利用烟道气培养微藻的机制与应用
2015-10-26杜奎梁芳耿亚洪李夜光
杜奎梁芳耿亚洪李夜光
(1. 中国科学院武汉植物园植物种质创新与特色农业重点实验室,武汉 430074;2. 中国科学院大学,北京 100049;3. 郑州师范学院,郑州 450044)
利用烟道气培养微藻的机制与应用
杜奎1,2梁芳3耿亚洪1李夜光1
(1. 中国科学院武汉植物园植物种质创新与特色农业重点实验室,武汉 430074;2. 中国科学院大学,北京 100049;3. 郑州师范学院,郑州 450044)
微藻生物柴油是唯一有潜力代替传统化石燃料解决交通用油问题的可再生生物能源,但其产业化主要受到微藻培养高成本的制约。工业废气(烟道气)不仅含有大量CO2,还含有硫氧化物(SOx)和氮氧化物(NOx)。因此,利用烟道气培养产油微藻既可以降低微藻生物柴油的生产成本,又可以减少温室气体和污染气体的排放。综述了微藻液体悬浮培养系统吸收、转化CO2、SOX和NOx的机理和利用烟道气培养微藻的研究与实践,基于微藻细胞具有高效吸收、转化CO2、SO2和NOx的能力,提出了建立微藻产油、固碳、脱硫、除硝一体化模式来帮助解决当前能源和环境问题的设想。
微藻;烟道气;生物柴油;固碳;脱硫;除硝
微藻通常是指个体较小(2-200 μm)、能进行光合作用(少数为异养生长)的、以单细胞或群体形式存在的水生(或陆生、气生、共生)低等植物[1]。微藻不仅种类多、分布广、繁殖快,而且光合效率和单位面积的产率高[2,3],多种微藻既能产油又能生产高附加值产品,因此,微藻具有很高的开发利用价值。
碳元素是构成微藻细胞的主要元素,含量占细胞干重的36%-65%[4,5],碳源成本在微藻的培养过程中占有比较大的比例。规模化培养微藻,可以使用NaHCO3或CO2作为碳源。以NaHCO3为碳源培养螺旋藻,碳源成本约占培养基原料成本的60%,而以CO2代替NaHCO3,碳源成本可降低90%[6]。
工业废气(烟道气)是大气中CO2的重要来源。以烟道气中的CO2为碳源培养产油微藻不仅可以降低微藻生物柴油约15%的原料成本[7],而且固定了CO2,实现了微藻生物柴油的环境效益。因此,利用烟道气中的CO2为碳源培养产油微藻,已经成为微藻生物柴油研发的重要指导思想之一。
烟道气不仅含有大量CO2,还含有大气污染物硫氧化物(SOx)和氮氧化物(NOx)[8-10]。本文综述了微藻液体悬浮培养系统吸收、转化CO2、SOX和NOx的机理和利用烟道气培养微藻的研究与实践,基于微藻细胞具有高效吸收、转化CO2、SO2和NOx的能力,提出了建立微藻产油、固碳、脱硫、除硝一体化模式来帮助解决当前能源和环境问题的设想,以期为微藻研究同行提供参考。
1 微藻液体悬浮培养系统吸收、转化CO2的机制与应用
微藻主要通过光合作用和钙化作用两种方式固定CO2[11]。如图1所示,富含CO2的烟道气被通入微藻生物反应器后,部分CO2通过自由扩散进入藻细胞[12,13],部分溶解在藻液中的CO2,形成H2CO3,再转化为碳酸氢根或(和)碳酸根离子被微藻吸收[12],某些蓝藻还可吸收作为碳源[14],但是的吸收都需要转运分子和能量。经碳酸酐酶(CA)转化为CO2后被卡尔文循环的第一个酶Rubisco(核酮糖-1,5-二磷酸羧化酶/加氧酶)固定,形成3-磷酸甘油酸脂(3-phosphoglycerate,3-PGA)。

图1 微藻吸收、转化CO2的模型[11]
微藻还可以通过钙化作用固定CO2,生成的CaCO3储存于细胞壁中(方程1,2)[15,16]:

CO2不仅可以作为碳源供微藻生长,还能在一定条件下影响微藻的生理结构和生化组成。Tsuzuki等[17]研究表明,用含2% CO2的空气培养小球藻Chlorella vulgaris比纯空气培养下不饱和脂肪酸含量低,油脂成分也发生了变化。徐敏等[18]报道,通入极高浓度CO2(20%、40%,空气作平衡气体)后,被甲栅藻Scenedesmus armatus细胞的光系统II(PSII)最大光化学效率(Fv/Fm)在24 h内明显下降;其后,随培养时间的增长而逐渐恢复正常。极高CO2浓度下培养6 d后,藻细胞体积稍膨大、颗粒化,色素体结构相对不完整,类囊体膜结构略显松散,蛋白核消失,细胞内的液泡数目增多。Xia和Gao[19]的研究表明,碳酸酐酶活性随着CO2浓度增加而下降;当CO2充足时,Chlamydomonas reinhardtii和Chlorella pyrenoidosa的硝酸还原酶活性降低,小球藻Chlorella pyrenoidosa的叶绿素a与叶绿素b比率增大。Ota等[20]的研究表明:当CO2浓度从5%升至50%时(N2平衡),氮源充足时,脂肪酸含量无变化,但当氮胁迫时,Chlorococcum littorale的总脂肪酸含量下降。对部分微藻,高浓度CO2(30%-50%)有利于总脂和不饱和脂肪酸的积累[21,22]。目前,CO2对微藻生物质化学组成的影响尚不十分清楚。
微藻对CO2的耐受程度受藻种[22-25]、光照[26]、pH值[24]、CO2补充速率[26]和细胞密度[21]等因素影响。微藻的固碳效率则受温度[27,28]、光强[28,29]、光质[30]、光暗周期[31]、通气速率、CO2浓度[28,32]、细胞密度、反应器类型[30,33]、培养基成分[34]、鼓气孔径[32]、藻液深度、碳源浓度和pH值、培养液运动状态[35]等影响。CO2的低吸收率是微藻固碳的瓶颈,气液接触时间短、接触面小是重要原因。如何在微藻培养中实现CO2的高效利用,一直是微藻规模化培养的研究课题之一[35,36]。李夜光等[37]发明了微藻高效利用CO2的专利技术,其装置提高了藻液对CO2吸收率,该技术适合于跑道式培养池和环形培养池养殖各种微藻时补充CO2。微藻固碳是一个生物化学过程,无机碳浓缩机制(Carbon concentration mechanism,CCM)在固定CO2中发挥重要作用,其效率与藻的生长状态和生长阶段密切相关,通常,在藻种生理适应的范围内,增加CO2充气深度,保持较高的pH值,可以提高CO2吸收率。
2 微藻液体悬浮培养系统吸收、转化SOx的机制与应用
SO2为无色气体,是烟道气中SOx的主要形式,在纯水中溶解度极高[10]。如图2所示,微藻液体悬浮培养系统吸收、转化SO2(脱硫)的主要化学和生物化学过程如下[11]:
(1)SO2被碱性藻液吸收:

(2)SO32-被藻细胞光合作用释放的高浓度溶解氧氧化:

硫元素是氨基酸和含硫类囊体脂质的必须元素,因此微藻生长需要充足的硫源。SO2本身可以作为硫源而不影响微藻的生长[25],笔者向光生物反应器中鼓入与BG11培养基等量的SO2(唯一硫源)后,与对照相比,小球藻生物量无明显差异。然而,更多研究表明,SO2抑制微藻的生长,其原因为高浓度SO2导致的基质酸化(方程3)[8,23,25,38],这种抑制常被称为毒性。SO2对微藻的毒性强弱因藻种[39]和SO2浓度而异[40]。一般认为烟道气需要脱硫后才能供微藻利用[40]。
3 微藻液体悬浮培养系统吸收、转化NOx的机制与应用

相对NO2而言,NO为无色较稳定的气体,在水中溶液度极低,在标准大气压下,25℃的纯水中溶解度仅为0.032 g/L[10]。当NO进入藻液中后,分以下3种情况[41-43]:
(1)部分NO逃逸出反应体系。
(2)溶解于藻液中的NO在被氧化为NO2和HNO3,反应如下:
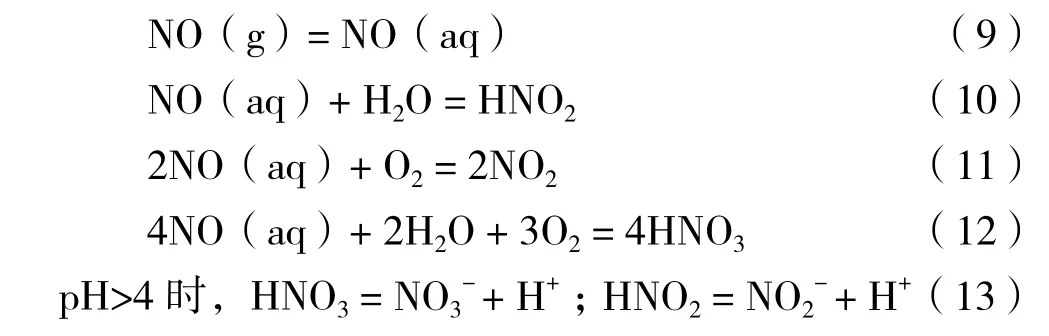

图2 微藻吸收、转化硫氧化物(SOX)的模型[11]
(3)NO被藻细胞表面吸附,并通过自由扩散进入细胞膜,在细胞内被转化为

NOx可以作为氮源被利用而不影响藻的正常生长[25]。Nagase等[42]将NO通入2 m深的杜氏藻Dunaliella tertiolecta藻液中,在光照培养条件下杜氏藻能够不断地吸收利用NO,维持NO去除率50%-60%长达15 d,添加NO与否对微藻的脂类、淀粉和蛋白质含量无影响。
微藻对NOx的耐受性因细胞密度[47]、NOx浓度[8]、反应器类型[43]和藻种[38,40,48,49]而异。
藻细胞和氧气的存在对NO的去除非常重要[42],但高溶氧浓度(如大于35 mg/L)则抑制微藻的生长[50]。NO在藻液中的低溶解度直接影响其去除效率,因此,NO的溶解是去除NO的关键。较小的鼓气气泡和合适的鼓气速率不仅可以促进NO的溶解,还能搅拌藻液,使藻细胞均匀地接收光照和营养,防止沉积和结团。Nagase等[51]采用小气泡逆流型气升式反应器NO去除率最高可达96%,但此反应器设计较复杂,不便于清洗。此外,添加螯合剂Fe(II)EDTA可显著提高NO的去除率。Jin等[52]利用气升式光反应器培养栅藻Scenedesmus sp.,当向培养基中添加5 mmol/L Fe(II)EDTA时提高了NO的溶解度,NO去除率维持在40%-45%长达12 d,但Fe(II)EDTA容易被氧化为不能螯合NO的Fe(III)EDTA,而且Fe(II)EDTA价格较贵,此法推广将受到限制。参与形成氨基酸。NO可以经自由扩散进入细胞,微藻

图3 微藻吸收转化氮氧化物(NOX)的模型[11]
藻液酸化是微藻生长受到抑制的主要原因[53]。为了克服由烟道气引起的基质酸化问题,人们筛选出耐受NOX和SOX或嗜酸性的藻种[23,38,54],但这些藻只能在SO2浓度不高于0.015%的条件下持续生长。此外,酸性基质也不利于烟道气的溶解,因此,嗜酸性藻种对烟道气的去除效果是有限的。保持藻液合适的pH值是克服烟道气对藻酸化抑制的有效途径。向培养液中添加碱性化学物质,如NaOH、CaCO3,虽然可以避免基质酸化,但是添加NaOH溶液,不仅导致高离子压而抑制微藻的生长,而且增加了培养程序的复杂性和成本[39,55];若添加CaCO3,后续分离碳酸钙、硫酸钙沉淀较为麻烦[23]。较小的通气量可以减弱或消除烟道气对微藻生长的抑制作用[56],往往需要额外补充营养物质才能满足微藻的生长。目前,pH自动反馈调节是克服烟道气对藻酸化抑制的理想途径[57],因为它可以根据藻的生长状态灵活控制烟道气的补充量,简便快捷,同时节约了人工成本。
4 利用烟道气培养微藻的研究与实践
1993年,Negoro等[58]在跑道池中培养微绿球藻Nannochloropsis sp. NANNP-2和三角褐指藻Phaeodactylum sp. PHAEO-2,两种微藻直接用烟道气和CO2培养后生物质产率均没有显著差异,Zeiler等[56]也得出了类似的结论。1995年,Maeda等[23]筛选出微藻Chlordu sp. T-l,该藻可以利用燃煤发电厂排放的烟道气进行生长。Lee等[8]研究了SO2和NO对小球藻Chlorella sp. KR-1生长的影响,结果表明:0.01% NO对Chlorella sp. KR-1的生长没有影响,但SO2抑制Chlorella sp. KR-1的生长,当通入0.006% SO2后,生物质产率比对照降低了25%,而且随着SO2的浓度增加,抑制作用更加明显。Doucha等[59]利用天然气燃烧后的烟道气培养小球藻Chlorella sp.,4.4 kg CO2可生产1 kg小球藻(干重),CO2利用率约50%,但此烟气中不含有硫氧化物。Douskova等[60]的研究表明,用烟道气培养的小球藻的生长速率高于用纯CO2和空气混合的对照组。
利用烟道气大规模培养微藻也有少量报道。位于美国夏威夷的Cyanotech公司利用自身的发电厂培养螺旋藻,并商业化运营。发电厂产生的电力为67个螺旋藻养殖池的叶轮搅拌器提供动力,从发电厂分离收集的CO2作为螺旋藻生长所需的碳源[61]。
之前的研究,大多从环境角度,强调对烟道气的去除,微藻仅仅作为生物固碳和去除其他有害气体的工具之一,然而,近年来能源危机日益凸显。因此,利用微藻资源来帮助同步解决环境和能源问题备受瞩目。
1990-2000年,日本国际贸易和工业部就资助了一项名为地球研究更新技术计划的项目,该项计划共有20多家私人公司和政府的研究机构参与,10年间共投资大约25亿美元。他们利用微藻吸收火力发电厂烟道气中的CO2以生产高附加价值的生物质能源微藻,并着力开发密闭光合生物反应器技术。最终分离出10 000多种微藻,筛选出多株耐受高CO2浓度和高温、生长速度快、能形成高细胞密度的藻种,建立了光合生物反应器的技术平台,以及微藻生物质能源开发的技术方案[61]。
5 小结
已有的研究表明,微藻细胞具有吸收、转化CO2、SO2和NOx的代谢途径,能够将烟道气中CO2、SO2和NOx作为碳源、硫源和氮源供细胞生长繁殖。微藻光合自养液体悬浮培养系统是唯一有潜力实现高效产油、固碳、脱硫、除硝一体化的生物系统。与物理和化学脱硫、除硝工艺相比,这一生物系统具有变废为宝,没有二次污染的特点;与微生物脱硫、除硝工艺相比,在脱硫、除硝的同时,实现了生物固碳和微藻油脂的积累,为生物柴油提供了原料。
我国近年来废气中SO2年均排放量约2×107t,NOX年均排放量超过1.5×107t,而且呈逐年增长趋势。废气排放量巨大,其中工业废气占主导地位。以2012年为例,工业二氧化硫和氮氧化物的排放量分别占各自总量的70.9%和90.3%[62]。利用微藻光合自养液体悬浮培养系统建立产油、固碳、脱硫、除硝一体化工艺,不仅能解决利用烟道气中的CO2为碳源培养产油微藻所面临的关键科学问题和技术难题,还能有效抑制温室气体和污染气体的排放,最大限度地实现微藻生物柴油的经济效益和环境效益,推动微藻生物柴油的产业化进程。因此,微藻光合自养液体悬浮培养系统在应对能源危机和环境危机两方面都具有广阔的应用前景。
[1] Greenwell HC, Laurens LML, Shields RJ, et al. Placing microalgae on the biofuels priority list:a review of the technological challenges[J]. Journal of the Royal Society Interface, 2010, 7(46):703-726.
[2] Li Q, Du W, Liu D. Perspectives of microbial oils for biodiesel production[J]. Appl Microbiol Biotechnol, 2008, 80(5):749-756.
[3] Chisti Y. Biodiesel from microalgae[J]. Biotechnology Advances,2007, 25(3):294-306.
[4] Chae SR, Hwang EJ, Shin HS. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photobioreactor[J]. Bioresource Technology, 2006, 97(2):322-329.
[5] Sydney EB, Sturm W, de Carvalho JC, et al. Potential carbon dioxide fixation by industrially important microalgae[J]. Bioresource Technology, 2010, 101(15):5892-5896.
[6] 李夜光, 胡鸿钧, 张良军, 等. 以CO2为碳源工业化生产螺旋藻工艺技术的研究[J]. 武汉植物学研究, 1996, 14(4):349-356.
[7] Acien FG, Fernandez JM, Magan JJ, et al. Production cost of a real microalgae production plant and strategies to reduce it[J]. Biotechnology Advances, 2012, 30(6):1344-1353.
[8] Lee JS, Kim DK, Lee JP, et al. Effects of SO2and NO on growth of Chlorella sp. KR-1[J]. Bioresource Technology, 2002, 82(1):1-4.
[9] Pires JCM, Alvim-Ferraz MCM, Martins FG, et al. Carbon dioxide capture from flue gases using microalgae:Engineering aspectsand biorefinery concept[J]. Renewable and Sustainable Energy Reviews, 2012, 16(5):3043-3053.
[10] Dora J, Gostomczyk MA, Jakubiak M, et al. Parametric studies of the effectiveness of No oxidation of process by ozone[J]. Chemical and Process Engineering-Inzynieria Chemiczna I Procesowa, 2009, 30(4):621-633.
[11] Hende SVD, Vervaeren H, Boon N. Flue gas compounds and microalgae:(bio-)chemical interactions leading to biotechnological opportunities[J]. Biotechnology Advances,2012, 30(6):1405-1424.
[12] Giordano M, Beardall J, Raven JA. CO2concentrating mechanisms in algae:mechanisms, environmental modulation, and evolution[J]. Annual Review of Plant Biology, 2005, 56:99-131.
[13] Badger MR, Price GD. CO2concentrating mechanisms in cyanobacteria:molecular components, their diversity and evolution[J]. Journal of Experimental Botany, 2003, 54(383):609-622.
[14] Mikhodyuk OS, Zavarzin GA, Ivanovsky RN. Transport systems for carbonate in the extremely natronophilic cyanobacterium Euhalothece sp.[J]. Microbiology, 2008, 77(4):412-418.
[15] Jansson C, Northen T. Calcifying cyanobacteria-the potential of biomineralization for carbon capture and storage[J]. Current Opinion in Biotechnology, 2010, 21(3):365-371.
[16] Obst M, Wehrli B, Dittrich M. CaCO3nucleation by cyanobacteria:laboratory evidence for a passive, surface-induced mechanism[J]. Geobiology, 2009, 7(3):324-347.
[17] Tsuzuki M, Ohnuma E, Sato N, et al. Effects of CO2concentration during growth on fatty-acid composition in microalgae[J]. Plant Physiology, 1990, 93(3):851-856.
[18] 徐敏, 陈珊, 刘国祥, 等. 极高CO2胁迫对被甲栅藻(Scenedesmus armatus)生理活性和细胞结构影响[J]. 武汉植物学研究,2004, 22(5):439-444.
[19] Xia JR, Gao KS. Impacts of elevated CO2concentration on biochemical composition, carbonic anhydrase, and nitrate reductase activity of freshwater green algae[J]. Journal of Integrative Plant Biology, 2005, 47(6):668-675.
[20]Ota M, Kato Y, Watanabe H, et al. Fatty acid production from a highly CO2tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate[J]. Bioresource Technology, 2009, 100(21):5237-5242.
[21] Chiu SY, Kao CY, Chen CH, et al. Reduction of CO2by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor[J]. Bioresource Technology, 2008, 99(9):3389-3396.
[22] Tang D, Han W, Li P, et al. CO2biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2levels[J]. Bioresource Technology,2011, 102(3):3071-3076.
[23] Maeda K, Owada M, Kimura N, et al. CO2Fixation from the flue gas on coal-fired thermal power plant by microalgae[J]. Energy Conversion and Management, 1995, 36(6-9):717-720.
[24] Olaizola M. Microalgal removal of CO2from flue gases:changes in medium pH and flue gas composition do not appear to affect the photochemical yield of microalgal cultures[J]. Biotechnology and Bioprocess Engineering, 2003, 8(6):360-367.
[25] Matsumoto H, Hamasaki A, Sioji N, et al. Influence of CO2, SO2and NO in flue gas on microalgae productivity[J]. Journal of Chemical Engineering of Japan, 1997, 30(4):620-624.
[26] Soletto D, Binaghi L, Binaghi L, et al. Effects of carbon dioxide feeding rate and light intensity on the fed-batch pulse-feeding cultivation of Spirulina platensis in helical photobioreactor[J]. Biochemical Engineering Journal, 2008, 39(2):369-375.
[27] Chinnasamy S, Ramakrishnan B, Bhatnagar A, et al. Biomass production potential of a wastewater alga Chlorella vulgaris ARC 1 under elevated levels of CO2and temperature[J]. International Journal of Molecular Sciences, 2009, 10(2):518-532.
[28] Jacob-Lopes E, Scoparo CHG, Franco TT. Rates of CO2removal by a aphanothece microscopica Nageli in tubular photobioreactors[J]. Chemical Engineering and Processing, 2008, 47(8):1371-1379.
[29] Li FF, Yang ZH, Zeng R, et al. Microalgae capture of CO2from actual flue gas discharged from a combustion chamber[J]. Industrial & Engineering Chemistry Research, 2011, 50(10):6496-6502.
[30] Fan LH, Zhang YT, Cheng LH, et al. Optimization of carbon dioxide fixation by Chlorelia vulgaris cultivated in a membranephotobioreactor[J]. Chemical Engineering & Technology, 2007, 30(8):1094-1099.
[31] Jacob-Lopes E, Scoparo E, Queiroz CHG, et al. Biotransformations of carbon dioxide in photobioreactors[J]. Energy Conversion and Management, 2010, 51(5):894-900.
[32] Ryu HJ, Oh KK, Kim YS. Optimization of the influential factors for the improvement of CO2utilization efficiency and CO2mass transfer rate[J]. Journal of Industrial and Engineering Chemistry, 2009,15(4):471-475.
[33] Kumar K, Dasgupta CN, Nayak B, et al. Development of suitable photobioreactors for CO2sequestration addressing global warming using green algae and cyanobacteria[J]. Bioresource Technology,2011, 102(8):4945-4953.
[34] Jin HF, Lim BR, Lee K. Influence of nitrate feeding on carbon dioxide fixation by microalgae[J]. Journal of Environmental Science and Health, Part A:Toxic/Hazardous Substances and Environmental Engineering, 2006, 41(12):2813-2824.
[35] 李夜光, 胡鸿钧. 螺旋藻培养液吸收CO2特性的研究[J]. 武汉植物学研究, 1996, 14(3):253-260.
[36] 李夜光, 胡鸿钧, 龚小敏. 螺旋藻培养液pH值变化的机理和碳源利用率的研究[J]. 生物工程学报, 1996, 12(增刊):242-248.
[37] 李夜光, 耿亚红, 殷大聪, 等. 微藻养殖池补充二氧化碳的装置:中国, CN 100419066C[P].2008-09-17.
[38] Hauck JT, Olson GJ, Scierka SJ, et al. Effects of simulated flue gas on growth of microalgae[J]. Abstracts of Papers of the American Chemical Society, 1996, 212:1391-1396.
[39] Lee JH, Lee JS, Shin CS, et al. Effects of NO and SO2on growth of highly-CO2-tolerant microalgae[J]. Journal of Microbiology and Biotechnology, 2000, 10(3):338-343.
[40] Negoro M, Shioji N, Miyamoto K, et al. Growth of Microalgae in high CO2gas and effects of SOx and NOx[J]. Applied Biochemistry and Biotechnology, 1991, 28-29(1):877-886.
[41] Miller Y, Finlayson-Pitts BJ, Gerber RB. Ionization of N2O4in contact with water:mechanism, time scales and atmospheric implications[J]. Journal of the American Chemical Society,2009, 131(34):12180-12185.
[42] Nagase H, Yoshihara K, Eguchi K, et al. Uptake pathway and continuous removal of nitric oxide from flue gas using microalgae[J]. Biochemical Engineering Journal, 2001, 7(3):241-246.
[43] Nagase H, Yoshihara K, Eguchi K, et al. Characteristics of biological NOx removal from flue gas in a Dunaliella tertiolecta culture system[J]. Journal of Fermentation and Bioengineering,1997, 83(5):461-465.
[44] Vermeiren J, Van de Wiele T, Verstraete W, et al. Nitric oxide production by the human intestinal microbiota by dissimilatory nitrate reduction to ammonium[J]. Journal of Biomedicine and Biotechnology, 2009, 2009:1-10.
[45] Sakihama Y, Nakamura S, Yamasaki H. Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii:an alternative NO production pathway in photosynthetic organisms[J]. Plant and Cell Physiology, 2002, 43(3):290-297.
[46] Mallick N, Rai LC, Mohn FH, et al. Studies on nitric oxide(NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum[J]. Chemosphere, 1999, 39(10):1601-1610.
[47] Yoshihara KI, Nagase H, Eguchi K, et al. Biological elimination of nitric oxide and carbon dioxide from flue gas by marine microalga NOA-113 cultivated in a long tubular photobioreactor[J]. Journal of Fermentation and Bioengineering, 1996, 82(4):351-354.
[48] Radmann EM, Costa JAV. Lipid content and fatty acids compostition variation of microalgae exposed to CO2, SO2and NO[J]. Quimica Nova, 2008, 31(7):1609-1612.
[49] Yanagi M, Watanabe Y, Saiki H. CO2fixation by Chlorella sp. HA-1 and its utilization[J]. Energy Conversion and Management,1995, 36(6-9):713-716.
[50] Singh UB, Ahluwalia AS. Microalgae:a promising tool for carbon sequestration[J]. Mitigation and Adaptation Strategies for Global Change, 2013, 18(1):73-95.
[51] Nagase H, Eguchi K, Yoshihara K, et al. Improvement of microalgal NOx removal in bubble column and airlift reactors[J]. Journal of Fermentation and Bioengineering, 1998, 86(4):421-423.
[52] Jin HF, Santiago DEO, Park J, et al. Enhancement of nitric oxide solubility using Fe(II)EDTA and its removal by green algae Scenedesmus sp.[J]. Biotechnology and Bioprocess Engineering,2008, 13(1):48-52.
[53] Wang X, Hao CB, Zhang F, et al. Inhibition of the growth of two blue-green algae species(Microsystis aruginosa and Anabaena spiroides) by acidification treatments using carbon dioxide[J]. Bioresource Technology, 2011, 102(10):5742-5748.
[54] Kurano N, Ikemoto H, Miyashita H, et al. Fixation and utilization of carbon dioxide by microalgal photosynthesis[J]. EnergyConversion and Management, 1995, 36(6-9):689-692.
[55] Westerhoff P, Hu Q, Esparza-Soto M, et al. Growth parameters of microalgae tolerant to high levels of carbon dioxide in batch and continuous-flow photobioreactors[J]. Environmental Technology,2010, 31(5):523-532.
[56] Zeiler KG, Heacox DA, Toon ST, et al. The use of microalgae for assimilation and utilization of carbon dioxide from fossil fuel fired power plant flue gas[J]. Energy Conversion and Management,1995, 36(6-9):707-712.
[57]Jiang Y, Zhang W, Wang J, et al. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus[J]. Bioresource Technology, 2013, 128:359-364.
[58] Negoro M, Hamasaki A, Ikuta Y, et al. Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler[J]. Applied Biochemistry and Biotechnology, 1993, 39:643-653.
[59] Doucha J, Straka F, Lívanský K. Utilization of flue gas for cultivation of microalgae(Chlorella sp.) in an outdoor open thinlayer photobioreactor[J]. Journal of Applied Phycology, 2005,17(5):403-412.
[60] Douskova I, Doucha J, Livansky K, et al. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs[J]. Applied Microbiology and Biotechnology, 2009, 82(1):179-185.
[61] Pedroni P, Davison J, Beckert H, et al. A proposal to establish an international network on biofixation of CO2and greenhouse gas abatement with microalgae[R]. Proceedings of 1st National Conference on Carbon Sequestration, Washington DC, USA. 2001.
[62] 中华人民共和国环境保护部. 2013年环境统计年报 [ED/OL]. 2014-11-24, http://zls.mep.gov.cn/hjtj/nb/2013tjnb/201411 /t20141124_291867.htm.
(责任编辑 狄艳红)
Cultivation of Microalgae with Flue Gas:Mechanism and Application
Du Kui1,2Liang Fang3Geng Yahong1Li Yeguang1
(1. Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture,Wuhan Botanical Garden,Chinese Academy of Sciences,Wuhan 430074;2. University of Chinese Academy of Sciences,Beijing 100049;3. Zhengzhou Normal University,Zhengzhou 450044)
Biodiesel from microalgae is considered as the only renewable biofuel that has the potential to displace traditional petroleumderived transport fuels and meet the global demand for transport fuels. However, its more widespread use is limited by high cost of microalgal cultivation. Industrial waste gas(flue gas) contains not only a lot of CO2, but also considerable sulfur oxides(SOx) and nitrogen oxides(NOx),so utilization of flue gas for microalgal cultivation to reduce the cost of microalgal biodiesel has atracted more and more attention. In this paper,the mechanism of absorption and metablization of CO2, SO2and NOx by microalgae and the applications of flue gas in cultivation of microalgae were reviewed. Based on the unique capacity of microalgal suspention culture system in utilization of CO2, SO2and NOx, the idea of simultaneous lipid production, CO2fixation, SO2and NOx removal by microalgae was established.
microalgae;flue gas;biodiesel;CO2fixation;SO2removal;NOx removal
10.13560/j.cnki.biotech.bull.1985.2015.02.001
2014-07-09
国家自然科学基金项目(31272680),国家高技术发展计划(“863计划”)(2013AA065803,SS2014AA022001)
杜奎,男,博士研究生,研究方向:植物生物技术;E-mail:dukui@wbgcas.cn
李夜光,男,研究员,研究方向:植物生物技术;E-mail:yeguang@wbgcas.cn
