Ankaflavin ameliorates steatotic liver ischemiareperfusion injury in mice
2015-12-24
Nanjing, China
Ankaflavin ameliorates steatotic liver ischemiareperfusion injury in mice
Hao-Jun Yang, Li-Ming Tang, Xian-Ju Zhou, Jun Qian, Jie Zhu, Ling Lu and Xue-Hao Wang
Nanjing, China
BACKGROUND: It is well-known that steatotic liver is more susceptible to ischemia-reperfusion (I/R) injury during liver transplantation, liver resection and other liver surgeries. The increasing incidence of non-alcoholic fatty liver disease (NAFLD) decreases the availability of liver donors. Although steatotic liver is now accepted as a source of liver for transplantation, NAFLD exacerbates the liver injury after liver surgery. The present study was to investigate the protective role of ankaflavin in steatotic liver I/R injury.
METHODS: The model of fatty liver mice was induced with high fat diet in four weeks, ankaflavin or vehicle (saline) was administrated by gavage once a day for one week. The animals were subjected to partial hepatic I/R. Blood samples were collected to measure serum aminotransferases. The liver tissues were used to examine liver steatosis, apoptosis of hepatocytes, hepatic oxidative stress, Kupffer cells and inflammatory cytokines. The effects of ankaflavin on inflammatory cytokines were evaluated in isolated Kupffer cells from the steatotic liver.
RESULTS: Ankaflavin reduced liver steatosis in high fat diet mice. Compared with normal mice, I/R induced more damage to the mice with steatosis, such as hepatocyte apoptosis, inflammatory cytokines (TNF-α, IL-6 and IL-1β), serum aminotransferases and thiobarbituric acid reactive substances. Importantly, ankaflavin administration significantly attenuated these changes. In addition, ankaflavin significantly decreased the proliferation of Kupffer cells and the expression of TNF-α, IL-6 and IL-1β protein in isolated Kupffer cells stimulated by TNF-α.
CONCLUSION: Ankaflavin has protective effects against I/R injury through anti-inflammatory, anti-oxidant and antiapoptotic mechanisms in fatty livers, these effects are at least partially mediated by inhibiting Kupffer cell functions.
(Hepatobiliary Pancreat Dis Int 2015;14:619-625)
ankaflavin;
ischemia-reperfusion;
Kupffer cells;
steatotic liver
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a common disease worldwide, especially in developed countries and its incidence is rapidly increasing.[1]In the transplant community, the disparity between the number of patients on the waiting list and available donor organs remains substantial, and steatotic livers have been considered as grafts based on extended donor criteria.[2]Notably, steatotic livers are more prone to hepatic ischemia-reperfusion (I/R) injury, suggesting a higher risk of graft dysfunction and damage after transplantation.[3]
A large amount of data indicates that reactive oxygen species (ROS) play a pivotal role in hepatic I/R injury by damaging organic compounds and increasing the expression of pro-inflammatory cytokines.[4]Additionally, it is believed that Kupffer cells, the liver resident macrophages, are activated during the development of steatotic livers and further by I/R injury.[5]The toxic mediators released by Kupffer cells, including superoxide radicals, tumor necrosis factor (TNF), and cytokines, lead to hepatocyte damage.[6]Also, there is evidence that the downregulation of Kupffer cell function can significantly attenuate hepatic I/R injury in steatotic livers.[7]
Ankaflavin is a traditional food additive that has been used in Eastern Asia, including China, for the last few centuries. Recently, some reports[8-13]have revealed its beneficial anti-obesity, anti-tumor and anti-inflammatory effects, and its ability to prevent cardiovasculardiseases and NAFLD. Lee and co-workers[8]reported that ankaflavin prevented fatty acid accumulation in hepatocytes by inhibiting the uptake of fatty acids and lipogenesis, promoting fatty acid beta-oxidationin vitro, as well as reducing total cholesterol, triglyceride and free fatty acids in the plasmain vivo. Recently, it was shown that ankaflavin reduced the expression of cytokines, such as TNF-α, IL-6 and IL-1β.[10]Based on these previous data, we proposed that ankaflavin protects steatotic livers against I/R injury. We also tried to figure out the potential mechanisms. ture and a fresh liver sample were collected for future analysis.
Methods
Animals and experimental protocol
Four-week-old inbred C57BL/6J male mice (Model Animal Research Center of Nanjing University, Nanjing, China) were caged in a constant temperature room (25 ℃) with a 12:12-hour light-dark cycle, and free access to tap water and food. The fatty liver model was developed by feeding the mice with high fat diet (HFD; D12492, Yongli Co., Shanghai, China) from the age of 4 weeks to 8 weeks old.[14]In contrast, the control mice were fed with normal diet (ND). Firstly, three mice were fed ND, HFD and HFD+ankaflavin individually for testing the role of ankaflavin. They were killed after one night fasting. Secondly, eighteen mice were equally divided into 3 groups for I/R: the control group, the mice were fed with ND, and administered with normal saline before I/R; the HFD group, the mice were fed with HFD, and administered with normal saline before I/R; the HFD+ankaflavin group, the mice were fed with HFD, and administered with 0.624 mg/kg ankaflavin (ANPEL Scientific Instrument, Shanghai, China; Molecular formula: C23H30O5, Molecular weight: 386.48, CAS number: 50980320) before I/R. The saline or ankaflavin was administrated by gavage once a day for one week. The ankaflavin dose in this study was based on a reference dose for a human adult with a weight of 65 kg and a height of 170 cm according to a previous study.[12]Our pilot studies showed that this dosage is optimal in the protection of I/R-induced injury in steatotic mice. The experimental protocol was approved by the Nanjing Medical University Institutional Animal Care and Use Committee (NJMU08-092).
Surgery
The animals were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal injection). The left portal vein was isolated and clamped with a micro-vessel clamp for one hour and then released for reperfusion, the incision was then sutured. The mice were killed 3 hours after reperfusion; a blood sample via cardia punc-
Serum aminotransferases
The serum was separated for aminotransferases (ALT and AST) analyses with a diagnosis kit (Bioassay, Wiener Laboratories, Rosario, Argentina).
Histology
The liver tissues were embedded in paraffin, cut and stained with hematoxylin and eosin (HE). The percentage of the steatosis area in the livers was evaluated by MetaMorph software.
Immunohistochemistry
Paraffin sections were stained with a rat monoclonal antibody (R&D Systems, Minneapolis, MN, USA) against F4/80, an important surface marker of macrophages, and then the activation of Kupffer cells in the livers were assessed.
Quantitative real-time PCR
Total RNA was extracted from frozen liver tissues using RNA-Bee reagent (Bio-Connect, Huissen, the Netherlands). A Super-Script First-Strand Synthesis System (Invitrogen, CA, USA) was then used for reverse transcription. Real-time PCR was performed by using the Light-Cycler System (Roche, Indianapolis, IN, USA) to determine the relative amounts of cDNA molecules as previously described.[15]The following primers were used: TNF-α (5'-TGT CTA CTG AAC TTC GGG TGA T-3' and 5'-AAC TGA TGA GAG GGA GGC CAT-3'); IL-6 (5'-CTG CAA GTG CAT CAT CGT TGT-3' and 5'-TGT CTA TAC CAC TTC ACA AGT CGG A-3'); IL-1β (5'-TGG CAG TCC TCT GTC CTT G-3' and 5'-GAT CTT TCA CAG ACA CTG CTG C-3'); GAPDH (5'-GGT CAC CAG GGC TGC CAT TTG-3' and 5'-CTG GTA CTC CAT ACA CTG GCT-3').
Thiobarbituric acid reactive substances (TBARS)
TBARS was measured to evaluate the hepatic oxidative stress using a QuantiChrom™ TBARS kit (BioAssay Systems, USA).
Isolation of mouse Kupffer cells and hepatocytes
Primary Kupffer cells were isolated from normal and steatotic livers through the portal vein perfusion. Briefly, livers were perfused with Gibco Liver Perfusion Media (Invitrogen) and Gibco Liver Digestion Media (Invitrogen)in situ. After excised, minced and strained through a steel mesh sieve, the hepatocytes were centrifuged, col-lected and washed with Williams' media (Invitrogen). The Kupffer cells were pelleted and re-suspended with sterile Ca2+- and Mg2+-free Hank's balanced salt solution. Next, the cells were fractioned by elutriation, and the viability of the cells was checked using trypan blue exclusion.
Flow cytometry
Flow cytometry was employed to determine hepatocyte apoptosis. Apoptosis of isolated hepatocytes was evaluated using an Annexin V-FITC/PI apoptosis detection kit (BIO-BOX Biotech, Nanjing, China). Briefly, approximately 2×106cells were collected, washed with precold PBS and then re-suspended. Next, 5 μL of Annexin V-FITC and PI was added and then incubated at room temperature in darkness for 10 minutes. Finally, apoptosis was detected by using a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Kupffer cells were distributed at a concentration of approximately 2×105cells/500 μL/well and incubated overnight. Then, 50 ng/mL of TNF-α was added to the cells for 24 hours, and cytokine expression was measured. Based on a previous study[10]and given the dose-effect of ankaflavin, an ankaflavin dose of 30 μmol/L was used for cell treatment. The media were collected and evaluated for the expression of TNF-α, IL-6 and IL-1β (R&D Systems).
Statistical analysis
All data are shown as mean±standard error (SE). Statistical analysis was performed by one-way ANOVA followed by Fisher's protected least-significance difference test or the Mann-WhitneyUtest. AP<0.05 was considered to be statistically significant.
Results
Ankaflavin attenuates HFD-induced steatosis and I/R-induced injury in mouse steatotic liver
First, histologically no steatosis was observed in liver sections from the control group (Fig. 1A), the steatotic liver model was successfully created in the HFD mice (Fig. 1B), and ankaflavin administration significantly attenuated HFD-induced liver steatosis (Fig. 1C). Moreover, we found that HFD markedly elevated the ALT levels, but not the AST levels, and ankaflavin significantly reduced ALT levels in the HFD-mice (Fig. 1E and F). Next, we examined the effects of ankaflavin on I/R-induced injury in the steatosis livers. I/R triggered a substantial increase in cell apoptosis in the liver of the HFD mice compared to that of the ND mice (Fig. 2A-D). The administration of ankaflavin before I/R significantly prevented apoptosis in the liver of the HFD mice, suggesting that ankaflavin alleviated the I/R-induced steatosis liver impairment.Moreover, the levels of serum ALT and AST were significantly increased in the HFD group after I/R compared with those in the control group. Also, ankaflavin administration significantly reduced the levels of serum ALT and AST in the HFD+ankaflavin group (Fig. 2E and F). Taken together, these results showed that ankaflavin ameliorated I/R injury in steatotic liver.
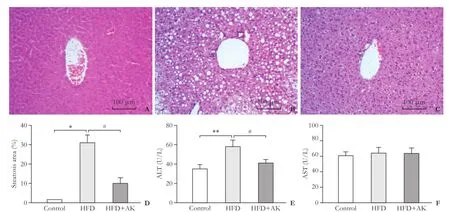
Fig. 1. Ankaflavin alleviates HFD-induced steatosis and I/R-induced injury in the steatotic liver. Representative morphological images of the liver from the control group (n=3) (A) or the HFD group (n=3) (B) or the HFD+ankaflavin group (n=3) (C) by HE staining (original magnification ×200). D: Qualification of steatosis area for (A), (B) and (C) by MetaMorph software. Scale bars represent 100 μm. Measurements of the serum levels of ALT (E) or AST (F) in the control, HFD and HFD+ankaflavin groups; HFD: high fat diet; AK: ankaflavin. *:P<0.001, **:P<0.01, compared to the control group; #:P<0.01, compared to the HFD group.
Ankaflavin suppresses oxidative stress and inflammatory responses to I/R injury
Oxidative stress plays a crucial role in I/R-induced liver injury.[16]It is widely accepted that TBARS is a marker of oxidative stress which is commonly used to estimate I/R injury in the liver. In addition, various inflammatory cytokines are involved in I/R injury. Thus, we first wanted to know whether the protective role of ankaflavin was associated with the inhibition of oxidative stress. Our data showed that following I/R, TBARS was significantly higher in the HFD group than in the control group; ankaflavin administration significantly decreased the TBARS level in the HFD+I/R mice, suggesting the inhibitory effects of ankaflavin on oxidative stress (Fig. 3A). Next, we evaluated the effect of ankaflavin on the expression of inflammatory cytokines usingreal-time PCR. Following I/R, the mRNA levels of cytokines, such as TNF-α, IL-6 and IL-1β were significantly up-regulated in the liver from the HFD group compared with those in the control group (Fig. 3B-D), pretreatment with ankaflavin significantly down-regulated these cytokines in HDF+I/R mice.

Fig. 2. Ankaflavin attenuates I/R-induced injury in the steatotic liver. Representative flow cytometry results of hepatocytes apoptosis in the control group (n=6) (A), the HFD group (n=6) (B) and the HFD+ankaflavin group (n=6) (C) after 1 hour of left portal vein occlusion followed by 3 hours of reperfusion. D: Statistical results of apoptosis from the three groups. Measurements of the serum levels of ALT (E) or AST (F) in the control, HFD and HFD+ankaflavin groups following I/R. HFD: high fat diet; AK: ankaflavin; I/R: ischemic-reperfusion. *:P<0.001, compared to the control group; #:P<0.01, compared to the HFD group.
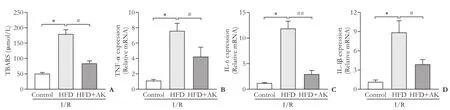
Fig. 3. Ankaflavin suppresses the oxidative stress response and inflammatory responses. A: ankaflavin decreases the level of TBARS in steatotic livers following I/R as detected with a TBARS assay kit. B-D: ankaflavin reduces the inflammatory cytokine expression in steatotic livers following I/R as determined by real-time PCR. HFD: high fat diet; AK: ankaflavin; I/R: ischemic-reperfusion. *:P<0.001, compared to the control group; #:P<0.01, ##:P<0.001, compared to HFD group (n=6 for each group).

Fig. 4. The protective role of ankaflavin is achieved by inhibition of Kupffer cell function. The representative F4/80 immunostaining is shown in the livers from the control group (n=6) (A), the HFD group (n=6) (B), and the HFD+ankaflavin group (n=6) (C) following 1 hour of left portal vein occlusion followed by 3 hours of reperfusion (original magnification ×200). D: Qualification for the three groups. Ankaflavin suppressed the expression of TNF-α (E), IL-6 (F) and IL-1β (G) stimulated by TNF-α in isolated Kupffer cells from the steatotic liver (n=3 for each group). Arrows indicate F4/80-positive cells in the livers. HFD: high fat diet; AK: ankaflavin; I/R: ischemic-reperfusion. *:P<0.001, compared to the control group; #:P<0.01, compared to the HFD group.
Ankaflavin suppresses Kupffer cell-mediated inflammatory responses to I/R injury
Kupffer cells are liver resident macrophages and play an important role in liver steatosis[17]and I/R injury.[7]Additionally, these cells mediate oxidative stress and inflammatory responses in the liver.[18]Thus, we proposed that the protective effects of ankaflavin are via inhibiting Kupffer cell functions. Our immunohistochemical staining showed that F4/80, a specific surface biomarker of Kupffer cells, was significantly increased in the HFD+I/R mice compared with that in the ND+I/R mice (Fig. 4A, B and D); the administration of ankaflavin significantly reduced F4/80 (+) cells (Fig. 4C and D). Next, to investigate the influence of ankaflavin on inflammatory responses of Kupffer cells, we separated Kupffer cells from the livers and stimulated them with TNF-α. We found that ankaflavin at 30 μmol/L effectively inhibited the TNF-α-induced inflammatory responses (data not shown). Compared with the non-treated mice, the TNF-α-treated Kupffer cells exhibited significantly elevated levels of TNF-α, IL-6 and IL-1β protein (Fig. 4E-G). The ankaflavin pretreatment significantly prevented TNF-α, IL-6 and IL-1β elevation in TNF-α-treated Kupffer cells (Fig. 4E-G). Collectively, these data suggest that ankaflavin exerts its protective effects by inhibiting Kupffer cell functions.
Discussion
The steatotic livers are more prone to acute I/R injury; severe impairment of liver functions may occur in the liver transplantation and surgery through enhanced oxidative stress and inflammatory cytokine release.[19-21]Therefore, it is of practical significance to protect the steatotic livers against I/R using some feasible approaches. Ankaflavin has been used as a traditional food additive for centuries in Eastern Asia. Recently, investigators have focused on its clinical value. Lee et al[8]demonstrated that ankaflavin reduces differentiation and lipogenesis in adipose tissue, disrupts lipid absorption in the small intestine, and alleviates NAFLDin vivo. In thein vitroexperiment, ankaflavin decreases lipid accumulation by activating peroxisome proliferator-activated receptor (PPAR)-α and AMP-activated kinase (AMPK)[13]in FL83B hepatocytes. Moreover, Hsu and colleagues[10]showed that ankaflavin plays an anti-inflammatory rolein vivoandin vitroby suppressing the LPS-induced inflammatory cytokines. The present study found that the administration of ankaflavin significantly reduced fatdroplets in mice with HFD and protected steatotic liver from I/R injury. The protective effects of ankaflavin were at least partially via the inhibition of oxidative stress and inflammatory responses, especially related to Kupffer cells.
The critical roles of ROS in I/R injury are well established, and lipid accumulation in the liver leads to considerable lipid peroxidation[22]and generates ROS after I/R.[23]Our data showed that after I/R in the steatotic livers, TBARS was significantly increased, ankaflavin substantially attenuated the TBARS levels in HDF+I/R mice. Consistent with our report, Lin et al[24]found that ankaflavin reduces ROS in TNF-α-treated human aortic endothelial cells. Hsu and colleagues[25]showed that ankaflavin attenuates lung inflammation and injury by up-regulating the anti-oxidant enzymes. Thus, the protective roles of ankaflavin were at least partially achieved by inhibition of oxidative stress. It is well-known that activated macrophages are key mediators of liver injury. Kudo et al[26]showed that the activation of macrophages is involved in the progression of steatohepatitis in the methionine and choline-deficient HFD mice. Teramoto et al[27]reported that steatotic livers displayed an increase in the number and function of Kupffer cells after transplantation. Similarly, our study suggested that steatosis and I/R injury were mediated by the infiltration and activation of Kupffer cells. First, I/R in the steatotic livers significantly increased the number of Kupffer cells; second, inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, were also substantially elevated, these data were consistent with previously studies in both NAFLD and I/R models,[28,29]suggesting the activation of Kupffer cells. In our study, ankaflavin markedly decreased the elevated levels of TNF-α, IL-6 and IL-1β in the TNF-αtreated Kupffer cells. As a result, ankaflavin reduced infiltration of Kupffer cells by down-regulation of expression of these cytokines. Additionally, it is known that Kupffer cells may be directly activated by ROS. Our study showed that ankaflavin significantly inhibited ROS formation. Thus it is possible that the decrease in the infiltration of Kupffer cells in steatotic liver is partially related to the reduction of ROS production.
There is evidence that activated macrophages have an adverse effect on hepatocytes, stellate cells and endothelial cells in a NAFLD model.[30]In our study, hepatocyte apoptosis was associated with the activation of macrophages. Pretreatment with ankaflavin significantly prevented inflammatory responses following I/R, including infiltration of Kupffer cells and the release of inflammatory cytokines. In thein vitroexperiments, we showed that ankaflavin inhibited TNF-α-induced inflammatory cytokine expression in the steatotic liver. Altogether, Kupffer cells play a vital role in I/R injury of the steatotic liver.
In conclusion, our study showed that steatotic livers are prone to I/R injury compared with normal controls, and pretreatment with ankaflavin effectively alleviated the injury of steatotic livers subjected to I/R injury. We also showed that the initial mechanisms of the protection involved inhibition of oxidative stress and inflammatory responses. Furthermore, ankaflavin acted as a novel anti-inflammatory compound to inhibit Kupffer cells recruitment and to reduce the expression of inflammatory cytokines in steatotic livers after I/R. Therefore, ankaflavin may be a promising strategy to protect steatotic livers against I/R injury.
Contributors:LL and WXH proposed the study. YHJ, QJ and ZJ performed the experiments. TLM and ZXJ analyzed the data. YHJ wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. WXH is the guarantor.
Funding:This study was supported by grants from the International Collaboration Foundation of Jiangsu Province (BZ2011041), the Special Funds of Ministry of Health for Health Research (201302009) and the National Natural Science Foundation of China (81273262).
Ethical approval: The experimental protocol was approved by the Nanjing Medical University Institutional Animal Care and Use Committee (NJMU08-092).
Competing interest:The authors do not choose to declare any conflict of interest related directly or indirectly to the subject of this article.
1 Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci 2011;48:97-113.
2 Said A. Non-alcoholic fatty liver disease and liver transplantation: outcomes and advances. World J Gastroenterol 2013;19: 9146-9155.
3 Perez-Daga JA, Santoyo J, Suárez MA, Fernández-Aguilar JA, Ramírez C, Rodríguez-Cañete A, et al. Influence of degree of hepatic steatosis on graft function and postoperative complications of liver transplantation. Transplant Proc 2006;38:2468-2470.
4 Llacuna L, Marí M, Lluis JM, García-Ruiz C, Fernández-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol 2009;174:1776-1785.
5 Leroux A, Ferrere G, Godie V, Cailleux F, Renoud ML, Gaudin F, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol 2012;57:141-149.
6 Tamura T, Kondo T, Pak S, Nakano Y, Murata S, Fukunaga K, et al. Interaction between Kupffer cells and platelets in the early period of hepatic ischemia-reperfusion injury--anin vivostudy. J Surg Res 2012;178:443-451.
7 Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, et al. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: anin vivomicroscopy study. Microcirculation 2011;18:205-213.
8 Lee CL, Wen JY, Hsu YW, Pan TM. Monascus-fermented yellow pigments monascin and ankaflavin showed antiobesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet. J Agric Food Chem 2013;61:1493-1500.
9 Hsu WH, Lee BH, Pan TM. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J Sci Food Agric 2010;90:2709-2715.
10 Hsu LC, Liang YH, Hsu YW, Kuo YH, Pan TM. Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568. J Agric Food Chem 2013;61:2796-2802.
11 Lee CL, Tsai TY, Wang JJ, Pan TM.In vivohypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol 2006;70:533-540.
12 Lee CL, Hung YP, Hsu YW, Pan TM. Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages. J Agric Food Chem 2013;61:143-150.
13 Hsu WH, Chen TH, Lee BH, Hsu YW, Pan TM. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in highfat diet-fed C57BL/6 mice. Food Chem Toxicol 2014;64:94-103.
14 Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl 2009;15:1101-1109.
15 Jiang R, Xia Y, Li J, Deng L, Zhao L, Shi J, et al. High expression levels of IKKalpha and IKKbeta are necessary for the malignant properties of liver cancer. Int J Cancer 2010;126:1263-1274.
16 Fang J, Qin H, Seki T, Nakamura H, Tsukigawa K, Shin T, et al. Therapeutic potential of pegylated hemin for reactive oxygen species-related diseases via induction of heme oxygenase-1: results from a rat hepatic ischemia/reperfusion injury model. J Pharmacol Exp Ther 2011;339:779-789.
17 Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology 2010;51: 511-522.
18 Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, et al. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology 2004;39:1544-1552.
19 de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol 2012;27:540-546.
20 Ben Mosbah I, Roselló-Catafau J, Alfany-Fernandez I, Rimola A, Parellada PP, Mitjavila MT, et al. Addition of carvedilol to University Wisconsin solution improves rat steatotic and nonsteatotic liver preservation. Liver Transpl 2010;16:163-171.
21 Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105-113.
22 Vairetti M, Ferrigno A, Carlucci F, Tabucchi A, Rizzo V, Boncompagni E, et al. Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl 2009;15:20-29.
23 Luo XY, Takahara T, Hou J, Kawai K, Sugiyama T, Tsukada K, et al. Theaflavin attenuates ischemia-reperfusion injury in a mouse fatty liver model. Biochem Biophys Res Commun 2012;417:287-293.
24 Lin CP, Lin YL, Huang PH, Tsai HS, Chen YH. Inhibition of endothelial adhesion molecule expression by Monascus purpureus-fermented rice metabolites, monacolin K, ankaflavin, and monascin. J Sci Food Agric 2011;91:1751-1758.
25 Hsu WH, Lee BH, Huang YC, Hsu YW, Pan TM. Ankaflavin, a novel Nrf-2 activator for attenuating allergic airway inflammation. Free Radic Biol Med 2012;53:1643-1651.
26 Kudo H, Yata Y, Takahara T, Kawai K, Nakayama Y, Kanayama M, et al. Telmisartan attenuates progression of steatohepatitis in mice: role of hepatic macrophage infiltration and effects on adipose tissue. Liver Int 2009;29:988-996.
27 Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation 1993;56:1076-1082.
28 Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, Somers EP, et al. Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharidein vitroorin vivo. J Immunol 2000;164:1046-1054.
29 Wu YJ, Ling Q, Zhou XH, Wang Y, Xie HY, Yu JR, et al. Urinary trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat Dis Int 2009;8:53-58.
30 Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol 2002;282:G1-5.
Received July 11, 2014
Accepted after revision November 6, 2014
Author Affiliations:Liver Transplantation Center, First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China (Yang HJ, Lu L and Wang XH); Department of General Surgery (Yang HJ, Tang LM, Qian J and Zhu J) and Department of Neurology, Laboratory of Neurological Diseases (Zhou XJ), Changzhou No. 2 People's Hospital, Affiliated Hospital of Nanjing Medical University, Changzhou 213003, China
Xue-Hao Wang, MD, Liver Transplantation Center, First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing 210029, China (Tel: +86-25-83718836ext6476; Fax: +86-25-83672106; Email: wangxh@njmu.edu.cn)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60361-7
Published online April 24, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Gut microbiota and non-alcoholic fatty liver disease
- Risk factors of metabolic syndrome after liver transplantation
- Combined Hangzhou criteria with neutrophillymphocyte ratio is superior to other criteria in selecting liver transplantation candidates with HBV-related hepatocellular carcinoma
- Warm HTK donor pretreatment reduces liver injury during static cold storage in experimental rat liver transplantation
- Intrahepatic distant recurrence following complete radiofrequency ablation of small hepatocellular carcinoma: risk factors and early MRI evaluation
- Oncogenic role of microRNA-423-5p in hepatocellular carcinoma
