酰腙席夫碱铜单核配合物的合成、结构及性质
2015-11-27张旭艳李建定刘宝林陶偌偈
张旭艳,李建定,刘宝林*,陶偌偈
(1.河南大学 化学化工学院,河南 开封 475004;2.河南省医药学校,河南 开封 475004)
随着配位化学的发展,人们不再一味追求结构新颖的晶体结构,而是开始关注配合物的性能.由于形成有机配合物后有机配体与金属之间存在微妙的作用,使两者之间的优点相辅相成,同时可以借助有机配体骨架结构的灵活多变使金属离子的特性发挥到最大,这是一般无机材料所无法比拟的.随着研究的深入,金属配合物在生命科学[1]、光电材料[2-16]、生物活性技术[17]、气体存储[18-23]等领域受到广泛关注.含-CONHN=CH-基团的酰腙类化合物因其具有优异的配位能力、多样的配位形式、独特的生物和药物活性,在农药、医药、分析和催化等领域被广泛应用[24-27].而酰腙类席夫碱及其金属配合物也显示了良好的抑菌、抗病毒和抗肿瘤的生物活性[28-29].铜作为生命起源的必需元素之一,在生命体中起着重要的作用,它的配合物在抗癌、抗溃疡、抗糖尿病、抗诱变剂、模仿酶活性、诊断成像或治疗应用等方面有重要的应用价值[30-36].本文作者以水杨酸乙酯缩水合肼和2-吡啶甲醛反应合成了配体H2L(水杨酸乙酯缩水合肼)缩2-吡啶甲醛,该配体与Cu(CH3COO)2、NH4SCN反应合成了一个铜单核配合物Cu(HL)2,并对其结构和性质进行了研究.
1 实验部分
1.1 仪器与试剂
红外光谱用Avater-360型傅立叶红外光谱仪(KBr压片)在400~4 000cm-1范围内测定;UVVis用UV-550型光谱仪在400~800nm范围内测定(DMF作溶剂);水杨酸乙酯缩水合肼为自制试剂,2-吡啶甲醛、水合肼等其他试剂均为市售分析纯.
1.2 配体H2L的合成
水杨酸乙酯缩水合肼的合成:称取1.661 8g(10mmol)水杨酸乙酯加入20mL甲醇中,然后逐滴加入20mL含有0.05g(10mmol)水合肼的甲醇溶液,搅拌回流2h,冷却至室温,得到黄色沉淀,真空抽滤,乙醚洗涤,真空干燥,得黄色粉末1.26g,产率为82.9%.
配体H2L的合成:称取1.521 5g(10mmol)水杨酸乙酯缩水合肼加入25mL甲醇中,然后逐滴加入25mL含有1.071 1g(10mmol)2-吡啶甲醛的甲醇溶液,搅拌回流2h,冷却到室温,得到黄色沉淀,真空抽滤,用乙醚洗涤,真空干燥,得黄色粉末2.03g,产率为84.2%.
1.3 配合物Cu(HL)2的合成
将配体H2L(0.1mmol,0.024 1g)加入10mL甲醇中,再加入10mL Cu(CH3COO)2(0.1mmol,0.019 9g)的甲醇溶液和10mL NH4SCN(0.05 mmol,0.003 8g)的甲醇溶液,室温下搅拌1h,得浅黄色澄清溶液,然后过滤,静置约半个月后得到绿色块状晶体.
1.4 配合物的结构解析
选取尺寸大小为0.17mm×0.18mm×0.23 mm的晶体用Bruker Smart APEX衍射仪进行测试,采用CCD探测器,并用石墨单色化的Mo Kα射线(λ=0.071 073nm)于296(2)K下收集衍射数据,共收集1 744个衍射点,其中可观测的独立衍射点[I>2σ(I)]为1 365个.全部衍射数据用SADABS[37]程序经Lp因子和Multi-scan吸收校正.晶体结构运用SHELXL-97[38]程序采用直接法解出,并用全矩阵最小二乘法进行精修,对所有非氢原子做了各向异性精修,配合物的主要的晶体学数据列于表1中(CSD:1036908).

表1 配合物的晶体学数据Table 1 Crystallographic data of complex
2 结果与讨论
2.1 红外光谱分析
从红外数据可以看出,配体在3 428cm-1处的吸收峰归属为νN-H的振动吸收,在1 608cm-1处的吸收峰归属为νC=N的振动吸收,在1 632cm-1处的吸收峰归属为νC=O的吸收峰.当形成配合物后,Schiff碱的特征官能团νC=N的振动吸收峰红移至1 564cm-1,说明-C=N-参与了配位;配合物中νC=O的吸收峰则红移至1 598cm-1,说明-C=O参与了配位,这与化合物的晶体结构相符;配合物在3 430cm-1处的吸收峰归属于νN-H的振动吸收,与配体相比基本上没变化,说明亚胺基上的氮原子没有参与配位,这也与晶体结构相一致.
2.2 配合物的UV-Vis光谱
以DMF作溶剂,在500~800nm范围内进行了液体紫外光谱测试,结果如图1所示.配合物在712nm处出现吸收峰,可归属于Cu(II)离子的d-d跃迁[39-40].
2.3 晶体结构描述
配合物的晶体结构如图2所示,主要键长键角列于表2.在配合物中,中心原子Cu1处于六配位(N4O2)的变形八面体构型中,分别与来自两个配体上的吡啶N原子(N1,N1#1)、C=N双键上的N原子(N2,N2#1)和羰基上的O原子(O1,O1#1)配位.变形八面体的赤道位置由N1#1,N2,N2#1,和O1#1 4个原子占据(其中,Cu1-N1#1键长为0.222 6(8)nm,Cu1-N2的键长与Cu1-N2#1的键长相等,均为0.196 8(7)nm,Cu1-O1#1键长为0.215 5(7)nm),轴向位置由O1和N1占据(Cu1-O1键长为0.215 5(7)nm,Cu1-N1键长为0.222 6(8)nm).N1#1、N2、N2#1、和O1#1近似在一个平面上,4个原子偏离平面的距离分别为0.006 4、-0.004 9、-0.008 4和0.006 9nm,Cu1原子偏离平面的距离为-0.001 6nm,N1和O1偏离平面的距离依次为0.213 2和-0.212 1nm.N2、Cu1和N2#1近似在一条直线上(N2-Cu1-N2#1夹角为175.7(6)°).

图1 配合物的紫外-可见吸收光谱Fig.1 Ligand UV-Vis spectrum of the complex
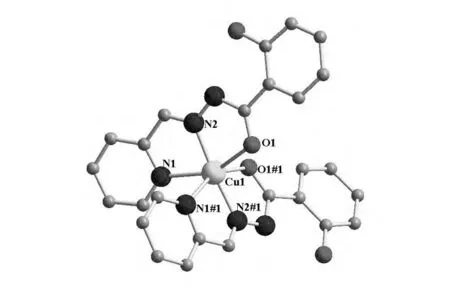
图2 配合物Cu(HL)2的晶体结构Fig.2 Molecular structure of Cu(HL)2

表2 配合物的主要键长和键角Table 2 Bond lengths and angles of the complex
3 结论
利用配体水杨酸乙酯缩水合肼与含有杂环的2-吡啶甲醛反应,得到了配体H2L,然后再与Cu(CH3COO)2和NH4SCN反应得到了一个铜单核配合物Cu(HL)2.利用X射线单晶衍射、红外光谱、紫外光谱对这个配合物的结构和性质进行了表征.结果表明,杂环中的氮原子参与了配位,形成了一个十字交叉型单核化合物.
[1]FAULKNER S,POPE S J A,BURTON-PYE B P.Lanthanide complexes for luminescence imaging applications[J].Appl Spectrosc Rev,2005,40(1):1-31.
[2]KLINK S I,KEIZER H,VEGGEL F C J M V.Transition metal complexes as photosensitizers for near-infrared lanthanide luminescence[J].Angew Chem Int Ed,2000,39(23):4319-4321.
[3]JANG H S,IM W B,LEE D C.Enhancement of red spectral emission intensity of Y3Al5O12:Ce3+phosphor via Pr Co-doping and Tb substitution for the application to white LEDs[J].J Lumin,2007,126(2):371-377.
[4]PARK J K,LIM M A,KIM C H.White light-emitting diodes of GaN-based Sr2SiO4:Eu and the luminescent properties[J].Appl Phys Lett,2003,82(5):683-685.
[5]LI P L,WANG Z J,YANG Z P,et al.Emission features of LiBaBO3:Sm3+red phosphor for white LED[J].Mater Lett,2009,63(9):751-753.
[6]IM W B,KIM Y I,FELLOWS N N,et al.A yellow-emitting Ce3+phosphor,La1-xCexSr2AlO5,for white light-emitting diodes[J].Appl Phys Lett,2008,93(9):1905-1907.
[7]KLINK S I,HEBBINK G A,GRAVE L,et al.Nearinfrared and visible luminescence from terphenyl-based lanthanide(III)complexes bearing amido and sulfonamide pendant arms[J].Eur J Org Chem,2000,10:1923-1931.
[8]STEEMERS F J,WERBOOM W,HOFSTRAAT J W,et al.Near-infrared luminescence of Yb3+,Nd3+,and Er3+azatriphenylene complexes[J].Tetrahedron Lett,1998,39(41):7583-7586.
[9]BO S H,HU J,WANG Q,et al.Near-infrared luminescence properties of erbium complexeswith the substituted phthalocyaninato ligands[J].Photochem Photobiol Sci,2008,7(4):474-479.
[10]WOLBERS M P O,VEGGEL F C J M V,HOFSTRAAT J W,et al.Photophysical studies of M-terphenyl-sensitizad visible and near-infrared emission from orange 1∶1lanthanide ion complexes in methanol solutions[J].J Chem Soc Perkin Trans 2,1998:2141-2150.
[11]COMBY S,IMBERT D,CHAUVIN A S,et al.Stable 8-hydroxyquinolinate-based podates as efficient sensetizers of lanthanide near-infrared luminescence[J].Inorg Chem,2006,45(2):732-743.
[12]DAVIES G M,AARONS R J,MOTSON G R,et al.Structural and near-IR photophysical studies on ternary lanthanide complexes containing poly(pyrazolyl)borate and 1,3-diketonate ligands[J].Dalton Trans,2004,8:1136-1141.
[13]ARTIZZU F,DEPLANO P,MARCHIO L,et al.Structure and emission properties of Er3Q9(Q=8-Quinolinolate)[J].Inorg Chem,2005,44(4):840-842.
[14]WONG W K,HOU A,GUO J P,et al.Synthesis,structure and near-infrared luminescence of neutral 3d-4f Bi-metallic monoporphyrinate complexes[J].J Chem Soc Dalton Trans,2001:3092-3098.
[15]QUICI S,CAVAZZINI M,MARZANNI G,et al.Visible and near-infrared intense luminescence from water-soluble lanthanide[Tb(III),Eu(III),Sm(III),Dy(III),Pr(III),Ho(III),Yb(III),Nd(III),Er(III)]complexes[J].Inorg Chem,2005,44(3):529-537.
[16]SHAVALEEV N M,POPE S J A,BELL Z R,et al.Visible-light sensitization of near-infrared luminescence from Yb(III),Nd(III)and Er(III)complexes of 3,6-bis(2-pyridy)tetrazine[J].Dalton Trans,2003:808-814.
[17]PANDYA S,YU J H,PARKER D.Engineering emissive europium and terbium complexes for molecular imaging and sensing[J].Dalton Trans,2006:2757-2766.
[18]ZHANG J P,CHEN X M.Optimized acetylene/carbon dioxide sorption in a dynamic porous crystal[J].J Am Chem Soc,2009,131(15):5516-5521.
[19]ZHANG J P,CHEN X M.Exceptional framework flexibility and sorption behavior of a multifunctional porous cuprous triazolate framework[J].J Am Chem Soc,2008,130(18):6010-6017.
[20]CHENG X N,ZHANG W X,CHEN X M.Single crystal-to-single crystal transformation from ferromagnetic discrete molecules to a spin-canting antiferromagnetic layer[J].J Am Chem Soc,2007,129(51):15738-15739.
[21]ZHANG J P,LIN Y Y,ZHANG W X,et al.Temperature or guset-induced drastic single-crystal-to-singlecrystal transformations of a nanoporous coordination polymer[J].J Am Chem Soc,2005,127(41):14162-14163.
[22]ZHANG J P,LIN Y Y,HUANG X C,et al.Copper(I)1,2,4-triazolates and related complexes:Studies of the solvothermal ligand reactions,network topologies and photoluminescence properties[J].J Am Chem Soc,2005,127(15):5495-5506.
[23]HUANG X C,ZHANG J P,CHEN X M.A new route to supramolecular isomers via molecular templating;anosized molecular polygons of copper(I)2-methylimidazolates[J].J Am Chem Soc,2004,126(41):13218-13219.
[24]RAMAN N,JOSEPH J,POTHIRAJ C.Antifungalactives of biorelevant complexes of copper(II)with biosensitive macrocyclic ligands[J].Mycobiology,2006,34(4):214.
[25]祝心德,党元林,王成刚,等.2,4-二羟基苯甲醛缩硫脲及其配合物的合成与生物活性研究[J].无机化学学报,1997,13(1):68-72.
[26]张闻,罗勤慧.锰过氧化氢酶及模型物研究进展[J].化学通报,2000,63(10):7-10.
[27]MEBRIDE T J,PRESTON B D,LOEB L A.Mutagenic spectrum resulting from DNA damage by oxygen radicals[J].Biochem,1991,30(1):207-211.
[28]黄娟,崔紫宁,李映,等.Schiff碱铜配合物的生物活性[J].有机化学,2008,28(4):598-604.
[29]CHOHAN Z H,ARIF M,SARFRAZ M.Metal-based antibacterial and antifungal amino acid derived schiff bases:their synthesis,characterization and in vitro biological activity[J].Appl Organometal Chem,2007,21(4):294-302.
[30]SOLOMON E I,SUNDARAM U M,MACHONKIN T E.Multicopper oxidases and oxygen-ases[J].Chem Rev,1996,96(7):2563-2605.
[31]PRAVIN N,RAMAN N.DNA interaction and antimicrobial activity of novel tetradentate imino-oxalato mixed ligand metal complexes[J].Inorg Chem Commun,2013,36:45-50.
[32]FINNEY L A,O'HALLORAN T V.Transition metal speciation in the cell:Insights from the chemistry of metal ion receptors[J].Science,2003,300(5621):931-936.
[33]GOPALAKRISHNAN M,SENTHILKUMAR K,RAO P R,et al.Synthesis,crystal structure,DNA binding and molecular docking studies on new copper(II)salicylate[Cu(DTBSA)2(2,2′-bpy)](dmf)[J]. Inorg Chem Commun,2014,46:54-59.
[34]YAGHI O M,DAVIS C E,LI G M,et al.Selective guest binding by tailored channels in a 3-dporous zinc(Ⅱ)-benzene tricarboxylate network[J].J Am Chem Soc,1997,119(12):2861-2868.
[35]LIMA L M P,HALIME Z,MARION R,et al.Monopicolinate cross-bridged cyclam combin-ing very fast complexation with very high stability and inertness of its copper(II)complex[J].Inorg Chem,2014,53(10):5269-5279.
[36]ISLAS M S,LEZAMA L,WILLIAMS P A M,et al.Antitumoral,antihypertensive antimicrobial,and antioxidant effects of an octanuclear copper(II)-telmisartan complex with an hydrophobic nanometer hole[J].Inorg Chem,2014,53(11):5724-5737.
[37]BLESSING R H.An empirical correction for absorption anisotropy[J].Acta Cryst,1995,A51:33-38.
[38]SHELDRICK G M.SHELXL 97,Program for the refinement of crystal strueture[CP].Germany:University of Göttingen,1997.
[39]COSTES J P,LAURENT J P,SANCHEZ J M M,et al.Magnetic properties of a series of trinuclear complexes(CuL)2Mn·xB(L representing the deprotonated form of N-(4-methyl-6-oxo-3-azahept-4-enyl)oxamic acid and B representing respectively H2O(x=5,4.5,3,1),(CH3)2SO(x=2),and C5H5N(x=4)),crystal and molecular structure of(CuL)2Mn·2(CH3)2SO[J].Inorg Chem,1997,36(21):4641-4646.
[40]LAHIRI D,BHOWMICK T,PATHAK B,et al.Anaerobic photocleavage of DNA in red light by dicopper(II)complexes of 3,3′-dithiodipropionic acid[J].Inorg Chem,2009,48(1):339-349.
