Reevaluation of the Taxonomic Status of a Poorly Known Gecko, Gekko liboensis (Reptilia: Squamata)
2015-10-31TeppeiJONOLiDINGTakumaKAITOYezhongTANGandMamoruTODA
Teppei JONO, Li DING, Takuma KAITO, Yezhong TANG, and Mamoru TODA
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China
2Department of Chemistry, Biology and Marine Science, Graduate School of Engineering and Science, University of the Ryukyus, Nishihara, Okinawa 903-0213, Japan
3Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-0213, Japan
Reevaluation of the Taxonomic Status of a Poorly Known Gecko, Gekko liboensis (Reptilia: Squamata)
Teppei JONO1*, Li DING1, Takuma KAITO2, Yezhong TANG1, and Mamoru TODA3
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China
2Department of Chemistry, Biology and Marine Science, Graduate School of Engineering and Science, University of the Ryukyus, Nishihara, Okinawa 903-0213, Japan
3Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-0213, Japan
Gekko liboensis Zhou and Li, 1982 is a poorly known gecko originating from Libo, Guizhou, southern China. Since the original description based on a single female from Libo, only three specimens (two females and one juvenile) have been reported from the type locality. Because of its close morphological resemblance to G. hokouensis Pope, 1927, G. liboensis is required to be reevaluated for its taxonomic status by studies based on additional specimens. Here we report for the first time the morphological features of 31 newly obtained specimens of G. liboensis from type locality and others, including males, and compare them with those of related species, chiefy on the basis of information from the literature. In addition, specimen-based comparisons were also made with G. hokouensis and an undescribed cryptic species known from the Ryukyus Islands, Japan. Results showed that the species is distinguished from its congeners in having a larger body size (60–91 mm), 0–2 small internasals, 10–13 supralabials, 10–13 infralabials, smooth enlarged tubercles present on the dorsum forming 4–10 irregular rows at the midbody but absent on the four limbs and tail, both fingers and toes without developed interdigital webs, a single cloacal spur on each side, and 10-12 precloacal pores in males. It most resembles G. hokouensis and its cryptic species but is clearly differentiated by having a larger body size, a predominantly absent internasal, fewer number of dorsal tubercle rows, larger number of precloacal pores, and conspicuous white marks on the dorsum and head. Judging from these results, we confirmed the taxonomic validity of G. liboensis. In addition, the geographic distribution of this gecko on the basis of obtained data was briefy discussed.
China, East Asia, Gekkonidae, Gekko japonicus group, taxonomy, morphology
1. Introduction
Gekko liboensis Zhou and Li, 1982 is a gekkonid lizard described on the basis of a single female collected from Libo, Guizhou Province, southern China (Zhou and Li, 1982). Subsequent to the original description, only three specimens (two females and one juvenile) have been reported from the same locality (Zhao et al., 1999), and thus, the morphological features of the male, range of variation, and other biological aspects of this species are virtually unknown. Although Xu et al. (2007) reportedthis species in their list of the herpetofauna of Maolan and Chishui, Guizhou, the record from Chishui, which is >350 km northwest of the type locality, was not based on any specimens and may be erroneous because of misidentification (Wei Gang, personal communication).
Zhou and Li (1982) described this species on the basis of a series of characteristics: large body size [snout–vent length (SVL) of 85 mm]; absence of internasal (IN); approximately 40 interorbitals (IOs); paired postmental, twice as long as wide, contacted posteriorly by a pair of enlarged scales; 12 supralabials (SPLs); 11 infralabials (ILs); somewhat flattened dorsal tubercles, forming approximately 10 irregular longitudinal rows on the body, absent on four limbs and tail; both fingers and toes having faint interdigital webs; and single cloacal spur on eachside. However, most of these character states are shared with G. hokouensis Pope, 1927, another species of Gekko occurring in the eastern part of China (including mainland China and Taiwan) and Japan (Zhou et al., 1982; Zhao et al., 1999). Thus, Zhao and Adler (1993), Günther (1994), Kluge (2001), and Henkel and Schmidt (2003) listed G. liboensis as a junior synonym of G. hokouensis, whereas Kluge (1993), Bauer (1994), Welch (1994), Matsui and Ota (1995), Zhao et al. (1999), Rösler (2000), and Rösler et al. (2006) recognized G. liboensis as a valid species.
Rösler et al. (2011) assigned G. liboensis as a member of the Gekko japonicus species group and noted the several morphological differences of this form from G. hokouensis by summarizing literature information, including larger maximum SVL (85 vs. 70 mm), absence of INs, greater number of IOs (40 vs. 30-33), fat dorsal tubercles, fewer dorsal tubercle rows (DTRs; 10 vs. 12–18), and fewer lamellae below the forth toe (9 vs. 15–18). Considering the fact that the data collected for G. liboensis are from a single specimen, reevaluation of the character states using a larger sample is needed. Furthermore, Toda et al. (2001) reported the existence of a possibly undescribed species from the Ryukyu Islands, Japan, which shares identical states in all morphological diagnostic characteristics with G. hokouensis but is genetically distinctive, even in a sympatric area (henceforth referred to as Gekko sp. 1). Considering such a future taxonomic problem, thorough revision of the taxonomic status of G. liboensis is required to reevaluate its validity. For around 30 years, however, no study has been attempted to elucidate the taxonomic validity of G. liboensis because of the deficiency of available specimens, particularly those of male individuals (Rösler et al., 2011).
Through the course of our field surveys in Guizhou Province and Guangxi Zhuang Autonomous Region, southern China, we collected many geckos, including males, whose morphological features agreed well with the original description of G. liboensis. Based on these new specimens, we conducted morphological examinations of this form for comparison with other congeneric species, particularly with the morphologically similar G. hokouensis and the putative undescribed species Gekko sp. 1.
2. Materials and Methods
2.1 Sampling Field surveys were conducted in Guizhou Province and Guangxi Zhuang Autonomous Region, southern China in August 2014. A portion of the specimens (six males and three females) were fixed in 10% formalin and then transferred to 70% ethanol for gonad examination. After that they were deposited in the collections of the Chengdu Institute of Biology (CIB). The remaining specimens were kept alive for future behavioral examinations and will be also deposited in the collections of CIB.
2.2 Morphological examinations Sex of individuals was determined based on the presence or absence of precloacal pores (PPs) and the swollen tail base, which are found only in males (Bauer, 2013). Two females and one male of relatively small size (around 60 mm of SVL) in our sample were subjected to gonadal observation to determine sexual maturity. One female and five males that were seemingly large enough to attain sexual maturity were also examined for comparison. Evaluation of gonad condition was made following the criteria provided by Okada et al. (1992).
A total of 31 specimens including males and females mentioned above were examined morphologically. Because most adult geckos of both sexes needed to be kept alive for future study, we conducted scale counts and other morphological observations on live specimens. This treatment might induce slight difference in mensural values when compared to values taken after fixation, but it was not the case for meristic characters because scale counts were carefully conducted using a microscope and close-up photography when necessary. SVL and tail length (TL) were measured to the nearest 0.1 mm using a caliper. Regenerated tails were not measured. SVL was compared between adult males and females by Mann-Whitney U-test. Meristic characteristics measured includedthe number of IOs, DTRs around midbody, the number of SPLs, the number of ILs, the number of INs, the number of subdigital lamellae under first toe (SLI), the number of subdigital lamellae under the forth toe (SLIV), the number of precloacal pores in males (PP), the number of dorsal dark bands (DB), and the number of caudal dark band (CB). We counted the right side for bilateral characteristics.
Morphological data for the present samples were compared with those of other congeneric species. Rösler et al. (2011) proposed a six species group within the genus Gekko on the basis of molecular phylogeny, body size, scalation, color pattern, and geographic distribution, and assigned all East Asian species including G. liboensis into the Gekko japonicus species group. In light of such classification, we considered all species belonging to this group for comparisons. Morphological data for these species were collected from available literature (Ota et al.,1989; Toda, 2008; Zhang et al., 2009; Phung and Ziegler, 2011; Rösler et al., 2011; Yang et al., 2012; Nguen et al., 2013; Luu et al., 2014; Ngo et al., 2015). Because of the close morphological resemblance of G. hokouensis and Gekko sp. 1 to G. liboensis (see above), we made additional detailed comparisons using the original unpublished dataset of Toda (2008), in which 170 specimens of G. hokouensis and 115 specimens of Gekko sp. 1 (see Appendix 1 for the specimen list). The taxonomic identities of these specimens were confirmed by allozyme analysis (Toda, 2008). In comparisons between G. liboensis and G. hokouensis or Gekko sp. 1, we employed the Steel-Dwass test. All statistical tests were performed using R 2.14.0 (R Development Core Team, 2011).
3. Results
3.1 Field collection and incidental data A total of 31 specimens were collected from Libo and Maolan in Guizhou Province, and Nalao in Guangxi Zhuang Autonomous Region (Figure 1A; Table 1). Most of the geckos were collected during the night (20:25–02:57) on rocky cliffs along roads that run through hilly or mountainous regions (Figure 1B and C). One specimen was found during the daytime (10:30) on a rocky cliff in a similar environment but inside a crevice. One specimen from Libo and two from Nalao were collected from vegetation-covered walls of human housing situated adjacent to mountain forests.
In some places where the Gekko specimens were collected, gekkonid eggs were found affixed to depressions of the rock surface or inside a crevice, characterized by calcareous shells, an elliptical shape, and two eggs per clutch (Figure 1D). The diameters along the long and short axes of the six eggs (mean ± SD) were 16.3 ± 0.57 (range 15.6–17.2) mm and 12.4 ± 0.40 (range 12.0–13.0) mm, respectively.
3.2 Redescription of Gekko liboensis Scale counts and tubercle conditions of the gecko specimens are listed in Table 2. The characteristics of the specimens were: (1) a medium sized Gekko (SVL up to 91.0 mm); (2) the absence of the IN in many cases (Figure 2C); however, 1–2 small INs were present in a few individuals (Figure 3); (3) a nostril touching rostral; (4) 38–46 IOs; a paired postmental, with the length approximately 1.5–2 fold of the width, contacted posteriorly or posterolaterally by 1–2 rows of enlarged scales (Figure 2D); (5) 10–13 SPLs; (6) 10–13 infralabials; (7) smooth enlarged tubercles present on the dorsum (Figure 2F), forming 4–10 irregular rows at the midbody but absent on the four limbs and tail (Figure 2H); (8) digits dilated, under surfaces covered with single rows of lamellae along their entire lengths; (9) 11-15 SLIs; (10) 11-18 SLIVs; (11) both fingers and toes without developed interdigital webs (Figure 2G); (12) a single cloacal spur on each side; (13) 10–12 PPs in males (Figure 2E); (14) an original tail that is slender shaped and somewhat depressed at the base; and (15) broad and enlarged subcaudals (Figure 2I).
The specimens exhibited a characteristic color pattern as shown in Figure 2A: the color of the dorsal surface of the body is greenish gray or gold, with 7–11 middorsal blotches, each subdivided medially. In most cases, each blotch had many gold fakes on both sides. Conspicuous round-shaped white marks were scattered on the dorsum, sometimes forming irregular transverse bands interveningthe middorsal blotches. On the dorsal surface of the head, relatively small sized white spots occurred with a series of white spots usually forming a “W” shape on the neck. A white stripe ran through the orbit of the eye, with the iris of a gold color, sometimes slightly greenish. White marks occurred on the four limbs, often forming a few transverse bands with irregular outlines. The tail was banded, with dark parts becoming darker and broader posteriorly. The ventral surface of the head and abdomen was creamy with dark dots.
3.3 Gonadal observation Gonads of nine specimens were measured as 60.9, 65.9, 68.4, 72.1, 78.6, and 86.0 mm in SVL of six males, and 58.3, 60.6, and 79.8 mm in SVL of three females. Among the females, the largest individual possessed three (left) and two (right) yolked ovarian follicles and flaccid oviducts, whereas the two females smaller than 60.6 mm in SVL possessed neither oviductal egg nor yolked ovarian follicle, and their oviducts were not faccid. Similarly, five large males had well-developed epididymides with apparently distinct tubules, whereas the smallest male had undeveloped epididymides characterized by the absence of convoluted tubules. Based on these results, we considered the three specimens around 60 mm in SVL to be juveniles and the remaining 28 specimens larger than 65 mm in SVL to be adults. In adults, no significant difference in SVL and TL between sexes was detected (Mann-Whitney U-test, P = 0.452 in SVL, P = 0.512 in TL; Table 2).
3.4 Comparison of key characteristics with G. hokouensis and Gekko sp. 1 In comparison with G. hokouensis and Gekko sp. 1 using the literature information, the present form differed by having a larger SVL and more PPs, although their ranges slightly overlapped (Table 3).
In quantitative comparison, the number of INs was significantly lower in G. liboensis than that in either G. hokouensis or Gekko sp. 1 (Steel–Dwass test, P < 0.0001 in G. liboensis vs. G. hokouensis and G. liboensis vs. Gekko sp. 1). IN was absent in 77% of G. liboensis, but only in 17% of both G hokouensis and Gekko sp. 1 (Figure 3). The SVL was significantly larger in G. liboensis than in either G. hokouensis or Gekko sp. 1 (Steel–Dwass test, P < 0.0001 in G. liboensis vs. G. hokouensis and G. liboensis vs. Gekko sp. 1; Figure 4). The range of SVL did not overlap between G. liboensis and G. hokouensis, but overlapped between G. liboensis and Gekko sp. 1. The number of PPs was significantly higher in G. liboensis than in either G. hokouensis or Gekko sp. 1 (Steel–Dwass test, P < 0.0001 in G. liboensisvs. G. hokouensis and G. liboensis vs. Gekko sp. 1; Figure 4). The range of PPs did not overlap between G. liboensis and Gekko sp. 1, but did overlap between G. liboensis and G. hokouensis.
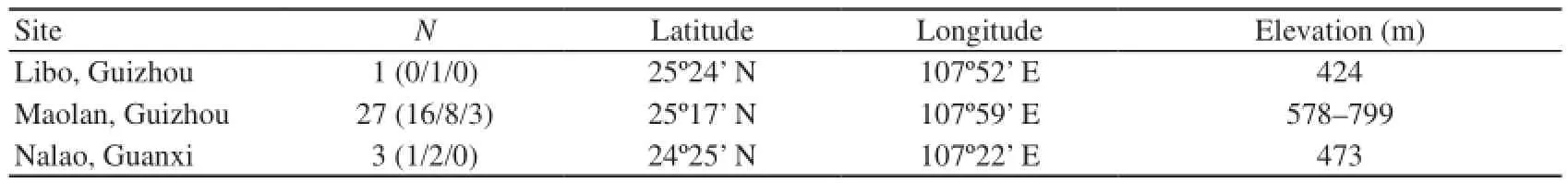
Table 1 Localities of Gekko liboensis examined in this study. N indicates the total number of individuals; number of males/females/juveniles are shown in parentheses. Latitude, longitude, and elevation were recorded by datum WGS84.
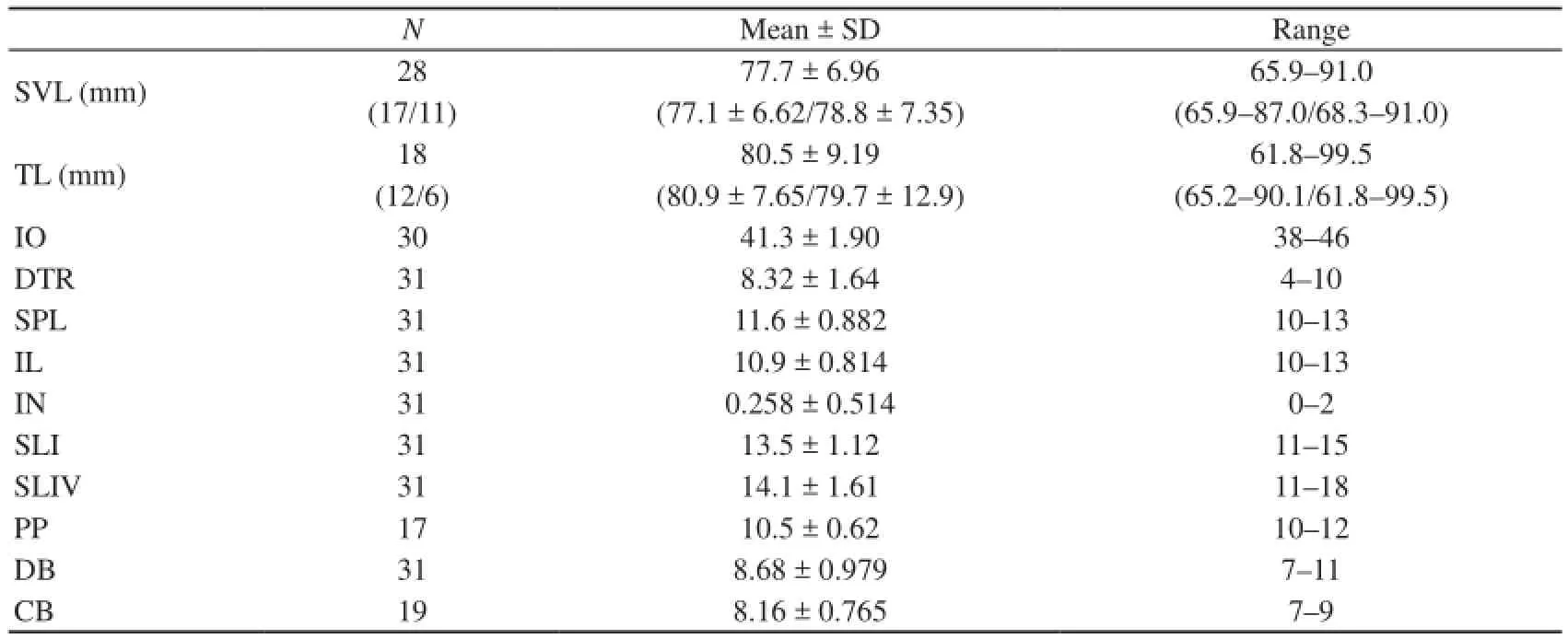
Table 2 Mensural and meristic data for the specimens of Gekko liboensis. Mean ± SD and range are shown. Abbreviations as in Materials and methods. Snout-vent length (SVL) and tail length (TL) data of adult males/females are shown in parentheses.
3.5 Comparison with all other species belonging to the G. japonicus group The series of Gekko specimens described in the current study differed from all other 23 members of the G. japonicus group (G. hokouensis, G.japonicus, G. tawaensis, G. yakuensis, G. vertebralis, G. shibatai, G. swinhonis, G. auriverrucosus, G. melli, G. scabridus, G. subpalmatus, G. palmatus, G. chinensis, G. taibaiensis, G. similignum, G. adleri, G. wenxianensis, G. canhi, G. truongi, G. scientiadventura, G. aaronbaueri, G. thakhekensis, and Gekko sp. 1) except for G. auriverrucosus, G. melli, G. scabridus, G. subpalmatus, and G. truongi by having 10–12 PPs. Moreover, the form was distinguished from G. auriverrucosus by a lower number of DTRs (4–10 vs. 16–20), absence of tubercles on the hind limbs, and a lower number of cloacal spurs (1 vs. 2–3); from G. melli by the presence of tubercles on the dorsum and undeveloped interdigital webs; from G. scabridus by a lower number of DTRs (4–10 vs. 17–21), and the presence of tubercles on the dorsal surface of the tail and the four limbs; from G. subpalmatus by the presence of tubercles on the dorsum and undeveloped interdigital webs; and from G. truongi by the presence of tubercles on the dorsum. In summary, the present form can be distinguished from all other members of the G. japonicus species group by more than one characteristic.
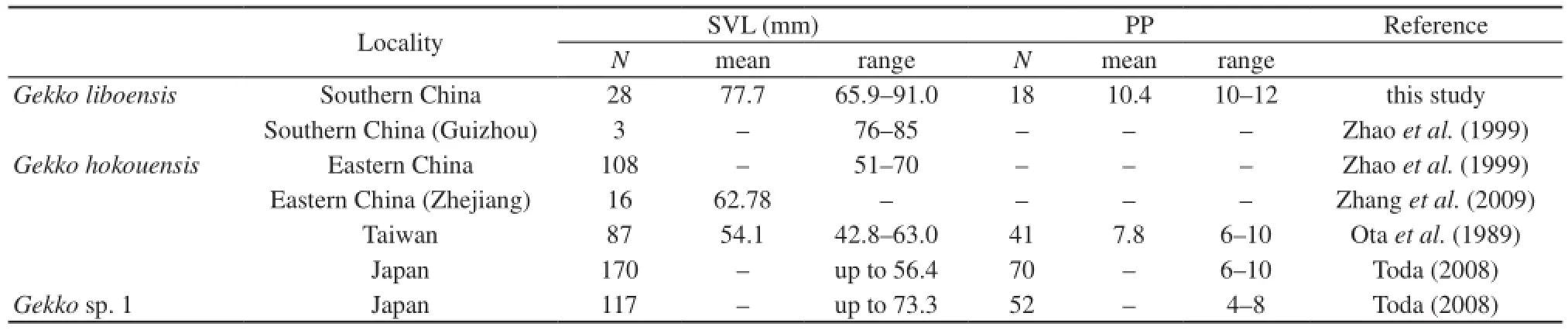
Table 3 Comparison of snout-vent length (SVL) and number of precloacal pores (PP) among Gekko liboensis, G. hokouensis, and Gekko sp. 1. according to four references and this study. N indicates the total number of individuals. Data from juveniles were removed from that of Zhao et al. (1999) and this study.
4. Discussion
To be best of our knowledge, our specimens are the third reported collection of G. liboensis following Zhou and Li (1982) and Zhao et al. (1999) and the first report of male specimens of the species. The comparisons with all other members of the G. japonicus group well support the taxonomic validity of G. liboensis. Morphologically, G. liboensis is most similar to G. hokouensis and Gekko sp. 1, a cryptic species of G. hokouensis. Despite some overlapping key character states, there are significant differences among these three species. In summary, G. liboensis can be reliably distinguished from G. hokouensis and Gekko sp. 1 by the presence of the following features: (1) a larger body size (65–91 mm in SVL [vs. 43–56 mm in G. hokouensis and 43–73 mm in Gekko sp. 1]); (2) an usual absence of IN (vs. usually present in the latter two species); (3) 4–10 DTRs (vs. 12–18 in G. hokouensis but data absent in Gekko sp. 1); (4) 10–12 PPs (vs. 6–10 in G. hokouensis and 3–8 in Gekko sp. 1); and (5) the presence of conspicuous white marks on the dorsum and head (vs. absence in the latter two species). Further studies by use of molecular techniques are desirable to verify our conclusion.
Our specimens of G. liboensis were collected from Libo and Maolan in Guizhou and Nalao in Guangxi, with those in Nalao the first report of the species from outside Guizhou. At Nalao we also found eggs of large size (the mean diameters along the long and short axes are 16.3 and 12.4 mm, respectively), where another gekkonid species, Hemiphyllodactylus dushanensis, has also been recorded (Zhou et al., 1981; Grismer et al., 2013). In comparison with the diameters along the long and short axes of the eggs of H. dushanensis (8.7–9.2 mm and 6.7–6.8 mm, respectively; Zhou et al., 1981), it is almost certain that the eggs we found at Nalao are of G. liboensis.
All specimens and the eggs were found on rocky outcrops in karstic mountains or hills, suggesting that such landscape that provides good shelter and suitable oviposition sites is a preferred habitat of the species G. liboensis. This kind of landscape continues around eastern Yunnan, Guizhou, and western Guangxi in southern China. Although the distribution range of G. liboensis requires clarification by further field survey, it is likely that the distribution of G. liboensis occurs more widely than is currently known. In contrast, during the current decade, a number of gecko species have been described from karstic outcrops and caves at single or a few localities in East and Southeast Asia (e.g., Ngo and Bauer, 2008; Ngo et al., 2009; Linkem et al., 2010; Ngo and Gamble, 2010, 2011; Ziegler and Nguyen, 2010; Grismer et al., 2014). In these regions, the erosion of karstic area has resulted in isolated karstic mountains and hills that are surrounded by alluvial plains that are not suitable habitat for the geckos (Ellis and Pauwels, 2012). This isolated distribution of potentially suitable habitatis likely to result in several local endemics. Further surveys of unexplored karstic mountains and hills and the comprehensive phylogenetic study of the Gekko japonicus group are required to understand the actual diversity of this group.
Acknowledgements We are grateful to K. Nishikawa for his help in taking the original photographs of the Figure 2; D. Han, Y. Liu, and X. Sun for assistance to keep geckos. This work was financially supported by a Fellowship for Young International Scientists of the Chinese Academy of Sciences (No. Y4J3011100) to TJ.
Bauer A. M. 1994. Gekkonidae (Reptilia, Sauria), Part I Australia and Oceania. Walter de Gruyter, Berlin, Germany: Das Tierreich 109 (Part)
Bauer A. M. 2013. Geckos: The Animal Answer Guide. Maryland, USA: Johns Hopkins University Press
Ellis M., Pauwels O. S. G. 2012. The bent-toed geckos (Cyrtodactylus) of the caves and karst of Thailand. Cave Karst Sci, 39: 16–22
Grismer L. L., Wood P. J. L., Anuar S., Muin M. A., Quah E. S. H., McGuire, J. A., Brown R. M., Ngo V. T., Hong T. P. 2013. Integrative taxonomy uncovers high levels of cryptic species diversity in Hemiphyllodactylus Bleeker, 1860 (Squamata: Gekkonidae) and the description of a new species from Peninsular Malaysia. Zool J Linn Soc, 169: 849–880
Grismer L. L., Wood P. J. L., Anuar S., Riyanto A., Ahmad N., Muin M. A., Sumontha M., Grismer J. L., Onn C. K., Quah E. S. H., Pauwels, O. S. A. 2014. Systematics and natural history of Southeast Asian Rock Geckos (genus Cnemaspis Strauch, 1887) with descriptions of eight new species from Malaysia, Thailand, and Indonesia. Zootaxa 3880: 1–147
Günther R. 1994. Eine neue Art der Gattung Gekko (Reptilia, Squamata, Gekkonidae) aus dem Süden Vietnams. Zool Anz 233: 57–67 (In Germany)
Henkel F. W., Schmidt W. 2003. Praxis Ratgeber Geckos. Frankfurt am Main, Germany: Edition Chimaira (In German)
Kluge A. G. 1993. Gekkonoid Lizard Taxonomy. San Diego, USA: International Gecko Society
Kluge A. G. 2001. Gekkotan lizard taxonomy. Hamadryad 26: 1–209
Linkem C. W., Siler C. D., Diesmos A. C., Brown R. M. 2010. A new species of Gekko (Squamata: Gekkonidae) from central Luzon Island, Philippines. Zootaxa 2396: 37–94
Luu, Q. V., Calame T., Nguyen T. Q., Le M. D., Bonkowski M., Ziegler T. 2014. A new species of the Gekko japonicus group (Squamata: Gekkonidae) from central Laos. Zootaxa 3895: 73–88
Matsui M., Ota H. 1995. On Chinese herpetology. Herpetologica 51: 234–250
Ngo V. T., Bauer A. M. 2008. Descriptions of two new species of Cyrtodactylus Gray 1827 (Squamata: Gekkonidae) endemic to southern Vietnam. Zootaxa 1715: 27–42
Ngo V. T., Bauer A. M., Wood Jr P. L., Grismer J. L. 2009. A new species of Gekko Laurenti, 1768 (Squamata: Gekkonidae) from Dong Nai Province, Southeastern Vietnam. Zootaxa 2238: 33–42
Ngo V. T., Gamble T. 2010. A new species of Gekko (Squamata: Gekkonidae) from Tà Kóu Nature Reserve, Binh Thuan Province, Southern Vietnam. Zootaxa 2346: 17–28
Ngo V. T., Gamble T. 2011. Gekko canaensis sp. nov. (Squamata: Gekkonidae), a new gecko from Southern Vietnam. Zootaxa 2890: 53–64
Ngo V. T., Pham, T. H., Anorath, P., Patrick, D., Alexandre, T. 2015. Gekko aaronbaueri, new gecko (Squamata: Gekkonidae) from central Laos. Zootaxa 3914: 144–156
Nguyen T. Q., Wang Y. Y., Yang J. H., Lehmann T., Le D. M., Ziegler T., Bonkowski M. 2013. A new species of the Gekko japonicus group (Squamata: Sauria: Gekkonidae) from the border region between China and Vietnam. Zootaxa 3652: 501–518
Okada S., Ota H., Hasegawa M., Hikida T., Miyaguni H., Kato J. 1992. Reproductive traits of seven species of lygosomine skinks (Squamata: Reptilia) from East Asia. Nat Hist Res 2: 43–52
Ota H., Lue K. Y., Chen S. H., Brown B. C. 1989. Taxonomic status of the Taiwanese Gekko, with comments on the synonymy of the Luperosaurus amissus Taylor. J Herp 23: 76–78
Phung T. M., Ziegler T. 2011. Another new Gekko species (Squamata: Gekkonidae) from southern Vietnam. Zootaxa 3129: 51–61
R Development Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rösler H. 2000. Kommentierte Liste der rezent, subrezent und fossil bekannten Gecko-Taxa (Reptilia: Gekkonomorpha). Gekkota 2: 28–153 (In German)
Rösler H., Siler C. D., Brown R. M., Demegillo A. D., Gaulke M. 2006. Gekko ernstkelleri sp. n. – a new gekkonid lizard from Panay Island, Philippines. Salamandra 42: 197–211
Rösler H., Bauer A. M., Heinicke M. P., Greenbaum E., Jackman T., Nguyen T. Q., Ziegler T. 2011. Phylogeny, taxonomy, and zoogeography of the genus Gekko Laurenti, 1768 with the revalidation of G. reevesii Gray, 1831 (Sauria: Gekkonidae). Zootaxa 2989: 1–50
Toda M., Hikida T., Ota H. 2001. Discovery of sympatric cryptic species within Gekko hokouensis (Gekkonidae: Squamata) from the Okinawa Island, Japan, by use of allozyme data. Zool Scr 30: 1–11
Toda M. 2008. External characters useful to discriminate Gekko hokouensis and its cryptic undescribed species of the Okinawa Group, Japan: a preliminary report. Akamata 19: 27–34 (In Japanese)
Yang J. H., Wang Y. Y., Zhang T. D., Sun Y. J., Lin S. S. 2012. Genetic and morphological evidence on the species validity of Gekko melli Vogt, 1922 with notes on its diagnosis and range extension (Squamata: Gekkonidae). Zootaxa 3505: 67–74
Zhang Y. P., Du W. G., Zhu L. J. 2009. Differences in body size and female reproductive traits between two sympatric geckos, Gekko japonicus and Gekko hokouensis. Folia Zool 58: 113–122
Zhao E., Adler K. 1993. Herpetology of China. Oxford, Ohio,USA: Society for the Study of Amphibians and Reptiles
Zhao E., Zhao K., Zhou K. 1999. Fauna Sinica Reptilia, Vol. 2, Squamata Lacertilia. Beijing, China: Science Press (In Chinese)
Zhou K. Y., Liu Y. Z., Yang G. P. 1981. Three new species of Hemiphyllodactylus yunnanensis (Boulenger) from China (Lacertiformes, Gekkonidae). Acta Zootaxonomica Sinica 6: 202–209 (In Chinese)
Zhou K. Y., Li D. J. 1982. Gekko liboensis. 1–2. In Zhou K. Y., Liu Y. Z., Li D. J. (Eds.), Three new species of Gekko and remarks on Gekko hokouensis (Lacertiformes, Gekkonidae). Acta Zootaxonomica Sinica 7: 438–446 (In Chinese)
Ziegler T., Nguyen Q. T. 2010. New discoveries of amphibians and reptiles from Vietnam. Bonn Zool Bull 57: 137–147
Welch K. R. G. 1994. Lizards of the World, 1, Geckos. Taunton,USA: R and A Research and Information Limited
Xu N., Gao X. M., Wu K. Y., Wei G. 2007. Study on the distribution of reptiles in eight natural reserves in Guizhou province. Chin J Zool 42: 106–113 (In Chinese)
Appendix 1 Catalogue numbers of specimens used in this study.
Gekko liboensis: CIB101225–101255 (101225–101236 for gonadal observation).
Gekko hokouensis: KUZ R30416–30422, 30471, 30473–30476, 30514–30521, 30570, 30571, 30573, 30574, 30600, 30601, 31172–31185, 31202, 31203, 31238–31242, 32257–32261, 32269–32275, 32309, 32310, 32323–32330, 32347, 32348, 32447, 32453, 32456–32459, 33336–33340, 33534–33539, 33631–33636, 34141, 34142, 45368, 45369, 45559–45561, 45563–45566, 47348–47351, 47355, 47357–47361, 47363, 47366–47369, 47371–47376, 47554–47565, 47567, 47568, 47570, 47571, 47573–47584, 47740–47745, 48071–48074, 48081–48086.
Gekko sp. 1: KUZ R30415, 30472, 30550–30559, 30572, 30575–30577, 31228, 31229, 32158–32161, 32263, 32264, 32266, 32268, 32314–32316, 32443–32446, 32448–32452, 32454, 32455, 32995–32998, 33269, 33270, 33297, 33299, 33300, 33540, 33541, 33573–33576, 34147–34150, 34214–34219, 34259–34266, 34558–34569, 45319–45321, 45325, 45327, 45330, 45371, 45463, 47370, 47377–47382, 47585–47596, 47736–47739, 50137.
Dr. Teppei JONO, from Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan, China, with his research focusing on evolutionary ecology and ethology of herptiles. E-mail: mjusinondo@gmail.com
12 February 2015 Accepted: 21 May 2015
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Japalura (Squamata: Sauria: Agamidae) from Upper Lancang (Mekong) Valley of Eastern Tibet, China
- Seasonal Dynamics of Male and Female Reproductive Systems in the Siberian Salamander, Salamandrella keyserlingii (Caudata, Hynobiidae)
- Oviposition Site Selection in the Malayan Giant Frog (Limnonectes blythii) in Singapore: Conservation Implications
- Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii (Reptilia, Squamata, Gekkonidae)
- Genetic and Morphological Variations within Laudakia microlepis (Blanford, 1874) (Sauria: Agamidae) Populations in Southeastern Iran with Description of a New Subspecies
- Potential Distribution Modeling and Morphology of Pelias barani (Böhme and Joger, 1983) in Turkey
