Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii (Reptilia, Squamata, Gekkonidae)
2015-10-31ShuangliHAOLiangliangPANZhouxiFANGandYongpuZHANG
Shuangli HAO, Liangliang PAN, Zhouxi FANGand Yongpu ZHANG*
1College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China
2College of Life Science, Wenzhou Medical University, Wenzhou 325035, China
Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii (Reptilia, Squamata, Gekkonidae)
Shuangli HAO1, Liangliang PAN2, Zhouxi FANG2and Yongpu ZHANG1*
1College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China
2College of Life Science, Wenzhou Medical University, Wenzhou 325035, China
We provide the first description of the ultrastructure of the spermatozoa of Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii for further understanding of the phylogenetic relationships of Gekkonidae. Mature spermatozoa of the three species differ in the occurrence and shape of epinuclear electron-lucent zone, nuclear space, neck cylinder and mitochondria. G. japonicus and G. chinensis have similar spermatozoan ultrastructure while H. bowrigii differs from these two species. In addition, these three species have neck cylinder with mitochondria in neck region and dense bodies arranged in grid with mitochondria in the midpiece, which may be the autapomorphies of the family Gekkonidae. Statistical analyses reveal that: total length of the spermatozoa was significantly different between G. japonicus and G. chinensis, as well as between G. japonicus and H. bowrigii (F2,57= 23.66, P<0.0001); G. japonicus and H. bowrigii differ in head length (F2,43= 4.64, P<0.05) and the width of nuclear base (F2,22= 3.97, P<0.05). In addition, the midpiece length (F2,33= 23.66, P<0.01) of the spermatozoa was significantly different between H. bowrigii and G. japonicus, and also between H. bowrigii and G. chinensis. Lengths of perforatorium, acrosomal complex and nuclear rostrum and the width of nuclear shoulder are similar in all three species. Our results indicated that the sperm ultrastructure contained intra and intergeneric variabilities which is helpful for better understanding their genetic relationships.
Spermatozoa, ultrastructure, Gekkonidae, phylogenetic relationship
1. Introduction
The sperm is a highly differentiated reproductive cell produced by testes and is a necessity in the fertilization process of Gekkonidae. Thus, any study on sperm ultrastructure is of great significance to help reveal the biological mechanisms of fertilization and reproductive biology. In addition, the ultrastructure of squamata sperm contains valuable phylogenetic information and provides important evidence for phylogenetic analyses (Jamiesion and Healy, 1992; Jamiesion, 1995; Oliver et al., 1996; Teixeira et al., 1999b, c; Vieira et al., 2004; Vieira et al., 2005; Zhang et al., 2006). The spermultrastructure of squamata has rich polymorphism within the family (Giugliano et al., 2002; Jamieson, 1995; Liu and Zhang, 2004; Oliver et al., 1996; Teixeira et al., 1999b, c; Tavares-Bastos et al., 2002). Knowledge of the sperm ultrastructure improves the accuracy of the phylogenetic analysis, and contributes to the phylogenetic reconstruction in the higher order of classification (Giugliano et al., 2002; Liu and Zhang, 2004; Tavares-Bastos et al., 2002).
The Gekkonidae is widely distributed around the world and contains more than 90 genera and over 1000 named species (Han et al., 2004). Other than Scincidae, it is the second-most-numerous family in sauria. However, detailed information on the ultrastructure of spermatozoa or spermiogenesis and studies clarifying the phylogenetic relationships of Gekkonidae remain obscure. Furieri (1970) briefly described the spermof Lygodactylus picturatus, Hemidactylus frenatus, Hemidactylus mabouia, and Tarentola mauritanica while Phillips and Asa (1993) reported the development of the midpiece section in Sphaerodactylus cinereus. Jamieson et al. (1996) has detailed study of the ultrastructure of spermatozoa of Heteronotia binoei and Röll and von Düring (2008) investigated the difference in the spermiogenesis of the normal and the phenotypic males. Rheubert et al. (2011) provided a detailed description of the formation of the sperm of Hemidactylus turcicus. There is a need for additional ultrastructural spermatozoa studies to develop a comprehensive phylogenetic hypothesis for this family.
Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii in our study are all oviparous and nocturnal animals. G. japonicus mainly distributes in the south of the Huaihe, west to Shanxi and Gansu in China, but also in Japan and Korea. G. chinensis is the endemic species of China, living in the wild or the structure gaps in Fujian, Guangdong, Hainan, Guangxi, while H. bowrigii distributes mainly in Fujian, Hainan, Sichuan, Guangdong, Guangxi, Taiwan and Yunnan in China, and is also common in India, Sikkim, Burma and Ryukyu islands in Japan (Zhao et al. 1999). Here we provided a detailed description of the mature spermatozoa of G. japonicus, G. chinensis and H. bowrigii for the first time. Our results were compared with the mature spermatozoa of previously examined gecko species. The main objectives of this study were to understand the spermatozoal autapomorphies in the family Gekkonidae and to provide a theoretical basis for detailed phylogenetic study on Gekkonidae. Specifically, we ascertained the degree of variability in sperm morphology of three Gekkonidae species common in China.
2. Materials and Methods
Mature spermatozoa from male G. japonicus, G. chinensis and H. bowrigii were collected from animals captured in three locations in China: Wenzhou, Zhejiang (27°23' N, 119°37' E), Quanzhou (24°30' N, 117°27' E) and Putian (25°45' N, 119°03' E), Fujian. All lizards used in this study were captured in early May 2014.
Four adult specimens of each species were quickly sacrificed in the laboratory. Epididymides of each experimental lizard were removed, and diced into 1–2 mm3pieces and placed in a petri dish with phosphatebuffered saline solution (PBS, pH 7.2). A small part of epididymides sample for sperm smear analysis was fixed using a solution containing 2.5% glutaraldehyde, 2% paraformaldehyde and 3% sucrose in 0.1M sodium cacodylate buffer, pH 7.2, for 10 min. Once fixed, each sample was stained for 30 s withtoluidine blue under an alcohol lamp. The morphology of entire sperm was observed under a light microscope (Olympus BX51, Japan) and morphological features were digitally captured with a CCD camera (Olympus DP71, Japan). Lengths of the head, midpiece and entire sperm of the three species were measured with a micrometer under the light microscope (Olympus BX51, Japan). The descriptions of sperm ultrastructure were based on the protocol described for the saurians (Colli et al., 2007; Giugliano et al., 2002; Jamieson et al., 1996; Scheltinga et al., 2001).
The rest of the epididymides samples were fixed at 4°C overnight using 2.5% glutaraldehyde solution. Tissue samples were then rinsed in 0.1M phosphate buffer at pH 7.2, post-fixed for 1h in buffered 1% osmium tetroxide,rinsed in 0.1M phosphate buffer, dehydrated through series of ascending contents of acetone (70–100%) and then finally embedded in epoxy resin. Ultrathin sections prepared by microtome were stained for 30 s in lead citrate, rinsed in distilled water, then in 6% aqueous uranyl acetate for 4 min, rinsed in distilled water, and further stained for 2 min in lead citrate before final rinse with distilled water. Electron micrographs were taken on a Hitachi 7500 transmission electron microscope (TEM). Using the TEM, the following morphometric were determined for each species following the techniques suggested by Teixeira et al. (2002) and Zhang et al. (2006): the length of perforatorium, midpiece, acrosome, nuclear rostrum, the width of nuclear shoulder and nuclear base. We used one-way analysis of variance (ANOVA) to statistically compare the various morphometric parameters (e.g., length of mid-piece, width of nucleus shoulder) of sperm collected from these three groups of Gekkonidae used in the study. Post-hoc comparisons of significantly different (P< 0.05) parameters were conducted by the Tukey’s test using Statistica version 6.0.
3. Results
3.1 Spermatozoal morphometrics Main sperm morphological dimensions and the statistical results of the three species of Gekkonidae are shown in Table 1. The total length (F2,57= 23.66, P<0.0001), head length (F2,43= 4.64, P<0.05) and nuclear base width (F2,22= 3.97, P< 0.05) of the spermatozoa were significantly different among species. G. japonicus had significantly shorter sperm than G. chinensis and H. bowrigii. The head length and nuclear base width follow the trend G. japonicus >G. chinensis > H. bowrigii. H. bowrigii had significantly longer midpiece than G. chinensis and G. japonicus (F2,33= 23.66, P<0.01). There were no statistical differences between any of the three species in the length of perforatorium (F2,10= 1.62, P = 0.25), acrosomal complex (F2,10= 0.34, P = 0.72) and nuclear rostrum (F2,18= 1.89, P = 0.18) or the width of nuclear shoulder (F2,20= 0.34, P = 0.72).
3.2 Ultrastructure of spermatozoa Spermatozoa of G. japonicus (Figure 1A), G. chinensis (Figure 1B) and H. bowrigii (Figure 1C) are filiform cells, consisting of head,midpiece and tail. The head is made up of acrosomal complex and the nucleus (Figures 2A; 4A and 6A) while the tail is subdivided into principal piece and endpiece (Figures 3E; 5F and 7N).
Acrosome complex In all three species, the acrosomal complex has a coniform and slightly curved shape and is composed of two caps: an external acrosomal vesicle and an internal subacrosomal cone. The acrosomal vesicle is the anterior terminal portion of the spermatozoon, also called acrosomal cap, which is distinctly divided into an external, moderately electrodense and thin cortex, and an internal denser medulla (Figures 2A, B, K; 4A, B, C; and 6A, B, G). Cross striations are seen in concentric circles (Figures 2B; 4C; and 6B). The posterior of acrosomal vesicle is very thin and sleeve-shaped, hence it is often referred to as acrosomal sleeve (Figures 2A; 4A, B; and 6A). Within the acrosomal medulla, the subacrosomal space contains a perforatorium that resembles a very narrow elongated cone with a pointed tip (Figures 2A, C, K; and 4B, C). The perforatorium begins at a stopperlike perforatorial base plate, embedded in the apex of the subacrosomal cone (Figures 2A, D; 4A, D; and 6G). The subacrosomal cone surrounds the tapered anterior end of the nucleus (Figures 2A, 4A, B; and 6A, G). There is a slightly densified subacrosomal space between the base of the perforatorium and the anterior extremity of the subacrosomal cone (Figures 2A; 4A, B; and 6A, G). The transverse sections of acrosomal vesicle are rounded. In G. japonicus and G. chinensis, an epinuclear electronlucent zone is present within the anterior region of the subacrosomal cone immediately anterior to the nuclearrostrum, while in H. bowrigii it is beginning at the tip of the nuclear space (Figures 2A, E, K; 4A, E; and 6C, G). However, the shape of epinuclear electron-lucent zone differs among the three species. G. japonicus is short rodlike with low electron density, G. chinensis is fusiform while H. bowrigii is long rod-like with high electron density. The nuclear space from the anterior to the nuclear rostrum in H. bowrigii was not observed in other two species that we examined (Figure 6G).
Nucleus The nucleus is elongated and composed of highly condensed, electron-dense chromatin. In transverse section, the nucleus is circular (Figures 2I, J; 4G; and 6E, F). Nucleus anteriorly tapers to form a slender cone, called nuclear rostrum, within the acrosome complex (Figures 2A; 4A, B; and 6A). Rounded nuclear shoulder is observed at the basal end of the rostrum (Figures 2A; 4B; and 6A). Nuclear shoulder has a diameter slightly smaller than the base of the nucleus. The basal pole of the nucleus has a conical depression called the nuclear fossa or implantation fossa associated with the neck elements(Figures 2L, J; and 6F, H).
Neck region The neck region occurs at the junction between nucleus and midpiece. It includes proximal and distal centrioles, neck cylinder or dense collar and mitochondria (Figures 2L; 3C; 5A; and 7A, I). The proximal centriole is located before the distal centriole, and it lies at approximately 90°to the distal centriole. The proximal centriole has nine short microtubule triplets arranged in a circular pattern (Figures 3C; and 7L). Electron-densed lamellar structures that are associated with pericentriolar material lie on both sides in the front of the proximal centriole (Figures 3C; 5A; 6H; and 7A, L). The distal centriole forms the basal body or basal granule of the axoneme and is characterized by the presence of a central electron-dense body (Figures 3F; 5G; and 7C, D). Nine triplets of microtubules and a pair of central microtubules from the axoneme are observed in a distal centriole. Nine peripheral fibers that partially cover each triplets and a central fiber connected to the centralmicrotubule are present. A special structure observed in all three species is the neck cylinder. However, the neck cylinders of different species have their distinct characteristics. The neck cylinders of G. japonicus and G. chinensis are thick, 0.19 μm and form a ring structure in cross-section containing a few mitochondria (Figures 3F; and 5G), while that of H. bowrigii is very thin, about 0.02 μm and looks like a seven-pointed star in cross-section. Besides, around the neck cylinder of H. bowrigii, there are seven mitochondria (Figures 7C, D).
Midpiece The midpiece of G. japonicus, G. chinensis and H. bowrigii begins with the neck cylinder and ends with the annulus. It includes the neck region, axoneme surrounded by mitochondrial sheath and the fibrous sheath (Figures 3A, B; 5B; and 7A, B, I).
The mitochondrial sheath surrounds the fibrous sheath and is composed of mitochondria and dense bodies arranged in grid. The mitochondria are in meshes formed by the dense bodies (Figures 3B; 5C; and 7I). In the species of G. japonicus and G. chinensis, the mitochondria are ovoid in oblique section while in H. bowrigii, the mitochondria have irregular shape. In transverse section, mitochondria are ovoid in shape composed of linear cristae (Figures 3G; 5H; and 7E). In addition, there are seven mitochondria without dense bodies before the annulus (Figures 3H; and 7F). The small ovoid annulus lies in the end of the midpiece and is close to the inner surface of the cell membrane (Figures 3D, I; 5B, I; and 7B). Axoneme is enclosed by the fibrous sheath that begins at the base of the distal centriole and extends into the midpiece (Figures 3A, G, H; 5D, H; and 7A, B E). Fibrous sheath appears as ring structures of dense material in transverse sections, and has regular shape composed of neatly arranged squares when viewed in the longitudinal section. Axoneme complex is composed of a pair of central microtubules (singlets) surrounded by nine doublets of microtubules associated with nine peripheral dense fibers (Figures 3A, G; 5D, H; and 7B). A central fiber connects with the two singlets, which posteriorly diminishes in size and is located centrally between the singlets of the axoneme. The central fiber is vestigial and not observable at the level of the granular cytoplasmic zone (Figures 3J; 5J; and 7G). In all species, the diameter of peripheral fibers rapidly decrease posteriorly except for the fibers at doublets 3 and 8 that form a double structure separated from their corresponding doublet and closely associated with the fibrous sheath (Figures 3G, H; 5H; and 7E, F). Posterior to the granular cytoplasmic zone, all nine peripheral fibers are vestigial or absent. The fibers at doublets 3 and 8 are absent at the anterior of the principal piece, which is the granular cytoplasmic zone (Figures 3J; 5J; and 7G).
Principal piece In all three species, the principalpiece has similar structure and is the longest part of the spermatozoon and occurs behind the midpiece. It consists of the axoneme surrounded by fibrous sheath, cytoplasm, and plasma membrane (Figures 3J, K; 5E, K; and 7H, M). In the anterior portion of the principal piece and immediately after the annulus, the diameter of the spermatozoon does not decrease compared to the annulus diameter and a thick region of granular cytoplasm widely separates the plasma membrane from the fibers sheath (Figures 3D; 5B; and 7B). Posteriorly, the plasma membrane becomes closely attached to the fibrous sheath (Figures 3E; 5E; and 7M). The thickness of the fibrous sheath becomes thinner and thinner and disappeared the junction with the endpiece, while the 9 + 2 pattern of the axonemal microtubules remains unaltered (Figure 7J).
Endpiece The three species have endpiece with similar structure. It is a short axoneme extending beyond the posterior limit of the fibrous sheath (Figures 3E; 5F; and 7N). The length of the endpiece is difficult to measure because the fiber sheath disappears and only the plasma membrane was observable. In the proximal section, the axoneme is still visible with the “9 + 2” structure that gradually becomes disrupted and shows irregular arrangement (Figures 3L; 5L; and 7K).
4. Discussion
4.1 Synapomorphies of the family Gekkonidae The spermatozoa of G. japonicus, G. chinensis and Hemidactylus bowrigii exhibit the following squamate synapomorphies: a acrosomal vesicle and subacrosomal cone; a single prenuclear perforatorium; absence of endonuclear canal; presence of a nucleus rostrum and intermitochondrial dense bodies; linear mitochondrial cristae; fibers at doublets 3 and 8 are thick and form a double structure separated from their corresponding doublet and fibrous sheath extending into midpiece but not into the neck region (Jamieson, 1995; Jamieson et al., 1996; Jamieson and Healy, 1992; Giugliano et al., 2002; Oliver, 1996; Teixeira et al., 1999b, c; Teixeira et al., 2002; Vieira et al., 2004; Zhang et al., 2006). Furthermore, rounded nuclear shoulder, a perforatorial base-plate, subacrosomal space were observed in the three species and all the gekkonid lizards reported (Furieri, 1970; Jamieson et al., 1996; Rheubert et al., 2011). The neck cylinder with mitochondria and densebodies arranged in grid with mitochondria were also observed in the three species, which were accordance to the Jamieson’s report (Jamieson et al., 1996). The similar results were observed in Furieri’s research about Hemidactylus frenatus, L. picturatus and T. mauritanica (Furieri, 1970). Hence, we concluded that the rounded nuclear shoulder, a perforatorial base-plate, subacrosomal space, the neck cylinder with mitochondria and dense bodies arranged in grid with mitochondria are synapomorphies of Gekkonidae. In lizards other than Gekkonidae, such as Tropiduridae, Phrynosomatidae, Polychrotidae, Crotaphytidae, Agamidae, Chamaeleonidae (Scheltinga et al., 2001), Hoplocercidae, Opluridae (Vieira et al., 2007), Varanidae (Oliver, 1996), Lacertidae (Zhang et al., 2005) and Scincidae (Liu and Zhang, 2004; Zhang et al., 2006), the neck cylinder with mitochondria and dense bodies arranged in grid with mitochondria have not been reported. Although the neck cylinder surrounding the distal centriole has been observed in serpents, there is no inset mitochondria around it (Colli et al., 2007; Cunha et al., 2008; Oliver et al., 1996; Tavares-Bastos et al., 2008; Tourmente et al., 2006; Teixeira et al., 2002; Vieira et al., 2007). In the midpiece, zigzagged mitochondria are tightly arranged and the dense body is scarce and even absent (Cunha et al., 2008; Jamieson and Koehler, 1994; Oliver et al., 1996; Tavares-Bastos et al., 2008; Tourmente et al., 2008; Tourmente et al., 2006). Therefore, our results showed that neck cylinder with mitochondria and dense bodies arranged in grid with mitochondria are autapomorphies of Gekkonidae.

Table 1 Sperm morphological dimensions of the three species of Gekkonidae
4.2 Polymorphic characters among the Gekkonidae species Our research, as well as previous studies on spermatozoa ultrastructure of Gekkonidae shows several distinct features in spermatozoa ultrastructure of the different species (Table 2). The subacrosomal cone in cross-section of Heteronotia binoei (Jamieson et al., 1996) is apically quadrangular rather than circular like observed in other species. The epinuclear electron-lucent zone is absent in Hemidactylus frenatus and Heteronotia binoei (Furieri, 1970; Jamieson et al., 1996) short, rodshaped and low electron dense in G. japonicus, fusiform in G. chinensis and is long, rod-like and highly electrondense in Hemidactylus bowrigii. The nuclear space is only observed in Hemidactylus bowrigii and not in others of Gekkonidae species. However, this nuclear space structure has been reported in four species of Tupinambis (Tavares-Bastos et al., 2002) and some serpents, such as Epicrates cenchria, Boa constrictor amarali, and Corallus hortulanus (Tavares-Bastos et al., 2008). Furthermore, the neck cylinders of G. japonicus and G. chinensis are thick and ring-shaped with few mitochondria in cross-section while those of Hemidactylus bowrigii and Heteronotia binoei are thin. In Hemidactylus bowrigii, the neck cylinder is like a seven-pointed star surrounded by seven mitochondria while in Heteronotia binoei, it is arranged as a six- or seven-pointed star surrounded by more mitochondria(Jamieson et al., 1996). The laminar structure observed in G. chinensis, G. japonicus and Hemidactylus bowrigii is absent in Heteronotia binoei ( Jamieson et al., 1996). In oblique section of the midpiece, mitochondria are ovoid in G. japonicus, G. chinensis and Hemidactylus frenatus, but are anomalous in Hemidactylus Bowrigii, Heteronotia binoei and Lygodactylus picturatus (Furieri, 1970; Jamieson et al., 1996). In addition, the mitochondrial abundance of different Gekkonidea species varies from 3 to 9 in transverse section of the midpiece with notable three species of Hemidactylus containing 6 (Furieri, 1970; Jamieson et al., 1996; Rheubert et al., 2011). The midpiece lengths of G. japonicus (5.77 μm)and G. chinensis (6.45 μm) are very much shorter than Hemidactylus bowrigii (14.28 μm).
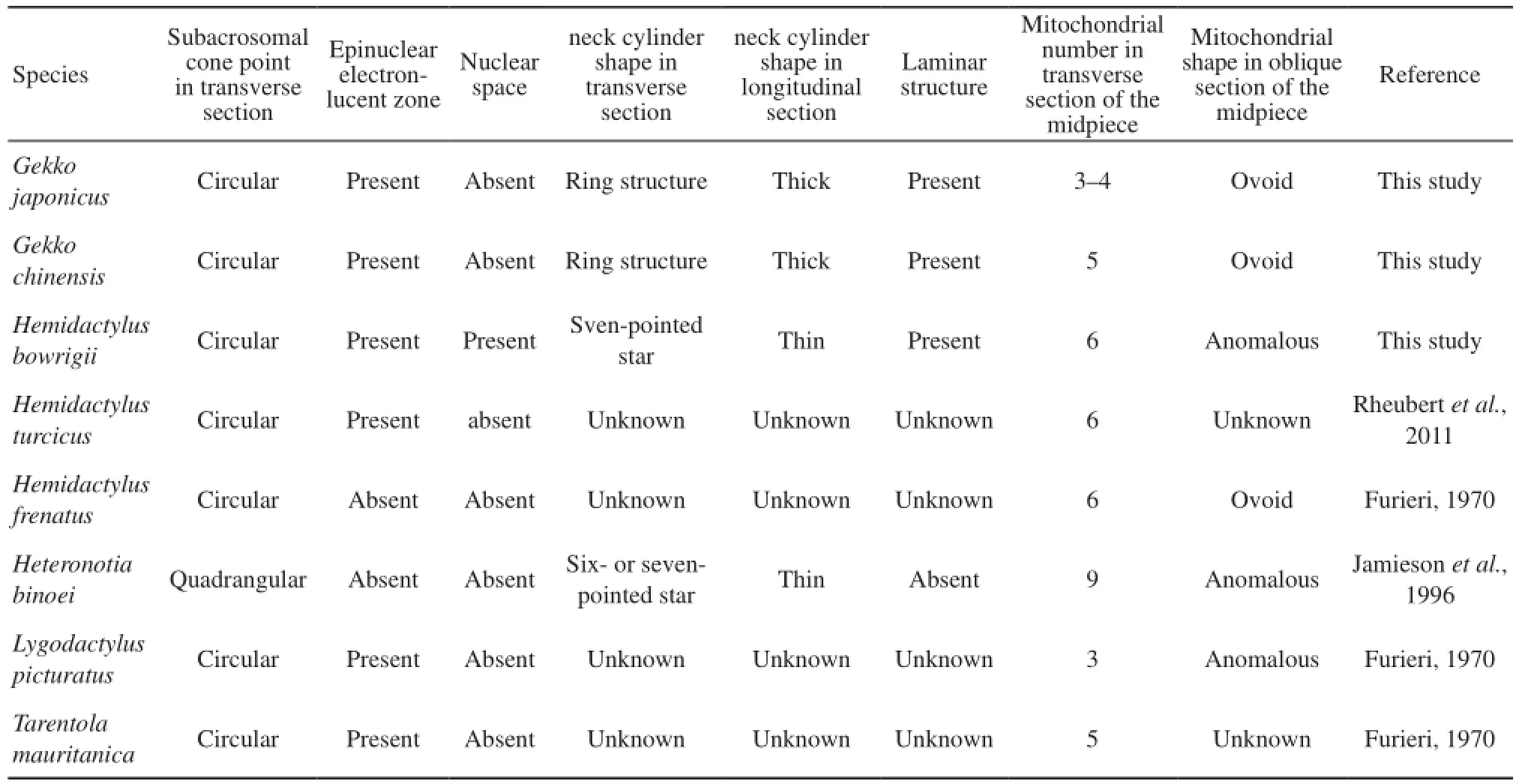
Table 2 Variability of spermatozoa ultrastructure among different species of Gekkonidae
The polymorphic traits in sperm ultrastructure among congeneric species had been documented in lizards such as Crotaphytidae(Scheltinga et al., 2001), Polychrotidae ( Teixeira et al., 1999a ; Scheltinga et al., 2001) and Tropiduridae ( Teixeira et al., 1999d). Polymorphic characters commonly occur in the acrosome complex and midpiece, which are believed to be infuenced by the fertilization processes and physiological environment demands. In fertilization processes, acrosome aids the sperm to penetrate the ovum using energy provided by the midpiece section. However, spermatozoa of different species have to acclimatize to various fertilization conditions including the penetration of various kinds of egg envelopes (Tavares-Bastos et al., 2002). Therefore, the knowledge on polymorphism of sperm ultrastructure can improve the veracity of the phylogenetic analysis, and it has great value on comparative biology, phylogenetic reconstruction, and evolution history.
4.3 Concluding remarks The sperm ultrastructure characteristics such as the shape of subacrosomal cone point in transverse section, the presence or absence of nuclear space and laminar structure, the shape of the epinuclear electron-lucent zone, the structure of the neck region and the forms of the mitochondria in the midpiece, and the sperm dimensions have certain intergeneric and interspecific differences. These features can cast light on the phylogenetic relationships of Gekkonidae. In addition, detailed description of the spermatozoa ultrastructure and statistical analyses of sperm dimensions of Gekkonidae provide quantitative estimates of the degree of variability in sperm ultrastructure between different genera in Gekkonidae. Moreover, our results provide basic information that are of value for further studies on molecular mechanism of reptile spermatogenesis.
For future study, the molecular mechanism of reptile spermatogenesis should be emphasized. Spermatogenesis is a complex process where spermatogonia undergo mitotic, meiotic, morphological transformations to finally become a functional mature sperm (Hou and Yang, 2013). There are three important steps in this process which include acrosome formation, nuclear shaping and fagellum formation (Hu et al., 2012; Hu et al., 2013). Future studies should include the following topics: formation of acrosome; factors affecting nuclear elongation; composition and function of motor protein and cytoskeleton during spermatogenesis of reptile.
Acknowledgements We thank Joselito M. Arocena, the full professor of University of Northern British Columbia, for his help in polishing the language of our manuscript. Our experimental procedures complied with the current laws on animal welfare and research in China. This work was supported by the grants from the National Natural Science Foundation of China (31170376).
Cunha L. D., Tavares-Bastos L., Báo S. N. 2008. Ultrastructural description and cytochemical study of the spermatozoon of Crotallus durissus (Squamata, Serpentes). Micron, 39: 915–925
Colli G. R., Teixeira R. D., Scheltinga D. M., Mesquita D. O., Wiederhecker H. C., Báo S. N. 2007. Comparative study of sperm ultrastructure of five species of teiid lizards (Teiidae, Squamata), and Cercosaura ocellata (Gymnophthalmidae, Squamata). Tissue Cell, 39: 59–78
Furieri P. 1970. Sperm morphology of some reptiles: Squamata and Chelonia. In Baccetti B. (Ed.), Comparative Spermatology. Rome: Accademia Nazionale dei Lincei, 115–131
Giugliano L. G., Teixeira R. D., Colli G. R., Báo S. N. 2002. Ultrastructure of spermatoxoa of the Lizard Ameiva ameiva, with considerations on polymorphism within the Family Teiidae (Squamata). J Morph, 253: 264–271
Hu J. R., Liu M., Wang D. H., Hu Y. J, Tan F. Q., Yang W. X. 2013. Molecular characterization and expression analysis of a KIFC1-like kinesin gene in the testis of Eumeces chinensis. Mol Biol Rep, 40: 6645–6655
Hu J. R., Xu N., Tan F. Q., Wang D. H., Liu M., Yang W. X. 2012. Molecular characterization of a KIF3A-like kinesin gene in the testis of the Chinese fire-bellied newt Cynops orientalis. Mol Biol Rep, 39(4): 4207–4214
Hou C. C., Yang W. X. 2013. Acroframosome-dependent KIFC1 facilitates acrosome formation during spermatogenesis in the caridean shrimp Exopalaemon modestus. PloS ONE, 8(9): 1–16
Han D. M., Zhou K. Y., Bauer A. M. 2004. Phylogenetic relationships among gekkotan lizards inferred from C-mos nuclear DNA sequences and a new classification of the Gekkota. Biol J Linn Soc, 83: 353–368
Jamieson B. G. M. 1995. The ultrastructure of spermatozoa of the Squamata (Reptilia) with phylogenetic considerations. In Jamieson B. G. M., Ausio J., Justine J. (Eds.), Advancesin Spermatozoal Phylogeny and Taxonomy. Paris: Muséum National d’Histoire Naturelle, 359–383
Jamieson B. G. M., Healy J. M. 1992. The phylogenetic position of the tuatara Sphenodon (Sphenodontida, Amniota), as indicated by cladistic analysis of the ultrastructure of spermatozoa. Philos Trans R Soc London (B), 335: 207–219
Jamieson B. G. M., Koehler L. 1994. The ultrastructure of the spermatozoon of the northern water snake, Nerodia sipedon (Colubridae, Serpentes), with phylogenetic considerations. Can J Zool, 72: 1648–1652
Jamieson B. G. M., Oliver S. C., Scheltinga D. M. 1996. The ultrastructure of spermatozoa of Squamata, I, Scincidae, Gekkonidae and Pygopodidae (Reptilia). Acta Zool, 77: 85–100
Liu Y. Z., Zhang Y. P. 2004. Ultrastructure of spermatozoa of Eumeces elegans. Zool Res, 25(5): 429–435 (In Chinese)
Oliver S. C., Jamieson B. G. M., Scheltinga D. M. 1996. The ultrastructure of spermatozoa of Squamata, II, Agamidae, Varanidae, Colubridae, Elapidae, and Boidae (Reptilia). Herpetologica, 52: 216–241
Phillips D. M., Asa C. S. 1993. Strategies for formation of the midpiece. In Baccetti B. (Ed.), Comparative Spemiauilogy 20 Years After Serono Symp. New York: Raven Press, 75
Rheubert J. L., Siegel D. S., Venable K. J., Sever D. M., Gribbins K. M. 2011. Ultrastructural description of spermiogenesis within the Mediterranean Gecko, Hemidactylus turcicus (Squamata: Gekkonidae). Micron, 42: 680–690
Röll B., von Düring M. U. G. 2008. Sexual characteristics and spermatogenesis in males of the parthenogenetic gecko Lepidoctylus lugubris (Reptilia, Gekkonidae). Zoology, 5: 385–400
Scheltinga D. M., Jamieson B. G. M., Espinoza R. E., Orrell K. S. 2001. Descriptions of the mature spermatozoa of the lizards Crotaphytus bicinctores, Gambelia wislizenii (Crotaphytidae) and Anolis carolinensis (Polychrotidae) (Reptilia, Squamata, Iguania). J Morph, 247: 160–171
Teixeira R. D., Colli G. R., Báo S. N. 1999a. The ultrastructure of spermatozoa of the lizard Polycbrus acutirostris (Squamata, Polychrotidae). J Submicrosc Cytol Pathol, 31 : 387–395
Teixeira R. D., Colli G. R., Báo S. N. 1999b. The ultrastructure of the spermatozoa of the lizard Micrablepharus maximiliani (Squamata, Gymnophthalmidae), with considerations on the use of sperm ultrastructure characters in phylogenetic reconstruction. Acta Zool, 80: 47–59
Teixeira R. D., Colli G. R., Báo S. N. 1999c. The ultrastructure of the spermatozoa of the worm lizard Amphisbaena alba (Squamata, Gymnophthalmidae), and the phylogenetic relationships of amphisbaenians. Can J Zool, 77: 1254–1264
Tavares-Bastos L., Colli G. R., Báo S. N. 2008. The evolution of sperm ultrastructure among Boidae (Serpentes). Zoomorphology, 127: 189–202
Teixeira R. D., Vieira G. H. C., Colli G. R., Báo S. N. 1999d. Ultrastructural study of spermatozoa of the neotropical lizards , Tropidurus semitaeniatus and Tropidurus torquatus (Squamata, Tropiduridae). Tissue Cell, 31: 308–317
Tourmente M., Cardozo G., Bertona M., Guidobaldi A., Giojalas L., Chiaraviglio M. 2006. The ultrastructure of the spermatozoa of Boa constrictor occidentalis, with considerations on its mating system and sperm competition theories. Acta Zool, 87: 25–32
Tourmente M., Giojalas L., Chiaraviglio M. 2008. Sperm ultrastructure of Bothrops alternatus and Bothrops diporus (Viperidae, Serpentes), and its possible relation to the reproductive features of the species. Zoomorphology, 127: 241–248
Teixeira R. D., Scheltinga D. M., Trauth S. E., Colli G. R., Báo S. N. 2002. A comparative ultrastructural study of spermatozoa of the teiid lizards Cnemidophorus gularis gularis, Cnemidophorus ocellifer, and Kentropyx altamazonica (Reptilia, Squamata, Teiidae). Tissue Cell, 34 (3): 135–142
Tavares-Bastos L., Teixeira R. D., Colli G. R., Báo S. N. 2002. Polymorphism in the sperm ultrastructure among four species of lizards in the genus Tupinambis (Squamata: Teiidae). Acta Zool, 83: 297–307
Vieira G. H. C., Colli G. R., Báo S. N. 2004. The ultrastructure of the spermatozoon of the lizard Iguana iguana (Reptilia, Squamata, Iguanidae) and the variability of sperm morphology among iguanian lizards. J Anat, 204 (6): 451–464
Vieira G. H. C., Colli G. R., Báo S. N. 2005. Phylogenetic relationships of corytophanid lizards (Iguania, Squamata, Reptilia) based on partitioned and total evidence analyses of sperm morphology, gross morphology, and DNA data. Zool Scr, 34: 605–625
Vieira G. H. C., Cunha L. D., Scheltinga D. M., Glaw F., Colli G. R., Báo S. N. 2007. Sperm ultrastructure of hoplocercid and oplurid lizards (Sauropsida, Squamata, Iguania) and the phylogeny of Iguania. J Zool Syst Evol Res, 45(3): 230–241
Zhang Y. P., Fang Z. X., Ji X. 2006. A comparison of the ultrastructure of spermatozoa of two species of skinks Mabuya multifasciata and Sphenomorphus indicus. Acta Zool Sin, 52(3): 591–602 (In Chinese)
Zhang Y. P., Hu J. R., Ji X. 2004. Ultrastructure of spermatozoa of the Chinese skink Eumeces chinensis. Acta Zool Sin, 50 (3): 431–441 (In Chinese)
Zhang Y. P., Ying X. P., Ji X. 2005. Ultrastructure of spermatozoon of the northern grass lizard (Takydromus septentrionalis) with comments on the variability of sperm morphology among lizard taxa. Zool Res, 26(5): 518–526 (In Chinese)
Zhao E. M., Zhao K. T., Zhou K. Y. 1999. Fauna Sinica: Reptilia, Vol. 2, Squamata: Lacertilia. Beijing: Science Press (In Chinese)
Prof. Yongpu ZHANG, Wenzhou University, Wenzhou, China, with his research focusing on physiological ecology and reproductive evolution of reptiles.
E-mail: zhangyp@wzu.edu.cn
18 October 2014 Accepted: 19 January 2015
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Japalura (Squamata: Sauria: Agamidae) from Upper Lancang (Mekong) Valley of Eastern Tibet, China
- Seasonal Dynamics of Male and Female Reproductive Systems in the Siberian Salamander, Salamandrella keyserlingii (Caudata, Hynobiidae)
- Oviposition Site Selection in the Malayan Giant Frog (Limnonectes blythii) in Singapore: Conservation Implications
- Genetic and Morphological Variations within Laudakia microlepis (Blanford, 1874) (Sauria: Agamidae) Populations in Southeastern Iran with Description of a New Subspecies
- Potential Distribution Modeling and Morphology of Pelias barani (Böhme and Joger, 1983) in Turkey
- Characterization of the Genetic Diversity of Trachemys dorbigni and Phrynops hilarii
