment with DMI and TMZ,as demonstrated by confocal microscopy.Following the treatment with both DMI and TMZ,U251/TR cells showed typical,apoptotic,morphological changes such as nuclear shrinkage and DNA condensation. This is supported by synergistic increases in caspase 3 activity produced by combination of both drugs.
CHOP,a crucial regulator of ER stress-related apoptosis signaling,plays an important role in DMI-induced apoptosis[14].In the present study, we demonstrated that combination of both DMI and TMZ synergistically activated CHOP expression in U251/TR cells in terms of CHOP mRNA and protein.In addition,knockdown of CHOP abrogated the synergistic effect of DMI in the presence of TMZ,suggesting that DMI enhances the apoptotic effect of TMZ through activation of CHOP.This is consistent with our previous study[14],which demonstrates that CHOP plays a key role in apoptosis induced by DMI in C6 glioma cells.
In conclusion,DMIsynergistically enhances the antitumor action of TMZ through activation of CHOP,as demonstrated in U251/TR cells.The results suggest that combination of DMI and TMZ can be a promising option for treatment of patients with glioma,in particular those with comorbidity of depression.
REFERENCES:
[1]Mrugala MM.Advances and challenges in the treatment of glioblastoma:a clinician′s perspective[J].Discov Med,2013,15(83):221-230.
[2]Stupp R,Hegi ME,Mason WP,Van Den Bent MJ, Taphoorn MJ,Janzer RC,et al.Effects of radio⁃therapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phaseⅢstudy:5-year analysis of the EORTC-NCIC trial[J].Lancet Oncol,2009,10 (5):459-466.
[3]Zhang Y,Ma JW,Liu HY,Wang SX,Yan YR,Liu ZH,et al.Inhibitory effect of temozolomide com⁃bined with tetrandrine on human glioblastoma U87 cells[J].Chin J Pharmacol Toxicol(中国药理学与毒理学杂志),2014,28(3):367-372.
[4] Nitta Y,Shimizu S,Shishido-Hara Y,Suzuki K, Shiokawa Y,Nagane M.Nimotuzumab enhances temozolomide-induced growth suppression of glioma cells expressing mutant EGFR in vivo[J].Cancer Med,2016,5(3):486-499.
[5]Yu Z,Zhao G,Xie G,Zhao L,Chen Y,Yu H,et al. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo[J].Oncotarget,2015,6(32): 32930-32943.
[6]Song T,Li H,Tian Z,Xu C,Liu J,Guo Y.Disruption of NF-κB signaling by fluoxetine attenuates MGMT ex⁃pression in glioma cells[J].Onco Targets Ther, 2015,20(8):2199-2208.
[7]Qi H,Chen HZ,Feng JM,Jin ZJ.Effect of desipra⁃mine alone and in combination with teniposide on proliferation in rat C6 glioma cells[J].China Oncol(中国癌症杂志),2000,10(1):62-64.
[8]Goldzweig G,Hasson-Ohayon I,Alon S,Shalit E. Perceived threat and depression among patients with cancer:the moderating role of health locus of control[J].Psychol Health Med,2016,21(5):601-607.
[9]BarberB,Dergousoff J,Slater L,Harris J, O′connell D,El-Hakim H,et al.Depression and survival in patients with head and neck cancer:a systematic review[J].JAMA Otolaryngol Head Neck Surg,2016,142(3):284-288.
[10] Mika J,Zychowska M,Makuch W,Rojewska E, Przewlocka B.Neuronal and immunological basis of action of antidepressants in chronic pain-clinical and experimental studies[J].Pharmacol Rep,2013, 65(6):1611-1621.
[11]Qi H,Chen HZ,Jin ZJ.Effect of desipramine on proliferation,inhibition and apoptosis induction in rat glioma C6 cells[J].Acta Pharmacol Sin,2002,23 (9):803-807.
[12]Kinjo T,Kowalczyk P,Kowalczyk M,Walaszek Z, Slaga TJ,Hanausek M.Effects of desipramine on the cell cycle and apoptosis in Ca3/7 mouse skin squamous carcinoma cells[J].Int J Mol Med,2010, 25(6):861-867.
[13]Chang HC,Huang CC,Huang CJ,Cheng JS,Liu SI,Tsai JY,et al.Desipramine-induced apoptosis in human PC3 prostate cancer cells:activation of JNK kinase and caspase 3 pathways and a protective role of[Ca2+]ielevation[J].Toxicology,2008,250 (1):9-14.
[14]Ma J,Qiu Y,Yang L,Peng L,Xia Z,Hou LN,et al. Desipramine induces apoptosis in ratglioma cells via endoplasmic reticulum stress-dependent CHOP pathway[J].J Neurooncol,2011,101(1):41-48.
[15]Jin ZJ.Addition in drug combination[J].Acta Pharmacol Sin(中国药理学报),1980,1(1):70-76.
地昔帕明逆转人脑胶质瘤耐药细胞U251/TR对替莫唑胺的耐药作用
马 健1*,杨艳茹1*,刘景景1,李芳芳1,陈美华1,王 浩1,王 蕾1,孙立立1,王凤泽2,王德才1,张汉霆1,3
(泰山医学院1.药理学研究所,2.生命科学学院,山东泰安 271016;3.Departments of Behavioral Medicine&Psychiatry and Physiology&Pharmacology,West Virginia University Health Sciences Center,Morgantown,WV 26506,USA)
目的 探讨抗抑郁药地昔帕明(DMI)对人脑胶质瘤耐药细胞(U251/TR)对替莫唑胺(TMZ)耐药作用的影响及其机制。方法 DMI 20~80 μmol·L-1或TMZ 0.5~10 mmol·L-1处理U251/TR细胞24 h,用CCK-8法测定DMI和TMZ对U251/TR细胞增殖抑制的半数有效量(IC50)。CCK-8检测TMZ(1和2 mmol·L-1)和DMI(20,30和40 μmol·L-1)联合或单独处理U251/TR细胞24 h对细胞增殖抑制的影响,用金正均法计算Q值来评价两种药物的协同效应。TMZ(1 mmol·L-1)和DMI(30 μmol·L-1)联合或单独处理U251/TR细胞24 h,以Hoechst33258染色观察细胞核形态,发光法检测胱天蛋白酶3活性;实时定量PCR和Western蛋白印迹法检测C/EBP同源蛋白(CHOP)的表达;小干扰RNA(siRNA)沉默CHOP的表达后,观察TMZ(1 mmol·L-1)和DMI(30 μmol·L-1)联合给药对U251/TR增殖抑制的影响。结果 DMI或TMZ单用对U251/TR细胞增殖均有明显的抑制作用,并且呈浓度依赖性(r2=0.983,0.982,P<0.05),IC50分别为(33.6± 0.5)μmol·L-1和(2.5±0.6)mmol·L-1。TMZ(1和2 mmol·L-1)和DMI(20,30和40 μmol·L-1)联合给药能24 h对U251/TR细胞的增殖抑制率明显强于单独给药,以金正均法计算联合给药的Q值均>1.15,证实TMZ和DMI联合给药具有协同效应。同时,DMI 30 μmol·L-1和TMZ 1 mmol·L-1联和应用可诱导U251/TR细胞凋亡,主要表现为细胞核固缩、染色质沉积和胱天蛋白酶3的激活。这种凋亡诱导作用明显好于单独给药的效果(P<0.05)。DMI和TMZ联合应用能显著激活细胞内质网应激标志蛋白CHOP的表达(P< 0.05)。以siRNA沉默CHOP表达后,DMI逆转TMZ耐药的作用明显减弱,联合给药组细胞生存率由51.8%升至62.2%(P<0.05)。结论 DMI能逆转人脑胶质瘤细胞U251/TR对TMZ的耐药性,其机制可能与激活CHOP表达有关。
脑胶质瘤;地昔帕明;替莫唑胺;逆转耐药;C/EBP同源蛋白
国家自然科学基金项目(81302202);国家自然科学基金项目(81272683);国家自然科学基金项目(81441111);山东省自然科学基金项目(ZR2011HQ055);山东省政府“泰山学者海外特聘专家”专项基金
张汉霆,E-mail:hzhang@hsc.wvu.edu,Tel:(0538)6231386
2016-04-20接受日期:2016-06-14)
R966
:A
:1000-3002-(2016)06-0620-07
10.3867/j.issn.1000-3002.2016.06.002
Foundation item:The project supported by National Natural Science Foundation of China(81302202);National Natural Sci⁃ence Foundation of China(81272683);National Natural Science Foundation of China(81441111);Natural Science Foundation of Shandong Province(ZR2011HQ055);and the Foundation of Overseas Distinguished Taishan Scholars of Shandong Province
Biography:MA Jian(1980-),female,associate professor,PhD,the main research field is tumor pharmacology;YANG Yan-ru(1990-),female,postgraduate,the main research field is tumor pharmacology.
Han-ting ZHANG,Tel:(0538)6231386,E-mail:hzhang@hsc.wvu.edu
*Co-first author.
*共同第一作者。
(本文编辑:乔 虹)
猜你喜欢
杂志排行
中国药理学与毒理学杂志的其它文章
- Brief Introduction of the Executive Committee of the Chinese Pharmacological Society North America Chapter(CPSNAC) and the Specialists in This Issue
- Developing high quality chemical probes targeting ubiquitin-specific proteases
- Role of calcium-activated potassium channels in neuronal pacemaker activity
- Effects of calcium channel blockers on growth cone and their clinical implications
- Pathological role of transient receptor potential melastatin member 2 channel in neurodegenerative diseases and Alzheimer disease
- Drug reward memory:implication from drug-induced conditioned place preference model
